Abstract
Rationale
Repeated nicotine exposure causes neuroadaptations in limbic cortico-striatal circuits involved in learning and motivation. Such alterations are relevant to addiction because they are suggested to mediate the ability of smoking-associated stimuli to control behavior and to enhance nicotine-seeking and -taking behaviors. Female smokers report higher cue reactivity relative to their male counter parts, yet little is known about putative gender-specific effects of adolescent nicotine exposure on reward-related learning. Prior repeated nicotine exposure in adult male rats enhances Pavlovian approach behavior and conditioned reinforcement
Objective
Given that smoking is typically initiated during adolescence, here we assessed the extent to which adolescent nicotine exposure impacts Pavlovian approach and conditioned reinforcement in male and female rats.
Methods
Rats were injected with nicotine on postnatal days 31–45 prior to training on Pavlovian approach behavior starting on day 51. They were trained to associate a conditioned stimulus (CS), illumination of a magazine light, and tone, with an unconditioned stimulus (US), the delivery of water, for 10-daily sessions, and then were tested on the acquisition of responding with conditioned reinforcement.
Results
Adolescent nicotine exposure selectively increased approach to the magazine during the CS in males but decreased approach to the magazine during the CS in female rats. Vehicle-exposed female rats, however, showed greater magazine approach during the CS than did male control rats. Prior nicotine exposure also enhanced conditioned reinforcement in both male and female rats.
Conclusions
Repeated exposure to nicotine during adolescence had opposite effects on Pavlovian approach behavior in male and female rats but produced enhancement of increases in acquisition of a new response with conditioned reinforcement. Novel information on how nicotine exposure influences reward-related learning during adolescence may increase our understanding of neurobiological mechanisms involved in the initiation of smoking behavior.
Keywords: nicotine, sex differences, Pavlovian approach, conditioned reinforcement, reward learning
1. INTRODUCTION
Reward-related learning plays an important role in drug addiction because stimuli and events associated with drugs can come to support and elicit drug-seeking and -taking behavior (Torregrossa et al., 2011). The external and sensory cues associated with tobacco regulate smoking behavior in multiple ways, and indeed, compulsive drug use is commonly associated with cue-dependent drug-seeking and –taking behaviors (Tiffany and Carter, 1998, Caggiula et al., 2001). In human smokers, nicotine-associated cues can elicit craving (Mucha et al., 1999, Dols et al., 2000, Brody et al., 2002, Due et al., 2002) and region-specific activation of limbic cortico-striatal regions (Mucha et al., 1999, Dols et al., 2000, Brody et al., 2002, Due et al., 2002). In animals, drug-associated stimuli support nicotine self-administration (Caggiula et al., 2001, Donny et al., 2011), produce reinstatement of drug-seeking (Grimm et al., 2001, See, 2002, Feltenstein et al., 2012) and elicit conditioned responses, such as approach to a location associated with delivery of an unconditioned stimulus (US) following onset of a conditioned stimulus (CS) (Everitt et al., 1999, Cardinal et al., 2002), which parallels aspects of smoking behaviors in humans (Donny et al., 2011). Recent work also suggests that pharmacological treatments, such as Olanzapine, a dopamine and serotonin antagonist, may exert therapeutic actions by reducing both cue-elicited craving and the neurobiological responses to smoking cues (Hutchison et al., 2004) Therefore, it is necessary to further investigate the mechanisms by which nicotine impacts reactivity to cues.
Reward-associated stimuli acquire their reinforcing properties through Pavlovian learning. The acquisition of appetitive Pavlovian approach behavior reflects the ability of a neutral stimulus to gain salience by virtue of its association with a reinforcer. Prior repeated exposure to nicotine or psychostimulants in adult rats facilitates the subsequent acquisition of cue-elicited Pavlovian approach behavior (Harmer and Phillips, 1998, Taylor and Jentsch, 2001, Olausson et al., 2003, 2004a) effects attributed to persistent alterations in neural systems involved in incentive learning, and behavioral control (Robinson and Berridge, 1993, Jentsch and Taylor, 1999, Berke and Hyman, 2000, Robinson and Berridge, 2000, Everitt et al., 2001). Nicotine has been shown to establish and enhance the incentive motivational properties of other reinforcers, including reward-associated cues (Balfour et al., 2000, Caggiula et al., 2001, Olausson et al., 2004b, a). Significantly, responding with conditioned reinforcement is potently enhanced following prior repeated nicotine exposure (Olausson et al., 2004b), as are other psychostimulants given acutely or chronically (Robbins, 1977, Taylor and Robbins, 1984, Taylor and Horger, 1999). Furthermore, enhancement of reward occurs following repeated exposure to nicotine and not in response to acute exposure to nicotine alone (Barrett and Odum, 2011). Together these observations argue that nicotine, like other drugs, can facilitate the incentive salience of reward-associated stimuli and that nicotine-induced alterations in cue-elicited behaviors and incentive motivational processes may be relevant to clinical aspects of smoking.
Importantly, the emotional and neuronal responses to smoking cues may differ in males and females. For example, female smokers experienced greater craving in response to nicotine cues than male smokers (Heishman et al., 2010) and gender differences in cue reactivity have been correlated with differential activation of craving- and reward-related regions of the brain (McClernon et al., 2008). Sex differences in the ability of nicotine-paired stimuli to enhance instrumental behavior also have been reported in rats. Female rats respond more for nicotine infusions when the infusion is accompanied by a visual stimulus than male rats (Chaudhri et al., 2005). This difference does not appear to be dependent on baseline levels of nicotine self-administration or cue-induced reinstatement (Feltenstein et al., 2012). Thus, it is necessary to further investigate sex-differences in nicotine modulated cue reactivity.
The initiation of smoking and other compulsive forms of drug use typically occurs during adolescence. Individuals that initiate smoking during adolescence have a high probability of developing a pattern of regular smoking a in adulthood (Patton et al., 1998). Indeed, adolescence has been argued to be a predisposing factor for addiction (Chambers et al., 2003). Here, we examined the impact of daily nicotine administration for 15 days during adolescence (postnatal days 31–45) on cue reactivity measured by Pavlovian discriminative approach behavior and conditioned reinforcement. We hypothesized that the behavioral consequences of adolescent nicotine exposure would be similar to our studies with adult-exposed animals but that this exposure would enhance cue reactivity to a greater extent in female rats than in male rats.
2. MATERIALS AND METHODS
2.1. Animals
Male and female Sprague-Dawley rats (n=40; male n=20, female n=20), aged 31 days at the start of the experiments, were supplied by Charles River (Portage, ME, motivation USA). The rats were housed in pairs under constant cage temperature (20–21°C), humidity (40–50%) and a controlled 12/12 h light-dark cycle (light on at 7 a.m. and off at 7 p.m.) and were initially allowed 7 days to adjust to the housing facilities. The rats had free access to food at all times. Water was available ad libitum until three days prior to the first day of training, and immediately after the 15 day training phase was completed. During the three days prior to the start of training, animals were restricted to 30 min access to water per day. During the testing period, water was intermittently available in the operant chambers according to the behavioral task protocol (see below) as well as in the home cage for 30 min, beginning 30 min after the daily testing session. The experiments in the present study were approved by the Yale University Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Drugs
(−)-Nicotine ditartrate (Sigma, USA) was dissolved in a sterile 0.9% sodium chloride solution, and the pH of the nicotine solution was neutralized with sodium bicarbonate. Nicotine was injected subcutaneously (s.c.) at 2 ml/kg. The dose of nicotine is expressed as the weight of the free-base of nicotine.
2.3. Experimental techniques
Locomotor activity
Locomotor activity was measured using automated activity meters (Digiscan animal activity monitor, Omnitech Electronics, USA). The activity meters were equipped with two parallel rows of infrared photosensors, each row consisting of 16 sensors placed 2.5 cm apart. The activity meters were controlled by and data from the activity meters collected by a PC using the Micropro software (Omnitech Electronics, USA).
Rats were placed in transparent plastic boxes that were fitted into the activity meters. The rats were initially allowed to habituate to the locomotor activity recording equipment for 30 min, after which they were taken out, injected with nicotine or vehicle, and placed back into the boxes. Locomotor activity was then recorded for 60 min starting 5 min after drug injection. All experiments were performed between 8 a.m. and 6 p.m.
Pavlovian discriminative approach behavior
Standard aluminum operant chambers with grid floors (MedAssociates Inc., USA) were used to study the acquisition of Pavlovian discriminative approach behavior and responding with conditioned reinforcement. Each operant chamber was housed in a sound attenuating outer box equipped with a white noise generator and a fan to reduce external noise. A liquid dipper (0.06 ml) delivered water as the reinforcer into the magazine. Head entries were detected by a photocell mounted within the magazine, above the reinforcer receptacle. Above the magazine was a 2.5 W, 24 V light. The operant chamber was illuminated by house light mounted on the back wall. A Sonalert tone (10 kHz) generator was mounted above the magazine. A PC with interface and the MedPC software (MedAssociates Inc., USA) controlled the boxes.
On the first day, rats were familiarized with water availability (the unconditioned stimulus [US]). Water dippers (0.06 ml) were presented for 5 sec on a fixed time 15-sec (FT-15) schedule and the session ended after the delivery of 100 USs. Pavlovian discrimination training sessions began on the second day. Rats received 30 pairings of a 5 sec compound conditioned stimulus (CS; light+tone) followed immediately by 5 sec access to 0.06 ml of water. The CS+US pairings were delivered on a random time 30 sec (RT-30) schedule. Head entries during the RT-30 interval resulted in a 3 sec delay during which time no reinforcement was given, and the RT-30 schedule was restarted. Training on this schedule results in a discriminated pattern of approach of the magazine during CS+US, but not during inter-CS+US, periods.
Acquisition of a New Response with Conditioned Reinforcement
After training on the Pavlovian approach behavior, animals were tested on responding with conditioned reinforcement. In this test, which was performed in the absence of primary reinforcement (i.e. extinction), two novel levers were introduced in the operant chambers. Testing utilized the behaviorally stringent acquisition of a new response with conditioned reinforcement (Taylor and Robbins, 1984). Responding on one lever (‘active’ or CR lever) resulted in the presentation of a 5-sec CS and elevation of the liquid dipper (without water). Responding on the other (‘inactive’ or NCR lever) had no programmed consequences and controlled for non-specific alterations in responding. The first three responses on the active lever elicited presentation of the CS, following which the CS was presented on a variable ratio (VR3) schedule. The session lasted for 30 min following the first response on the CR lever. The position of the CR and NCR levers (left/right) was balanced for all exposure groups.
2.4. Experimental design
Male and female rats were randomly divided into four experimental groups (n=10/group). Two groups (one male, one female) received daily injections (15 consecutive days) with nicotine (0.35 mg/kg s.c.) and the control groups (one male, one female) received the equal volume of a sterile 0.9% sodium chloride solution. The exposures were thus administered between postnatal day 31–45. Locomotor activity was recorded on exposure days 1 and 15 for 8 of the 10 rats in each group. After 5 days of withdrawal from the nicotine exposure, all rats were subjected to the Pavlovian discriminatory approach behavior task described above for 10 consecutive days, starting on postnatal day 51. Following completion of training, all animals were tested on the acquisition of responding with conditioned reinforcement.
2.5. Statistics
The data from the present experiments were evaluated using a two- or three-way analysis of variance (ANOVA) for repeated measures where appropriate. Follow-up comparisons were performed using paired t-test or one-way repeated measure ANOVA where appropriate. Training day or Lever [CR/NCR] was used as repeated measures and Exposure (vehicle/nicotine) and Sex [male/female] were the dependent factors. A probability value (p) equal to or less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Locomotor activity studies
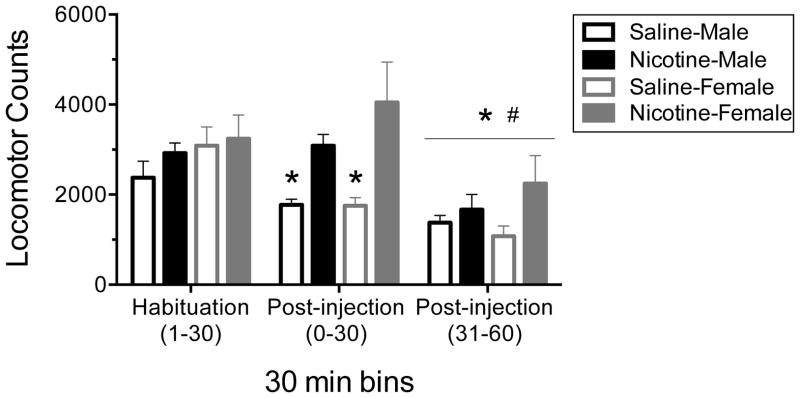
To evaluate the sensitivity to nicotine following the nicotine exposure paradigm in adolescence, we first examined locomotor activity. Here, there was a statistically significant interaction of Timepoint and Exposure (F2,56=10.105; p•0.01; Fig. 1) as well as a significant main effect of Timepoint. Follow-up paired samples t-tests indicate that for vehicle-treated rats locomotor activity was significantly lower in the first thirty minutes (t15=4.122: p<.01) and second thirty minutes (t15=5.302: p<.001) as compared to habituation in the vehicle treated rats and activity was greater in the first thirty minutes following an injection than in the second in the thirty minutes (p<.01). In nicotine-treated rats locomotor activity was equivalent during habituation and the first thirty minutes following nicotine (t15=−1.740: p=n.s.) and then decreased in the second thirty minutes (t15=6.743: p<.01). There was, however, no significant effect of Sex on nicotine-induced locomotor activity (F1,28=0.434; p=n.s.; Fig. 1). Importantly, there was not a statistical difference in locomotor activity between male and female rats overall (F1,28=1.014; p=n.s.; Fig. 1). Thus, on the final day of repeated nicotine exposure (0.35 mg/kg s.c.) during adolescence both male and female rats exhibited enhanced locomotor activity.
Fig. 1.
Effects of repeated nicotine exposure (0.35 mg/kg sc; 15 days) during adolescence (PD 35–50) on nicotine-induced locomotor activity following an acute nicotine (0.35 mg/kg sc) injection on exposure day 15. Statistics: ANOVA for repeated measures with follow-up t-tests for each timepoint comparison; n=8, all groups. There was a significant effect of nicotine exposure (p•0.001). * significant difference compared to habituation, # significant difference compared to the first thirty minutes after injection.
3.2. Appetitive Pavlovian approach behavior
We next examined the effects of prior repeated nicotine exposure during adolescence on appetitive Pavlovian approach. Prior nicotine exposure did not alter the approach of the magazine during the initial magazine training session, and there was no difference between male and female rats (data not shown). Thus, the repeated drug exposure does not appear to affect primary motivation for water or ability to obtain water from the dipper, and, importantly, there are no significant sex-differences in performing the baseline behavior.
We next performed a 2-way repeated measures ANOVA on magazine approach during the CS period (see Fig. 2A) to probe effects of Exposure and Sex across days of training (i.e. Training Day). There was a significant Exposure X Sex X Training day (F9,324=2.0473, p<0.05) interaction. The analysis also identified significant interactions between Exposure X Sex (F1,36=11.300, p<0.01) and Exposure X Training day (F9,324=1.972, p<0.05) along with a main effect of Training day (F9,324=104.556; p•0.001) that confirmed that CS-evoked magazine approach progressively increased as a function of training. In male rats, follow-up one way repeated measures ANOVA revealed a non-significant Training day X Exposure interaction (F9,162=.308, p= n.s.) and significant main effects of both Exposure (F1,18=5.587, p<.05) and Training day (F9,162=55.316, p<.001). In female rats, follow-up one way repeated measures ANOVA revealed a significant Training day X Exposure interaction (F9,162=3.269, p<.05) and significant main effects of both Exposure (F1,18=5.570, p<.05) and Training day (F9,162=50.744, p<.001). Therefore as illustrated in Fig. 2A, magazine approach during CS presentation in male rats was greater in adolescent nicotine treated rats than in vehicle treated rats. In contrast, in female rats magazine approach during CS presentation in male rats was lesser in nicotine treated rats than in vehicle treated rats. Of the vehicle-treated animals, female rats displayed increased Pavlovian approach compared to male rats (F1,18=10.810, p<.05)
Fig. 2.
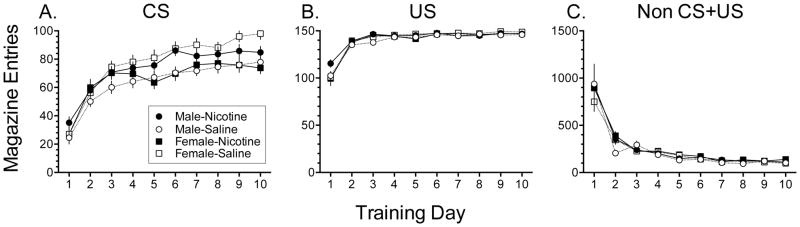
Effects of prior repeated nicotine exposure (0.35 mg/kg sc; 15 days) during adolescence (PD 36–50) on magazine entries during A) CS, B) US and C) non-CS+US periods.: ANOVA for repeated measures; n=10, all groups. There were significant effects of training day (p•0.001) but no significant effect of nicotine exposure.
There was a significant Exposure X Sex X Training day interaction (F9,324=2.625, p<0.05; Fig. 2B) in approach to the US, which suggests that nicotine exposure also impacted approach to the US depending on Sex across training days. There was also a main effect of Training day on approach of the magazine during US presentation (F9,324=151.524; p•0.001), and a significant Exposure X Sex interaction (F1,36=5.396; p•0.05). This interaction was driven primarily by a significant Exposure X Training day interaction in male rats (F1,18=3.556; p<.001).
In the present experiments there was also a main effect of Training day on non-specific approach to the magazine during the inter-CS+US period ((F9,324=85.824; p•0.001; Fig 2C) that was not affected by Exposure (F1,36=1.344; p= n.s.) or by Sex (F1,36=0.029; p= n.s.) in both cases suggestive that all groups were learning the CS-US association.
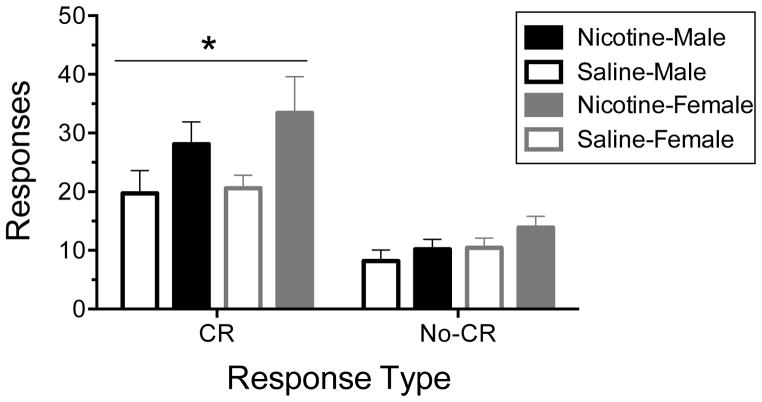
3.3. Conditioned reinforcement
In this experiment, the ANOVA analysis revealed an Exposure X Lever interaction (F1,36=5.837; p•0.05, Fig 3) as well as main effects of Exposure (F1,36=10.699; p•0.01) and Lever (F1,36=55.661; p•0.0001), but not Sex (F1,36=0.402; p=n.s.). Therefore, the basic conditioned reinforcement effect was observed in both the vehicle- and nicotine-treated rats: animals made significantly more responses on the CR lever (eliciting the presentation of the CS) compared to the NCR lever (that had no programmed consequences) during the test. This indicates that the Pavlovian training sessions successfully established the CS as a conditioned reinforcer. Furthermore, prior repeated nicotine (0.35 mg/kg sc) exposure during adolescence significantly increased responding on the CR lever, but not the NCR lever, compared to saline-exposed vehicle-control rats. This was true for both male and female rats. Prior repeated nicotine exposure produced an increase in the responding with conditioned reinforcement that was selective for the CR lever and, thus, behaviorally specific.
Fig. 3.
Effects of repeated daily nicotine exposure (0.35 mg/kg sc; 15 days) during adolescence (PD 36–50) on responding with conditioned reinforcement. Prior repeated nicotine exposure increased responding on the active (i.e., CR) lever but had no effect on responding on the inactive (NCR) lever. Statistics: ANOVA for repeated measures; n=10, all groups. In this experiment, there was a significant effect of lever (p•0.001) and a lever X nicotine exposure interaction (p•0.05). * p•0.05
4. DISCUSSION
The present study investigated the ability of repeated nicotine exposure given during adolescence to augment subsequent Pavlovian discriminative approach behavior and responding with conditioned reinforcement in male and female rats. These experiments demonstrate that daily nicotine exposure in adolescent male rats, prior to training on Pavlovian approach during adulthood, selectively increased head entries into the magazine during CS presentation. These findings confirm previous observations made in adult male rats after prior repeated administration of nicotine or the psychostimulants cocaine or amphetamine (Harmer and Phillips, 1998, Taylor and Jentsch, 2001, Olausson et al., 2003). The current findings support the hypothesis that repeated nicotine exposure produces long-lasting alterations in neurobiological functions that facilitate reward-related learning. Interestingly, the opposite pattern was observed in female rats. Here, adolescent nicotine exposure to female rats reduced Pavlovian CS approach behavior compared to vehicle-exposed controls. The reason for this sex difference is unknown but it occurred against a baseline where nicotine-naïve animals displayed accelerated Pavlovian conditioning and enhanced baseline performance in the task compared to their male counterparts. These differences are unlikely due to alterations in locomotor simulation as activity rates were equivalent in both male and female rats and there were no other behavioral differences. Nicotine exposure to females brought the number of magazine approach during CS presentations to the same level exhibited by vehicle-exposed male rats. It is possible that repeated exposure to nicotine during adolescence interferes with the processes that normally underlie this enhancement in female rats. For example, estrogen has been demonstrated to facilitate neuroplasticity and learning and chronic nicotine exposure markedly inhibits estrogen-response element binding in rats (Shingo et al., 2000). Presently we can only speculate about the possible mechanisms involved, but there are several testable hypotheses for future experiments to study the involvement of adolescent gonadal hormones in response to nicotine.
While many previous studies have shown augmented cognitive abilities in female rats and a positive effect of estrogen in models of learning and memory (Luine, 2008), we are not aware of a previous study demonstrating enhanced appetitive Pavlovian conditioning. Our laboratory has previously used a mouse model to dissociate the consequences of genetic and gonadal sex on reward-related learning processes. In our study by Quinn et al. (Quinn et al., 2007), female sex chromosome complement was associated with accelerated habit formation regardless of gonadal status. In a more recent study, the opposite was observed when alcohol habits were tested where chromosomal male rats displayed more habitual responding (Barker et al., 2010). The results in our present work add to our current understanding regarding the role of sex differences in learning and memory. While the precise neurobiological mechanisms that underlie these sex differences remain to be tested the availability of this models provides us with a model to examine these questions in detail.
Pavlovian approach behavior is dependent on limbic-striatal circuits, including the NAc core and the central nucleus of the amygdala (Everitt et al., 1999, Cardinal et al., 2002). Both of these brain regions receive dense afferent dopaminergic input originating from the ventral tegmental area. It is well established that repeated nicotine exposure produces long-lasting neuroadaptations in this pathway. These neuroadaptations have multiple consequences for the normal functioning of the mesolimbic dopamine system including alterations in dopamine receptors and reward processing in human smokers (Dagher et al., 2001, David et al., 2005, McBride et al., 2006, Rose et al., 2012). Previous studies have demonstrated that dopamine activation in both the NAc and the amygdala is implicated in Pavlovian approach behavior (Hitchcott and Phillips, 1998, Everitt et al., 1999, Parkinson et al., 1999, Cardinal et al., 2002, Phillips et al., 2002). Changes in dopamine neurotransmission have been argued to mediate the actions of repeated psychostimulant exposure on Pavlovian discriminative approach behavior (Harmer and Phillips, 1999, Jentsch and Taylor, 1999). The nicotine-induced alterations in dopamine-regulated signaling within these circuits likely contribute to the altered CS approach observed here. In support of this hypothesis we have previously reported that direct activation of cAMP/PKA signaling in the amygdala also facilitates CS approach behavior as does prior cocaine exposures in male rats (Taylor and Jentsch, 2001, Jentsch et al., 2002). Taken together with the observation that withdrawal from chronic nicotine treatment upregulates cAMP signaling in the amygdala (Tzavara et al., 2002), it is likely that these nicotine-induced enhancements are primarily attributed to drug-induced alterations in dopamine-regulated signaling pathways in male rats.
Nicotine exposure also augments the ability of cues to increase instrumental behavior and cues profoundly regulate smoking behavior. We have previously reported that acute and prior repeated nicotine exposure enhances the ability of cues to motivate responding thereby acting as conditioned reinforcers (Olausson et al., 2004b, a). Nicotine-associated increased responding with conditioned reinforcement can be blocked by mecamylamine, a general nicotinic acetylcholine receptor (nAChR) antagonist (Olausson et al., 2004a). We also found that in mice, the 2-subunit of the nAChR was required for this nicotine-induced enhancement (Brunzell et al., 2006). We have subsequently demonstrated that nAChR signaling is required for the conditioned reinforcing and dopamine-activating effects of both sucrose- or alcohol-associated cues in nicotine-naïve rats (Lof et al., 2007, Lof et al., 2010). Specifically, these experiments implicated 7 and/or 6/ 3 2 3* nAChRs as important mediators of both the dopamine-activating and behavioral effects of sucrose cues (Lof et al., 2010). Together, our prior and current results demonstrate that both nicotine exposure and underlying nAChR mechanisms modulate the ability of reward-associated conditioned stimuli to exert powerful control over behavior in both adult male and female rats during adolescence. There was, however, no effect of sex on enhanced responding with conditioned reinforcement that depends on dopamine and the NAc but not the amygdala (Taylor and Robbins, 1986, Cador et al., 1989, Robbins et al., 1989, Cador et al., 1991), and notably the amygdala is a highly sexually dimorphic nuclei. Our observed sex difference in CS approach behavior, but not conditioned reinforcement, suggests dissociable mechanisms that subserve appetitive Pavlovian approach behavior and responding with conditioned reinforcement (Everitt et al., 1999), and are in line with the lack of sex differences in nicotine self-administration or reinstatement in animals initiating self-administration in adulthood (Feltenstein et al., 2012). Human data also support these observations, as there were no differences in cue reactivity and craving to smoking-related cues shown to male and female smokers (Saladin et al., 2012). Thus, the gender differences in smoking may be more related to other components and drivers of smoking behaviors, such as stress, and negative affect, that are augmented in female smokers (Saladin et al., 2012).
The consequence of acute and repeated nicotine exposure on learning and cognitive functions has been extensively examined (Levin et al., 2006). Nevertheless, few preclinical studies have demonstrated a long-lasting facilitation of learning processes after withdrawal from repeated or sub-chronic nicotine administration as shown here. We, and others, have hypothesized that drug-induced changes in limbic-striatal function are causally involved in the ability of cues to acquire heightened abilities to control behavior, and in combination with reduced cortical inhibitory modulation of motivational impulses may contribute to compulsive and chronically relapsing patterns of drug use (Jentsch and Taylor, 1999, Everitt et al., 2001, Taylor and Jentsch, 2001, Jentsch et al., 2002, Everitt and Robbins, 2005, Everitt et al., 2008, Flagel et al., 2009, Taylor et al., 2009) – including in adolescence (Chambers et al., 2003). These present data support such hypothesis and extends previous research by the demonstration that repeated nicotine exposure during adolescence also enhances responding for conditioned reinforcement, and highlights some sex differences in Pavlovian approach behavior. These current results are relevant to nicotine abuse since smoking-related cues possess the ability to elicit craving and support smoking behavior in humans, and such cues can precipitate relapse to smoking after nicotine abstinence (Mucha et al., 1999, Dols et al., 2000, Mucha et al., 2000, Rose and Behm, 2004). Novel methods aimed at reducing the behavioral impact of cues should be a promising therapeutic target for smoking cessation (Franklin et al., 2011) and other addictions (for review, see (Taylor et al., 2009, Torregrossa et al., 2011)). Additional preclinical research is warranted to examine how sex differences in adolescent nicotine exposure contributes to compulsive behavior.
Research Highlights.
Repeated nicotine exposure during adolescence enhances reward-associated learning in males
Repeated nicotine exposure during adolescence reduces reward-associated learning in females
Female animals display enhanced acquisition of appetitive Pavlovian approach learning at baseline
Repeated nicotine exposure during adolescence augments responding with conditioned reinforement
The ability of nicotine to enhance responding with conditioned reinforcement is not influenced by sex
Acknowledgments
The authors gratefully acknowledge the valuable technical assistance of Ms. Valyphone Phantharangsy and Ms. Victoria Stewart. The present study was financially supported by Public Health Services grants DA11717, DA15222, AA15632 and a pilot grant from the Center for Nicotine and Tobacco Research at Yale that was funded by NCI/NIDA/Robert Wood Johnson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci. 2010;30:9140–9144. doi: 10.1523/JNEUROSCI.0548-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Odum AL. The effects of repeated exposure on the reward-enhancing effects of nicotine. Behav Pharmacol. 2011;22:283–290. doi: 10.1097/FBP.0b013e3283473c25. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of general psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology (Berl) 1991;104:377–385. doi: 10.1007/BF02246039. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dols M, Willems B, van den Hout M, Bittoun R. Smokers can learn to influence their urge to smoke. Addictive behaviors. 2000;25:103–108. doi: 10.1016/s0306-4603(98)00115-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol Behav. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR. Effects of varenicline on smoking cue-triggered neural and craving responses. Archives of general psychiatry. 2011;68:516–526. doi: 10.1001/archgenpsychiatry.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced conditioned inhibition following repeated pretreatment with d-amphetamine. Psychopharmacology (Berl) 1999;142:120–131. doi: 10.1007/s002130050870. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: Effects of tobacco deprivation and sex. Exp Clin Psychopharmacol. 2010;18:245–256. doi: 10.1037/a0019401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcott PK, Phillips GD. Double dissociation of the behavioural effects of R(+) 7-OH-DPAT infusions in the central and basolateral amygdala nuclei upon Pavlovian and instrumental conditioned appetitive behaviours. Psychopharmacology (Berl) 1998;140:458–469. doi: 10.1007/s002130050790. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Rutter MC, Niaura R, Swift RM, Pickworth WB, Sobik L. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology. 2004;175:407–413. doi: 10.1007/s00213-004-1837-3. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, Nestler EJ, Taylor JR. Stimulation of protein kinase a activity in the rat amygdala enhances reward-related learning. Biol Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, Stomberg R, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors are required for the conditioned reinforcing properties of sucrose-associated cues. Psychopharmacology (Berl) 2010;212:321–328. doi: 10.1007/s00213-010-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. Journal of neuroendocrinology. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Pauli P. Modulation of craving by cues having differential overlap with pharmacological effect: evidence for cue approach in smokers and social drinkers. Psychopharmacology (Berl) 1999;147:306–313. doi: 10.1007/s002130051172. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Stuhlinger M, Mundle G. Appetitve effects of drug cues modelled by pictures of the intake ritual: generality of cue-modulated startle examined with inpatient alcoholics. Psychopharmacology (Berl) 2000;151:428–432. doi: 10.1007/s002130000508. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004a;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004b doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. American Journal of Public Health. 1998;88:1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Harmer CJ, Hitchcott PK. Blockade of sensitisation-induced facilitation of appetitive conditioning by post-session intra-amygdala nafadotride. Behav Brain Res. 2002;134:249–257. doi: 10.1016/s0166-4328(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Reward enhancement by psychomotor stimulant drugs [proceedings] Neuropharmacology. 1977;16:529–530. doi: 10.1016/0028-3908(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis M, Stein EA. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71:206–213. doi: 10.1016/j.biopsych.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: pharmacological and behavioral treatments. Nicotine Tob Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–220. doi: 10.1111/j.1521-0391.2012.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Yonezawa M, Sakurai K, Kito S. Nicotine inhibits estrogen response element binding in the rat brain. J Neural Transm. 2000;107:1491–1495. doi: 10.1007/s007020070013. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? Journal of psychopharmacology (Oxford, England) 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Monory K, Hanoune J, Nomikos GG. Nicotine withdrawal syndrome: behavioural distress and selective up-regulation of the cyclic AMP pathway in the amygdala. Eur J Neurosci. 2002;16:149–153. doi: 10.1046/j.1460-9568.2002.02061.x. [DOI] [PubMed] [Google Scholar]