Background: The la ribonucleoprotein domain family member 6, LARP6, regulates collagen type 1 mRNA translation.
Results: IGF-1 increases LARP6 expression, resulting in increased LARP6-collagen type 1 mRNA complex and collagen synthesis in smooth muscle.
Conclusion: IGF-1 enhances collagen fibrillogenesis via induction of LARP6.
Significance: This report uncovers a critical mechanism whereby IGF-1 induces a more stable plaque phenotype in atherosclerosis.
Keywords: Akt, Atherosclerosis, Insulin-like Growth Factor (IGF), Protein Synthesis, Translation, Vascular Smooth Muscle Cells, 5′ Stem-loop, Acheron, Fibrillogenesis, Post-transcriptional
Abstract
Collagen content in atherosclerotic plaque is a hallmark of plaque stability. Our earlier studies showed that insulin-like growth factor-1 (IGF-1) increases collagen content in atherosclerotic plaques of Apoe−/− mice. To identify mechanisms we investigated the effect of IGF-1 on the la ribonucleoprotein domain family member 6 (LARP6). LARP6 binds a stem-loop motif in the 5′-UTR of the mRNAs encoding the collagen type I α-subunits (α1(I) and α2(I)), and coordinates their translation into the heterotrimeric collagen type I molecule. In human aortic smooth muscle cells (SMCs), IGF-1 rapidly increased LARP6 expression and the rate of collagen synthesis and extracellular accumulation. IGF-1 increased both LARP6 and collagen type I expression via a post-transcriptional and translation-dependent mechanism involving PI3K/Akt/p70S6k-signaling. Immunoprecipitation of LARP6, followed by qPCR indicated that IGF-1 increased the level of COL1a1 and COL1a2 mRNA bound to LARP6. Mutation of the 5′ stem-loop of Col1a1 mRNA, which inhibits binding of LARP6, abolished the ability of IGF-1 to increase synthesis of collagen type I. Furthermore, overexpression of a 5′ stem-loop RNA molecular decoy that sequesters LARP6, prevented the ability of IGF-1 to increase pro-α1(I) and mature α1(I) expression in cultured medium. IGF-1 infusion in Apoe−/− mice increased expression of LARP6 and pro-α1(I) in aortic lysates, and SMC-specific IGF-1-overexpression robustly increased collagen fibrillogenesis in atherosclerotic plaque. In conclusion, we identify LARP6 as a critical mediator by which IGF-1 augments synthesis of collagen type I in vascular smooth muscle, which may play an important role in promoting atherosclerotic plaque stability.
Introduction
Collagen type I is the most abundantly expressed protein in the human body and has crucial functions in growth, development, and disease (1). The collagen type I subunit molecule is a fibril-forming heterotrimeric protein composed of two α1 chains and one α2 chain, which fold into a stable and highly ordered triple-helix. Proper assembly of these subunit molecules is important for the collagen macroscopic fibril structure and is required for its tensile strength, stability, and biological function (2, 3). Mutations within the collagen type I genes can result in formation of collagen species with compromised structural integrity and are associated with various connective tissue disorders including osteogenesis imperfecta types I-IV, Ehlers-Danlos syndrome (type VIIA, VIIB), Caffey Disease, and osteoporosis (1–3).
Within the 5′ untranslated region (UTR)2 of the mRNA encoding the α chains, COL1a1 and COL1a2, there is an evolutionarily conserved sequence that forms a stem-loop secondary structure encompassing the translation start codon. This 5′ stem-loop (5′SL) motif has been shown to regulate collagen type I mRNA expression and translation (4–6). Mutation of the COL1a1 5′SL, which abolishes the stem-loop structure but does not affect the coding region of COL1a1, resulted in reduced collagen type I expression in vivo (7). In addition to promoting translation, the 5′SL has also been shown to be inhibitory of translation in vitro and in quiescent hepatic stellate cells in which there is an absence of protein binding to the 5′SL (4, 6). The physical constraints of the 5′SL motif dictate accessibility to the start codon and prevent uncontrolled translation (5, 8). Thus, the 5′SL allows for a tight regulation of initiation of collagen type I translation.
The 5′SL secondary structure is only present in the collagen type I and III (fibril-forming) mRNAs, and the la ribonucleoprotein domain family member 6 (LARP6) is the only protein identified to directly bind the 5′SL (5). LARP6 is a member of the La-related protein family of RNA-binding proteins (LARPs) (9) and coordinates efficient translation of COL1a1 and COL1a2 (5, 10–13). Suppressing LARP6 expression via targeted siRNA significantly reduced collagen type I synthesis and secretion (5).
While excessive collagen production can be detrimental, rapid up-regulation of collagen expression is critical in states such as in response to injury, wound healing, and growth. For instance in atherosclerosis, a deficit in collagen expression results in plaque vulnerability and significantly increases likelihood of plaque rupture (14). Therefore, collagen content in plaque is established as a major hallmark of plaque stability (15–18). Our previous studies in vivo have shown that IGF-1 infusion in Apoe−/− mice significantly reduced oxidative stress and atherosclerosis, and increased plaque collagen (19, 20). SMC-specific IGF-1 overexpression did not change atherosclerotic plaque burden, but did increase collagen content in atherosclerotic plaque together with other features of plaque stability (21). In the current study, we investigate the mechanisms by which IGF-1 increases collagen type I in vascular smooth muscle.
EXPERIMENTAL PROCEDURES
Cell Culture
Human aortic smooth muscle cells (Lonza) were cultured in SmBM medium (Lonza) supplemented with 5% fetal calf serum (FCS), antibiotics, human recombinant epidermal growth factor, insulin, and human recombinant fibroblast growth factor. Experiments were performed at cell passages 4 to 9 under serum-free conditions using a 1:1 mixture of Dulbecco's modified essential medium (DMEM) and F-12 nutrient solution (Invitrogen). Mouse embryonic fibroblasts (MEFs) from 5′ stem-loop mutant (5′SL−/−) or wild type (WT) C57/BL6 mice were obtained as previously described (7). The 5′SL−/− MEFs contained a 21-nt mutation in the 5′-UTR of the COL1a1 gene, which disrupted formation of the stem-loop structure, inhibited LARP6 binding, but did not affect the coding region of the COL1a1 gene (7, 10). MEFs were cultured in 10% FCS, DMEM, and experiments were performed at passages 4 to 8. Adenoviral vectors to express constitutively active Akt1 (ad-CMV-Akt1(Myr)) and Akt2 (ad-CMV-Akt2(Myr)) were purchased from Vector BioLabs (Philadelphia, PA) and cells were incubated at a multiplicity of infection (MOI) of 20 for 48 h.
Animals
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee. Apoe knock-out mice (Apoe−/−) of C57BL/6 background (Jackson Laboratory) were infused with saline or human recombinant IGF-1 (1.5 mg/kg/day) using subcutaneously implanted osmotic minipumps (ALZET, Cupertino, CA). After 5-day continuous infusion, mice were sacrificed and whole aortas were isolated and cleaned, followed by mechanical homogenization in RIPA cell-lysis buffer. Human and mouse IGF-1 levels in serum at time of sacrifice were determined via ELISA (Diagnostic Systems Laboratories, Webster, TX). SMP8-IGF-1 transgenic mice (in an FVB background) were obtained from Dr. James A. Fagin (University of Cincinnati, Cincinnati, Ohio) (22). SMP8-IGF-1 transgene-positive mice (SMP8) were identified by polymerase chain reaction (PCR) and bred to Apoe−/− (C57BL/6 background) mice for 8 generations before this line was used for experiments. SMP8/Apoe−/− and Apoe−/− mice 8 weeks of age were fed a Western-type diet (42% of total calories from fat, 0.15% cholesterol, Harlan, Indianapolis, Ind) for 12 weeks to generate atherosclerosis.
Western Blot
Western blot analysis was performed as previously described (9). In brief, cells were washed with PBS and lysed in RIPA buffer, containing 150 mm NaCl, 20 mm Tris-HCl, pH 7.2, 1 mm EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 0.1 m okadaic acid, 0.1 μm aprotinin, 10 μg/ml leupeptin, and 10 mm NaF. Lysates were quantified via bicinchoninic acid (BCA) protein assay (Thermo-Scientific) and subjected to 10% SDS-PAGE, after addition of loading buffer containing β-mercaptoethanol. β-Mercaptoethanol was not included in sample buffer for non-reducing Western blot. Polyclonal antibodies were used for detection of collagen type I (Rockland, 1:1000), LARP6 (Abnova, 1:500), lamin A (Abcam, 1:700), phospho-Thr308-Akt (Cell Signaling Technology, 1:1000), Collagen I α1 C-telopeptide (Rockland, 1:1000), GFP (abcam, 1:4000), and β-tubulin (abcam,1:500) as control for equal loading. Immunopositive bands were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences), and images were captured using a Bio-Rad GelDoc imager.
Real-time Reverse Transcriptase-Polymerase Chain Reaction
Total RNA extraction and quantitative PCR were performed as previously described (23). Briefly, total RNA was isolated using the TriPure Isolation Reagent (Roche) followed by purification with the RNeasy mini-kit (Qiagen). cDNA was synthesized using the First Strand cDNA Synthesis kit (SA Biosciences) and amplified using 40-cycle 2-step PCR with sequence-specific primer pairs (SA Biosciences) in the iCycler IQ Real-Time Detection System (Bio-Rad). Relative COL1a1 and COL1a2 mRNA expression were determined via normalizing to β-actin mRNA expression, and LARP6 mRNA expression was normalized to β-tubulin mRNA expression.
Collagen Synthesis and Extracellular Accumulation
Synthesis and extracellular accumulation of intact collagen was measured using the method described by Zhang et al. (24), with slight modifications. Cells were grown to ∼80% confluency, washed twice with serum-free medium, and incubated for 0–30 h in serum-free medium containing 2 mCi/ml l-[2,3,4,5-3H(N)]proline (Perkin Elmer). At designated time intervals after treatment with 100 ng/ml IGF-1, the cultured medium was removed and the cells were washed twice with ice-cold PBS. The separated cells and cultured medium were then digested with 1 mg/ml pepsin (in 0.1 m acetic acid) overnight at 4 °C with gentle rocking. Following pepsin-digestion, the remaining triple-helical core of intact collagen was precipitated in 30% trichloroacetic acid (TCA). Samples were then centrifuged and the pellet was resuspended and precipitated in 10% TCA, two times. Precipitant was then resuspended in 500 μl of 0.5 m NaOH, 0.1% Triton X-100, and radioactivity (CPM) was measured using Packard Tri-Carb Liquid Scintillation Counter (1600 CA).
Chemical Inhibitors
The following chemical inhibitors were dissolved in DMSO and added to culture medium 1 h prior to treatment with IGF-1: actinomycin D (1 μg/ml), cycloheximide (1 μg/ml), LY29004 (50 μm), Akt VIII (250 nm), rapamycin (100 nm), PF4708671 (160 nm), or 0.1% DMSO (vehicle).
LARP6 siRNA
siRNA targeting exon 1 of LARP6 (Sigma-Aldrich) were custom designed using the D2 siRNA sequence (5′-UCCAACUCGUCCACGUCCU-3′), previously shown to knock down LARP6 protein expression (5). scrRNA (5′-GGAGGGCUUCGAGUUAGGA-3′) was used as control. siRNA or scrRNA were transfected into HASMCs via electroporation followed by overnight incubation in 5% serum-containing SmBM (Lonza) culture medium. Cells were then placed in serum-free medium for an additional 48 h before treatment with IGF-1, and efficacy of the siRNA to knockdown LARP6 was assessed by Western blot.
Immunoprecipation of Ribonucleoprotein Complex
Messenger RNA-protein complexes have been immunoprecipitated as described (25–27). Briefly, mRNA-protein complexes were extracted from the cells using polysome lysis buffer (100 mm KCl, 5 mm MgCl2, 10 mm HEPES pH 7.0, 0.5% IGEPAL CA-630 (Sigma-Aldrich), 1 mm dithiothreitol, 100 units/ml RNase OUT (Invitrogen), and 0.2% Ribonucleoside Vanadyl Complex, protease inhibitor mixture (Halt Protease Inhibitor Cocktails, Thermo Scientific). Protein contents in the extract were determined using RC DC Protein Assay kit (Bio-Rad), and the equal amount of protein for each sample was subjected to an immunoprecipitation. Immunoprecipitation reaction was achieved by mixing the extract with anti-LARP6 antibody (Abnova) in 50 mm Tris pH 7.4, 150 mm NaCl, 1 mm MgCl2, 0.05% IGEPAL CA-630, 15 mm EDTA, 1 mm dithiothreitol, 100 units/ml RNase OUT (Invitrogen), and 0.2% Ribonucleoside Vanadyl Complex containing protease inhibitor mixture for 18 h at 4 °C. The antibody-LARP6-mRNA complexes were isolated via μMACS Protein G magnetic beads/columns kit (Miltenyi Biotec Inc.) according to the manufacturer's protocol. The collected immunoprecipitants were extracted for RNAs using Tripure reagent (Roche) and further purified using RNeasy kit (Qiagen). COL1a1 and COL1a2 mRNA levels were determined as described above by quantitative realtime-PCR. Mouse non-immune IgG was used in place of the anti-LARP6 antibody in the immunoprecipitation procedure to confirm specific precipitation of LARP6-mRNA complexes.
5′ Stem-loop Molecular Decoy for LARP6
p74WT and p74MUT decoys were designed as previously described (28). Briefly, double-stranded oligonucleotide constructs contained the identical sequence as the 5′SL structure of the COL1a1 gene (or a mutated sequence as control) and the optimal Sm binding site (p74) from the mouse U7 small nuclear RNA-derived gene (29), which allowed for accumulation in both the nucleus and cytoplasm. It was previously shown that LARP6 binds to the wild-type 5′SL sequence but does not bind to the mutated sequence (5). Decoy constructs were cloned into the pAdTrack vector containing green fluorescent protein (GFP) expression cassette under independent control of the cytomegalovirus promoter, as control for infection. Resulting adenoviruses express both GFP and the p74WT or p74MUT decoy, and were amplified by ViraQuest Inc. Cells were incubated in presence of viruses at an MOI of 1000 for 3 days prior to treatment. Between ∼95–100% transduction efficiency was achieved, as judged by GFP fluorescence viewed under fluorescent microscope.
Histochemical Assessment of Collagen Fibers in Atherosclerotic Plaque
After 12 weeks on western diet, SMP8/Apoe−/− and Apoe−/− animals were sacrificed, and serial 6 μm paraffin-embedded cross-sections were made through the aortic valve area. Sections were stained with picrosirius red, examined under polarized light with a Motic BA300 Pol polarized microscope, and collagen fibers in plaque were assessed as described in detail by Rich et al. (30). Picrosirius red dye stains thick, tightly packed collagen fibrils red/orange, intermediate fibrils yellow, and thin, loosely packed fibrils green (31–33). A hue selection method (30), performed via Image-pro software (Media Cybernetics), was used to select and quantify the proportions of different fibril color.
RESULTS
IGF-1 Increases Pro-α1(I) Expression in Human Aortic Smooth Muscle Cells via a Post-transcriptional Mechanism
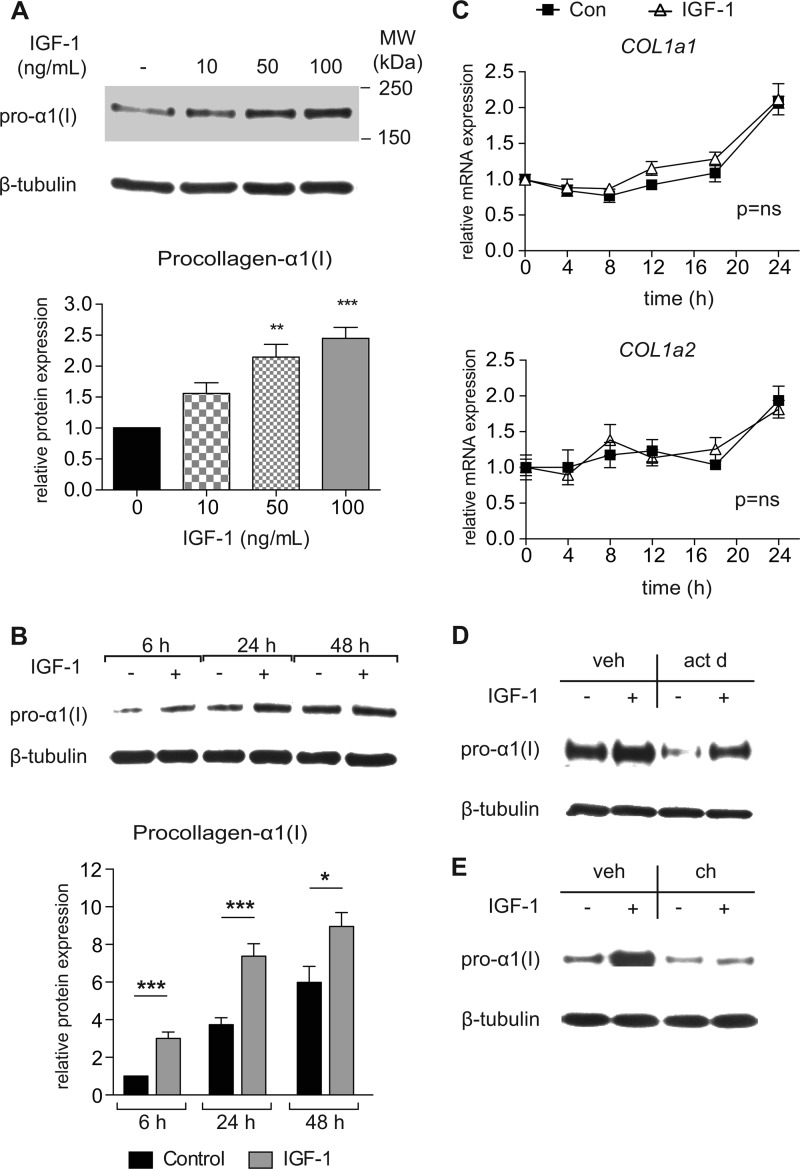
To identify potential mechanisms responsible for the IGF-1-induced increase in collagen expression in vivo, human aortic smooth muscle cells (HASMCs) were grown in primary culture and treated with IGF-1 for 0–48 h. IGF-1 induced a dose- and time-dependent increase in expression of pro-α1(I) (Fig. 1, A and B). Real-time RT-PCR showed that IGF-1 had no significant effect on COL1a1 or COL1a2 mRNA expression (Fig. 1C). Additionally, IGF-1 up-regulation of pro-α1(I) expression was not prevented by actinomycin D (p < 0.01, Fig. 1D), indicative of a post-transcriptional regulation. Furthermore, cycloheximide, an inhibitor of protein synthesis, blocked the ability of IGF-1 to increase pro-α1(I) expression, suggesting that IGF-1 increased collagen type I via a translational mechanism (p < 0.01, Fig. 1E).
FIGURE 1.
IGF-1 dose- and time-dependently increases procollagen-1α expression via a post-transcriptional and translation-dependent mechanism. A, Western blot from HASMCs of procollagen-α1(I) expression treated with different doses of IGF-1 for 18 h, or B, time-dependent expression in response to IGF-1 (100 ng/ml). C, real-time RT-PCR determined COL1a1 and COL1a2 time-dependent mRNA expression in response to IGF-1 (100 ng/ml). D, Western blot of HASMCs treated with IGF-1 (100 ng/ml) for 12 h in presence of actinomycin D (act D) or vehicle (veh), or E, 6 h IGF-1-treatment (100 ng/ml) in presence of cycloheximide (ch). Asterisks (*) indicate statistically significant difference from control (serum-free medium, 0 ng/ml IGF-1); n = 8–12, Mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001.
IGF-1 Increases the Rate of Collagen Type I Synthesis and Extracellular Accumulation
To gain insight into regulation of collagen synthesis, [3H]proline incorporation assays were performed to measure accumulation of intact triple-helical collagen in response to IGF-1. IGF-1 increased the rate of intracellular pepsin-resistant collagen accumulation by 2.2-fold within 4 h (p < 0.0001, n = 6, Fig. 2A). This rapid increase indicates that IGF-1 increased the rate of collagen translation. To determine if this was a result of stimulating general cap-dependent protein synthesis via the mTOR-pathway, experiments were performed in the presence of 100 nm rapamycin, a dose that is inhibitory of both mTORC1 and mTORC2. Rapamycin did not block the IGF-1-induced increase in the rate of 3H-proline accumulation (p < 0.0001, Fig. 2B), and Western blot confirmed that rapamycin did not block the ability of IGF-1 to increase pro-α1(I) expression (Fig. 2C), indicating that IGF-1 increased collagen synthesis via an mTOR-independent mechanism. It is noteworthy that the basal pro-α1(I) expression level was elevated by rapamycin (Fig. 2C, vehicle/-IGF-1 versus rapamycin/-IGF-1). This finding is consistent with feedback activation of Akt in response to mTOR inhibition (34), resulting in up-regulation of pro-α1(I) expression.
FIGURE 2.
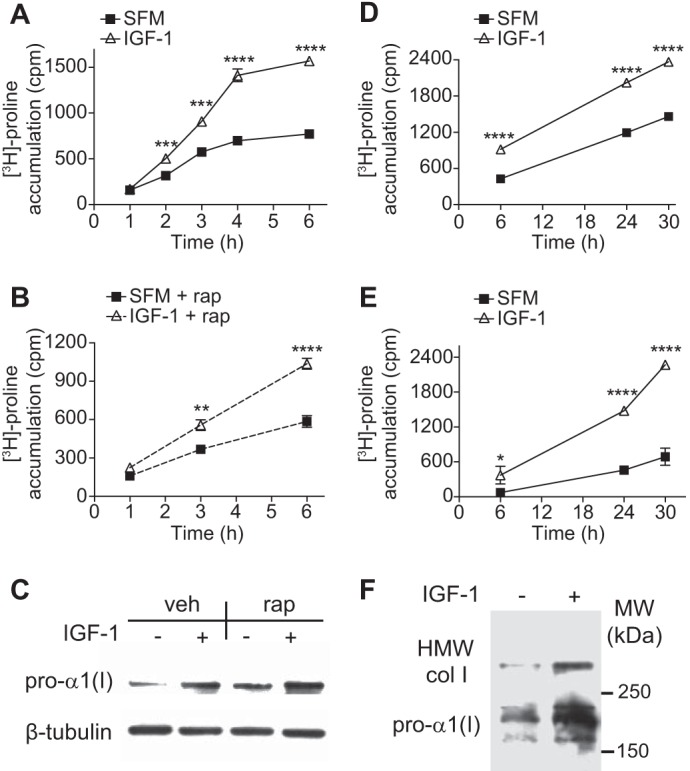
IGF-1 increases the rate of collagen synthesis and extracellular accumulation. A, collagen accumulation in lysate of HASMCs stimulated with IGF-1 (100 ng/ml) or serum-free medium (SFM) for the initial 6 h of stimulation. Collagen was determined via [3H]proline-recovery after overnight pepsin digestion (1 mg/ml) followed by 10% TCA-precipitation. B, same as in A, except IGF-1-stimulation was given in presence of 100 nm rapamycin (rap). C, Western blot of lysate from HASMCs treated with IGF-1 (100 ng/ml) in presence of 100 nm rapamycin. D, same as in A, and [3H]proline accumulation was chased up to 30 h after IGF-1 stimulation. E, same conditions as in A except [3H]proline recovery was measured in cultured medium, representing secreted collagen. F, non-reducing Western blot detecting covalently cross-linked collagen of high molecular weight (HMW) and procollagen-1α in cultured medium from HASMCs treated with IGF-1 for 24 h. Molecular weight is indicated to right of blot. Asterisks (*) indicate statistically significant difference between IGF-1 and SFM at specified time point. n = 4–6, Mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Between 6 and 30 h, IGF-1 steadily increased the rate of intracellular collagen accumulation by 41% (p < 0.0001, Fig. 2D), and IGF-1 increased collagen in cultured medium (3.1-fold at 24 h, p < 0.001, Fig. 2E). To confirm this increase in extracellular collagen, non-reducing Western blot was performed to detect covalently cross-linked collagen type I in cellular medium after 24 h of treatment (Fig. 2F). IGF-1 increased expression of both pro-α1(I) and disulfide-bonded collagen type I (of high molecular weight, HMW) (2.9-fold and 3.8-fold, p < 0.01 and p < 0.05, respectively), consistent with an increase in extracellular accumulation of intact collagen type I. Taken together, these results demonstrate that IGF-1 increases the rate of collagen type I synthesis, leading to enhanced extracellular collagen type I accumulation.
IGF-1 Induction of Pro-α1(I) Expression Correlates with an Increase in Expression of the mRNA-binding Protein, LARP6
Given our findings that IGF-1 post-transcriptionally regulates synthesis of collagen type I, we hypothesized that the mRNA-binding protein, LARP6, could play a role in the ability of IGF-1 to increase collagen type I. LARP6 binds to the 5′ stem-loop secondary structure present in COL1a1 and COL1a2 mRNAs, and regulates efficient translation of the collagen type I heterotrimer (5, 11–13). IGF-1 induced a significant increase in expression of LARP6 within 3 h (2.9-fold, p < 0.01), and this increase was sustained at 24 h (p < 0.01, Fig. 3A). IGF-1 also dose-dependently increased LARP6 expression, and this corresponded with a proportional increase in pro-α1(I) expression (p < 0.01, Fig. 3B). In an effort to determine if LARP6 expression is necessary for the IGF-1-induction of pro-α1(I) expression, HASMCs were transfected with siRNA targeting exon 1 of LARP6 or with scrambled RNA (scrRNA) as control, and treated with IGF-1 for 18 h (administered 3 days after transfection). Although the siRNA effectively suppressed LARP6 expression under non-stimulated conditions (reduced by 53%, p < 0.05, Fig. 3C), IGF-1 increased expression of LARP6 even in the presence of siRNA (2.4-fold, p < 0.01, Fig. 3C). Accordingly, the IGF-1-induction of LARP6 expression in presence of the siRNA corresponded with an IGF-1-induced increase in pro-α1(I) expression (p < 0.05, Fig. 3C). Thus, to identify a possible correlation between LARP6 expression and pro-α1(I) expression, we plotted the relative expression of LARP6 and pro-α1(I) as the fold-change compared with basal levels (i.e. scrRNA) (Fig. 3C). There was a highly significant correlation between the expression of LARP6 and expression of pro-α1(I) (Spearman r = 0.9432, p < 0.0001), indicating a tight association between LARP6 and pro-α1(I) expression levels.
FIGURE 3.
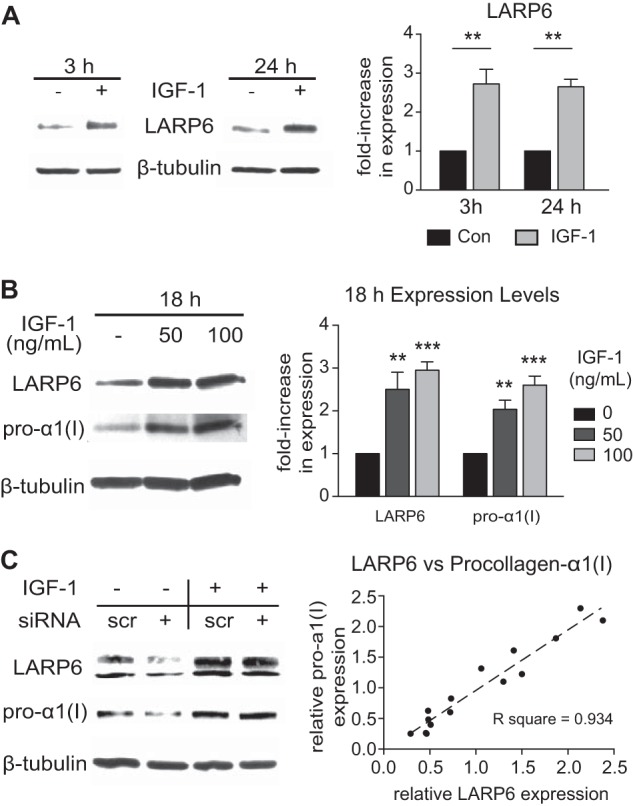
IGF-1 induces expression of LARP6 in HASMCs. A and B, Western blot from HASMCs lysate. C, Western blot from HASMCs after transfection with siRNA targeting LARP6. 3 days after electroporation of 100 nm siRNA or scrRNA (control), cells were stimulated with IGF-1 (100 ng/ml) for an additional 24 h, and lysate was collected for Western blot. D, correlation of LARP6 expression and procollagen-1α expression, from the experiment described in C. The relative expressions of LARP6 and procollagen-1α were determined as the fold-change in expression compared with control levels (i.e. scrRNA without IGF-1-stimulation) and normalized to β-tubulin expression. One data point was plotted for each sample using the relative LARP6 expression as the X-coordinate and the relative procollagen-1α expression as the y-coordinate. (Samples of the control group (scrRNA without IGF-1) were not plotted.) Spearman statistical test for correlation, r = 0.9429, p < 0.0001. n = 6–8, Mean ± S.E., **, p < 0.01; ***, p < 0.001
IGF-1 Increases LARP6 and Pro-α1(I) Expression via a PI3K/Akt/p70S6K-mediated Translational Mechanism
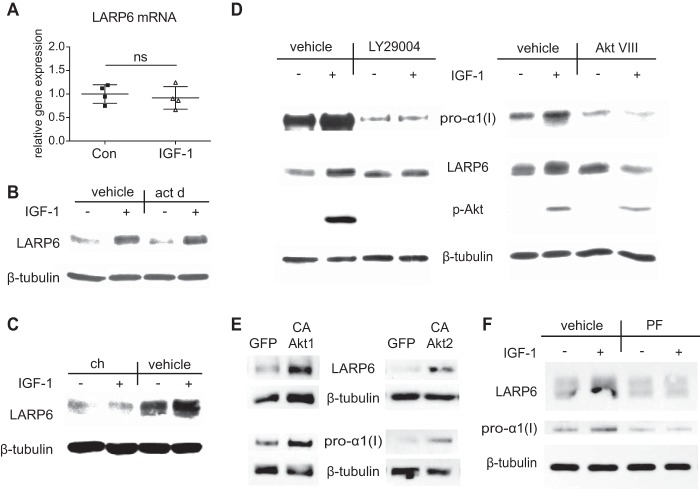
To gain insight into mechanisms whereby IGF-1 increased LARP6 expression, we analyzed LARP6 mRNA and protein levels after IGF-1 treatment with pharmacological inhibitors. IGF-1 had no effect on LARP6 mRNA expression, and actinomycin D did not block the ability of IGF-1 to increase LARP6 protein expression (p < 0.01), indicating that IGF-1 up-regulated LARP6 via a posttranscriptional mechanism (Fig. 4, A and B). In fact, cycloheximide completely inhibited LARP6 up-regulation by IGF-1, suggesting a translational mechanism (Fig. 4C). Furthermore, PI3K-inhibitor (LY2940032) and Akt1/2-inhibitor (Akt VIII) prevented the ability of IGF-1 to increase LARP6 and pro-α1(I) expression (Fig. 4D). To further assess potential involvement of Akt, we overexpressed a constitutively active form of Akt1 (35) and Akt2 (36), and then assessed LARP6 expression (Fig. 4E). Intriguingly, both Akt1 and Akt2 enhanced LARP6 expression (Fig. 4E), which was accompanied with increased pro-α1(I) expression (Fig. 4E). Moreover, inhibition of p70 S6 kinase (p70S6k) completely blocked the IGF-1-induced increase in expression of LARP6 (Fig. 4F). To determine whether p70S6K was required for IGF-1 regulation of pro-α1(I) we inhibited p70S6K activity and found that this abolished IGF-1-induced up-regulation of pro-α1(I) protein levels (Fig. 4F). Taken together these results indicate that IGF-1 increased LARP6 and pro-α1(I) expression via a PI3K/Akt/p70S6K-mediated translational mechanism.
FIGURE 4.
IGF-1 regulation of LARP6 expression. A, real-time RT-PCR determined expression of LARP6 mRNA after 6 h treatment with IGF-1 (100 ng/ml). n = 4, Mean ± S.E. B–D, Western blot of lysate from HASMCs treated for 6 h with IGF-1 (100 ng/ml) in presence of indicated chemical inhibitor: (B) 1 μg/ml actinomycin D (act d), (C) 1 μg/ml cycloheximide (ch), and (D) 50 μm LY29004 (PI3K-inhibitor) or 250 nm Akt VIII (Akt1/2-inhibitor). E, Western blot of lysate from HASMCs overexpressing constitutively active Akt1 and Akt2. Cells were infected with ad-CMV-Akt1 (Myr) (CA-Akt1) and ad-CMV-Akt2 (Myr) (CA-Akt2) at an MOI of 20 for 48 h. F, Western blot of lysate from HASMCs treated for 6 h with IGF-1 (100 ng/ml) in presence of 160 nm PF4708671 (PF, p70S6k inhibitor). Western blot results (B–F) are representative of three independent experiments.
IGF-1 Increases Levels of COL1a1 and COL1a2 mRNA Bound to LARP6
Because LARP6 has been shown to bind COL1a1 and COL1a2 mRNAs and regulate their translation, we analyzed whether IGF-1 alters level of LARP6/COL1a1 and COL1a2 mRNA complex. HASMCs were treated with IGF-1 for 3 h and immunoprecipitation of LARP6 was performed followed by measurement of COL1a1 and COL1a2 mRNA via real-time RT-PCR. Significantly more COL1a1 and COL1a2 mRNA was present in LARP6-immunoprecipitant from IGF-1-treated cells (Fig. 5A). Western blot of input immunoprecipitant shows significantly higher pull-down of LARP6 from IGF-1 treated cells (Fig. 5B), which reflects the IGF-1-induced increase in LARP6 expression (Fig. 3A), resulting in more mRNA binding. These findings suggest that the IGF-1-induction of LARP6 expression is responsible for the increased association of LARP6 with COL1a1 and COL1a2 mRNA.
FIGURE 5.
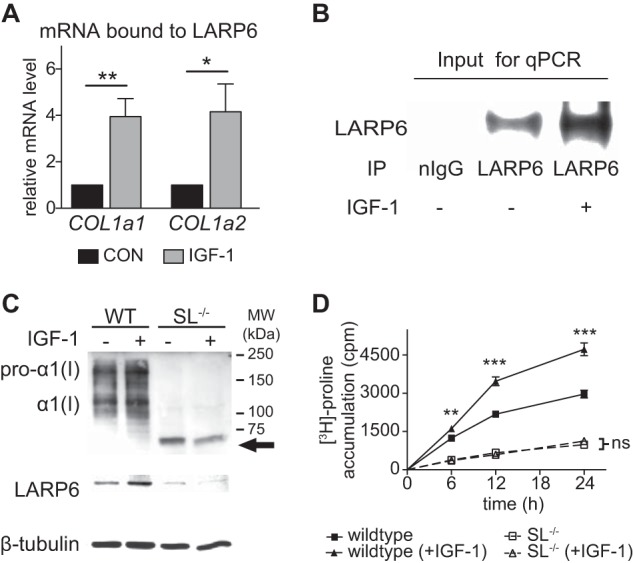
IGF-1 induces association of LARP6 with COL1a1 and COL1a2 mRNA, and enhances collagen synthesis in a 5′ stem-loop-dependent manner. A, COL1a1 and COL1a2 mRNA bound to LARP6. HASMCs were treated with IGF-1 (100 ng/ml) for 3 h and LARP6 was immunoprecipitated from cellular lysate followed by RNA extraction. Relative amount of COL1a1 and COL1a2 mRNA were subsequently determined via real-time RT-PCR (qPCR). B, LARP6 pull-down from immunoprecipitation in A, using a LARP6-antibody or normal IgG (negative control). C, Western blot of lysate from wild-type (WT) or 5′ stem-loop mutant (SL−/−) mouse embryonic fibroblasts (MEFs) treated with IGF-1 (100 ng/ml) for 18 h. Top, immunologic detection using anti-collagen type 1 antibody. An abnormal collagen species observed in SL−/− is indicated with an arrow. D, collagen accumulation in lysate from WT or SL−/− cells in response to IGF-1 (100 ng/ml). Intact triple-helical collagen was measured via [3H]proline recovery after overnight pepsin-digestion (1 mg/ml) followed by 10% TCA precipitation. n = 4–6, Mean ± S.E., *, p < 0.05; **, p < 0.01; ***, p < 0.001.
IGF-1 Stimulates the Rate of Collagen Type I Synthesis in a 5′ Stem-loop-dependent Manner
To determine if the 5′SL is necessary for IGF-1 to increase collagen type I expression, we measured collagen type I levels in mouse embryonic fibroblasts (MEFs) harboring a mutation in the 5′SL of the COL1a1 gene (SL−/−). The 21-nt mutation in the 5′-UTR does not change the coding region of the COL1a1 gene but abolishes the 5′SL secondary structure and prevents the binding of LARP6 to COL1a1 mRNA (11). SL−/− and WT cells were treated with IGF-1, and collagen type I expression was assessed by Western blot and by [3H]proline accumulation assay. IGF-1 had no effect on COL1a1 and COL1a2 mRNA expression in either cell type (data not shown). After 18 h treatment, IGF-1 increased expression of pro-α1(I) in WT cells (2.3-fold, p < 0.01), while IGF-1 failed to increase collagen type I in SL−/− cells (Fig. 5C). Interestingly, an altered collagen species of ∼72 kDa (indicated with arrow, Fig. 5C) was observed in SL−/− cells, indicating that mutation of the 5′SL resulted in abnormal processing and/or cleavage of the procollagen molecule. Intriguingly, basal LARP6 expression was significantly lower in SL−/− cells (74% reduction compared with WT, p < 0.05, Fig. 5A), and IGF-1 did not increase LARP6 expression in SL−/−, suggesting that LARP6 expression and its regulation by IGF-1 is dependent on an intact 5′SL. Overall, these results highlight the significance of the 5′SL for procollagen type I assembly and up-regulation by IGF-1. We also measured the effect of IGF-1 on collagen accumulation after pepsin-digestion, which selects for only the triple-helical core region of intact collagen. IGF-1 induced a 2.0-fold increase in the rate of collagen accumulation in WT cells within 12 h (p < 0.0001), and this induction was completely prevented in SL−/− cells (Fig. 5B). These findings demonstrate that the ability of IGF-1 to increase the rate of collagen synthesis is critically dependent on the 5′SL.
5′ Stem-loop RNA Molecular Decoy for LARP6 Inhibits the IGF-1 Induction of Collagen Type I Expression
A 108-nt RNA containing the identical sequence as the 5′SL structure of the COL1a1 gene was overexpressed in HASMCs using adenoviral delivery and served as a molecular decoy to sequester LARP6. The decoy RNA (p74WT) and a control RNA (p74MUT), which contained a mutated stem-loop sequence unable to bind LARP6, were specially designed to accumulate in both the nucleus and the cytoplasm (28). Both adenoviral vectors additionally expressed GFP (independently of p74MUT or p74WT expression), which served as a control for infection. IGF-1 increased expression of LARP6 in presence of either decoy or control RNA (∼2.5-fold, p < 0.05, Fig. 6A). After 18 h treatment with IGF-1, collagen type I expression was assessed in cellular lysate and in cultured medium. Because LARP6 is implicated in ensuring proper assembly of collagen type I (10–13), we also determined expression of mature collagen type I in the cultured medium using an antibody, which recognizes the α1(I) C-telopeptide. The α1(I) C-telopeptide is exposed after cleavage of the C'-terminal propeptide, and is crucial for cross-linking and fibril formation (37–39). In presence of the p74MUT control RNA, IGF-1 increased intracellular pro-α1(I) expression as well as pro-α1(I) and mature α1(I) expression in the cultured medium (Fig. 6, A and B). However, in the presence of the p74WT decoy, the ability of IGF-1 to increase both the intracellular and extracellular expression of pro-α1(I) was blocked (Fig. 6, A and B). The p74WT decoy also inhibited the ability of IGF-1 to increase expression of mature collagen type I in the cultured medium (Fig. 6B). These results demonstrate that sequestration of LARP6 prevents the ability of IGF-1 to increase both synthesis of pro-α1(I) and expression of extracellular mature collagen type I.
FIGURE 6.
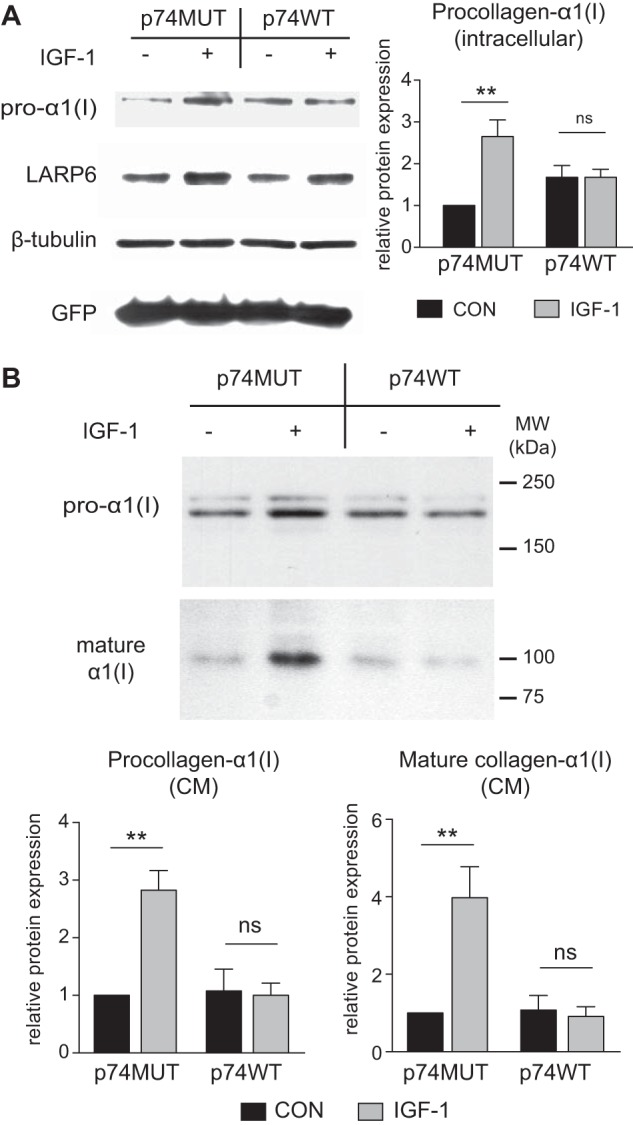
5′ Stem-loop molecular decoy inhibits IGF-1 induction of collagen type I expression. A, Western blot of lysate from HASMCs treated with IGF-1 (100 ng/ml) in presence of stem-loop RNA decoy (p74WT) or a mutated stem-loop RNA (p74MUT) as control. Decoys were overexpressed using adenoviral delivery and cells were treated with IGF-1 3 days after transfection. B, Western blot of cultured medium from conditions described in A. Quantification of Western blot is shown. n = 6, Mean ± S.E., **, p < 0.01.
IGF-1 Increases LARP6 Expression in Vivo
To ascertain whether IGF-1 regulates LARP6 expression in vivo, we infused Apoe−/− mice with human recombinant IGF-1 (1.5 mg/kg/day) or saline (control) and measured LARP6 expression in aortic lysates. Total IGF-1 (mouse + human) in serum at time of sacrifice was measured via ELISA, and the levels confirmed the successful delivery of IGF-1 (Fig. 7B). IGF-1 increased LARP6 by 3.1-fold at 5 days, (p < 0.05, n = 6, Fig. 7A) and this increase corresponded with a 2.3-fold-increase in pro-α1(I) (p < 0.05, n = 6, Fig. 7A).
FIGURE 7.
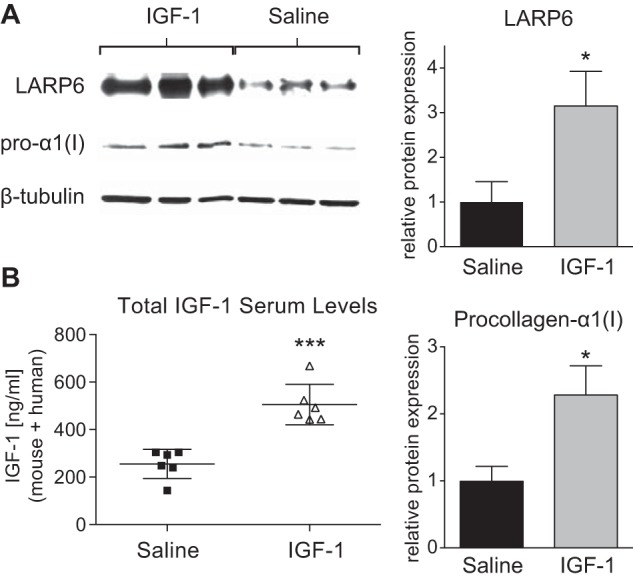
IGF-1-infusion increases LARP6 expression in vivo. A, Western blot of the aortic lysate from Apoe−/− mice given a 5-day continuous infusion of either human recombinant IGF-1 (1.5 mg/kg/day) or saline (control) via a subcutaneously implanted minipump. Quantification is shown to right. B, total IGF-1 (human IGF-1 + mouse IGF-1) levels in serum at time of sacrifice were determined via ELISA. n = 6, Mean ± S.E., *, p < 0.05; ***, p < 0.001.
Smooth Muscle Cell-specific IGF-1 Overexpression Increases Collagen Fibrillogenesis in Atherosclerotic Lesions of Apoe−/− Mice
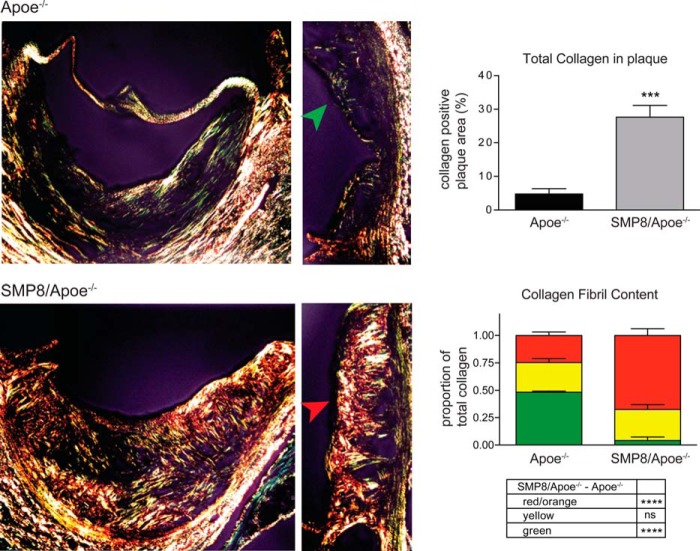
During the progression of atherosclerotic plaques, VSMCs migrate into the lesion and secrete collagen (mainly types I and III) as a means to prevent the necrotic core of the plaque from rupturing and triggering thrombosis (14). The secreted collagen fibrillar network can be examined histologically by using picrosirius red stain, which distinguishes thick collagen fibers (orange-red), fibers of intermediate thickness (yellow), and loosely-packed thin collagen fibers (green), as described previously (30, 40). To evaluate a potential effect of IGF-1 on collagen fibril composition in atherosclerotic plaque, we compared aortic valve cross-sections from Apoe−/− mice with sections from SMP8/Apoe−/− mice, which had an approximate 3-fold increase in aortic IGF-1 levels (22). SMP8/Apoe−/− mice had strikingly more total collagen fibrils within plaque compared with Apoe−/− control (Fig. 8), characteristic of having a more stable plaque phenotype. Furthermore, the ratio of thick (red) fibers to the total collagen fibers in plaque was significantly higher in SMP8/Apoe−/− mice than in Apoe−/− control mice (68% versus 25%, p < 0.0001, n = 4–5, Fig. 8), indicating that IGF-1 enhanced collagen fibrillogenesis.
FIGURE 8.
Smooth muscle-specific IGF-1 overexpression increases collagen fibril maturation in atherosclerotic plaque. Cross-sections of aortic valve atherosclerotic plaque from Apo E-deficient (Apoe−/−) mice or smooth-muscle cell specific IGF-1-overexpressing Apoe−/− mice (SMP8/Apoe−/−). Cross-sections were stained with picrosirius red, which stains thick, tightly packed collagen fibrils red/orange, intermediate fibrils yellow, and thin, loosely packed fibrils green. A hue selection method (performed via Image-pro software) was used to analyze the proportions of different fibril color within plaque. The green arrowhead points to the thin fibrous cap present in Apoe−/− animals, and the red arrowhead points to the thicker, more highly structured fibrous cap present in SMP8/Apoe−/− mice. Quantification of total collagen content in plaque and composition of collagen fiber thickness in each group is shown. n = 4–5, Mean ± S.E., ***, p < 0.001; ****, p < 0.0001.
DISCUSSION
We have shown previously that IGF-1 reduces atherosclerosis in ApoE-deficient mice (19, 20). IGF-1 infusion and SMC-specific IGF-1 overexpression increases features of plaque stability including up-regulation of plaque collagen levels (19, 21), however the mechanism mediating this effect has not been identified. Here we report that IGF-1 increases expression of LARP6 in vascular SMCs resulting in increased binding of LARP6 to the 5′SL of Col1a1 and Col1a2 mRNAs and enhanced synthesis of collagen type I. Furthermore, this augmentation of collagen type I synthesis by IGF-1 leads to enhanced extracellular accumulation of intact collagen and promotes fibril maturation.
The co-translational processing of the collagen type I α-polypeptides requires a coordinated mechanism to select and colocalize the three α chains in order to initiate heterotrimer formation and ensure proper assembly of the procollagen molecule (41). LARP6 is proposed to orchestrate the formation of the collagen type I heterotrimer via binding to the 5′SL and recruiting the appropriate molecular chaperones needed for alignment and maturation (5, 10–13). Our experimental results are consistent with a role of LARP6 in coordinating the synthesis and maturation of collagen type I. We found that IGF-1 increased LARP6 expression and induced accumulation of pepsin-resistant collagen (intact triple helical core) as well as covalently cross-linked collagen type I in cultured medium (Fig. 2F). Covalent cross-linking of collagen and fibrillogenesis is highly dependent on collagen's C-terminal telopeptide region, which is exposed after cleavage of the C-terminal propeptide in the process of maturation (37–39). Our experimental findings show that IGF-1 significantly increased expression of mature α1(I) (exposed C-telopeptide, Fig. 6B) under control conditions, while in presence of the 5′stem-loop decoy, IGF-1 failed to increase mature α1(I) in cultured medium (Fig. 6B). Therefore, this suggests that the ability of IGF-1 to increase extracellular collagen type I maturation is dependent on its ability to induce procollagen synthesis via LARP6.
Our experiments using 5′SL-mutant MEFs further imply a role of LARP6 binding to the 5′SL in the coordination of procollagen assembly. In the SL−/− cells, LARP6 expression was significantly reduced, and the cells produced a collagen species of an altered molecular weight (Fig. 5C), suggesting that altered processing and/or cleavage of the procollagen molecule had occurred. This may be explained by the fact that in SL−/− cells LARP6 was unable to bind COL1a1 mRNA and recruit the appropriate molecular chaperones for maturation. While apparently collagen translation did occur in the 5′SL−/− cells, mutation of the 5′SL resulted in substantially reduced production of intact-triple helical collagen and completely prevented regulation by IGF-1 (Fig. 5, A and B). These results demonstrate the importance of the 5′SL and binding by LARP6 for the proper assembly of procollagen and its regulation by IGF-1.
We observed a significant correlation in the expression levels of LARP6 and pro-α1(I) under conditions both with and without IGF-1-stimulation (Fig. 3C), suggesting that procollagen type I expression is dependent on LARP6 expression. This was consistent with previous findings in which siRNA-driven knockdown of LARP6 had been shown to cause a significant reduction in basal expression of collagen type I in human lung fibroblasts (5). Interestingly, we found that IGF-1 was able to increase LARP6 protein expression in presence of siRNA, and this was correlated with a proportional increase in pro-α1(I) expression (Fig. 3C). We also showed that increased LARP6 expression in response to IGF-1 resulted in an increase of COL1a1 and COL1a2 mRNA bound to LARP6 (Fig. 5, A and B), further suggesting a link between LARP6 expression and pro-α1(I) expression. Since correlation of expression does not necessarily mean cause and effect we confirmed that LARP6 was responsible for the IGF-1-induction of collagen type I expression, by using the 5′SL decoy to sequester LARP6, which completely inhibited the IGF-1-induction of collagen type I expression (Fig. 6). These experimental findings suggest that IGF-1 regulation of LARP6 is responsible for the ability of IGF-1 to increase collagen type I.
LARP6 is a recently characterized RNA-binding protein that is expressed in various human tissues (42); however, little is known about its regulation. In a model of wound healing, LARP6 expression was elevated after mechanical injury, although no specific mechanisms were identified (43). A different report showed that LARP6 (also known as Acheron) expression was increased in some human basal-like ductal carcinomas and that ectopic overexpression of LARP6 resulted in increased proliferation and angiogenesis (44). These reports suggest that increased expression of LARP6 is important for promoting growth and vascularization, however they do not address specific mechanisms by which LARP6 expression is regulated. Our experiments, in which we measured protein expression after treatment with actinomycin D and measured gene expression via quantitative PCR, demonstrate that IGF-1 regulates LARP6 expression post-transcriptionally (Fig. 3, B and C). Furthermore, we show that IGF-1 regulation of LARP6 expression is dependent on PI3k/Akt-signaling (Fig. 3E). Intriguingly, activation of either Akt1- or Akt2-signaling pathways led to an up-regulation of LARP6. We further demonstrated that p70S6k is required for IGF-1 to up-regulate LARP6 as well as pro-α1(I) expression levels (Fig. 4, F and G). On the other hand, inhibition of mTOR had no effect on the IGF-1-induction of pro-α1(I) expression (Fig. 2C), indicating that p70S6k-dependent but mTOR-independent signaling is responsible for the IGF-1 effect. Such a pathway is novel and will require further investigation in the future.
While our experiments show that the 5′SL positively regulates collagen type I expression via LARP6 and its induction by IGF-1, the 5′SL may be inhibitory of translation in the absence of LARP6. Experiments using 5′SL-reporter gene constructs have shown that the 5′SL inhibited expression of collagen type I in quiescent hepatic stellate cells (HSCs) but promoted expression in activated HSCs. Importantly, in quiescent HSCs, the 5′SL did not associate with LARP6, while in activated HSCs association did occur and collagen type I expression was significantly increased (4). Thus, it seems that the 5′SL-regulation of collagen type I synthesis is critically dependent on LARP6 binding. In agreement, our experiments showed that IGF-1 increased the association of COL1a1 and COL1a2 mRNA with LARP6 resulting in an increase in collagen type I expression, and when LARP6 was sequestered with the 5′SL decoy, the IGF-1-induction of collagen type I was inhibited. Therefore, the ability of IGF-1 to post-transcriptionally regulate LARP6 expression resulting in its increased association with COL1a1 and COL1a2 mRNA may be a critical mechanism by which rapid up-regulation of collagen synthesis can be achieved, at least in vascular smooth muscle.
Collagen type I is a major component of the vessel wall, and the ability of vascular smooth muscle cells to synthesize collagen is important in vascular homeostasis and relevant to the pathophysiology of cardiovascular diseases. We show that IGF-1 significantly increases LARP6 expression in aortic tissue, and this corresponds with an increase in procollagen type I expression. The structural integrity of the fibrous cap in atherosclerotic plaque is a major determinant of plaque stability. Unstable plaques, which are more prone to rupture, are characterized by a thinner fibrous cap, larger necrotic cores and less collagen expression (18). The striking increase in collagen content and fibril maturation observed in plaque from SMP8/Apoe−/− mice suggests that IGF-1 promotes plaque stability. Furthermore, the ability of IGF-1 to up-regulate collagen type I synthesis may have implications in various physiological settings such as development and wound healing, in which rapid up-regulation of collagen type I expression is critical for growth and repair.
This work was supported, in whole or in part, by grants from the National Institutes of Health: NHLBI R01-HL070241, R01-HL080682, and R21-HL113705–01A1, NCRR P20-RR018766, and NIGMS P20-GM103514, P30-GM103337, and U54-GM104940. This work was also supported by the American Heart Association (13GRNT17230069).
- UTR
- untranslated region
- SL
- stem-loop
- LARP
- La-related protein family of RNA-binding protein
- HASMC
- human aortic smooth muscle cell
- IGF
- insulin-like growth factor.
REFERENCES
- 1. Prockop D. J., Kivirikko K. I. (1995) Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 2. Chang S. W., Shefelbine S. J., Buehler M. J. (2012) Structural and mechanical differences between collagen homo- and heterotrimers: relevance for the molecular origin of brittle bone disease. Biophys. J. 102, 640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDaniel D. P., Shaw G. A., Elliott J. T., Bhadriraju K., Meuse C., Chung K. H., Plant A. L. (2007) The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys. J. 92, 1759–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stefanovic B., Brenner D. A. (2003) 5′ stem-loop of collagen alpha 1(I) mRNA inhibits translation in vitro but is required for triple helical collagen synthesis in vivo. J. Biol. Chem. 278, 927–933 [DOI] [PubMed] [Google Scholar]
- 5. Cai L., Fritz D., Stefanovic L., Stefanovic B. (2010) Binding of LARP6 to the conserved 5′ stem-loop regulates translation of mRNAs encoding type I collagen. J. Mol. Biol. 395, 309–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefanovic B., Hellerbrand C., Brenner D. A. (1999) Regulatory role of the conserved stem-loop structure at the 5′ end of collagen α1(I) mRNA. Mol. Cell Biol. 19, 4334–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parsons C. J., Stefanovic B., Seki E., Aoyama T., Latour A. M., Marzluff W. F., Rippe R. A., Brenner D. A. (2011) Mutation of the 5′-untranslated region stem-loop structure inhibits α1(I) collagen expression in vivo. J. Biol. Chem. 286, 8609–8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kozak M. (1987) At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 196, 947–950 [DOI] [PubMed] [Google Scholar]
- 9. Bayfield M. A., Yang R., Maraia R. J. (2010) Conserved and divergent features of the structure and function of La and La-related proteins (LARPs). Biochim. Biophys. Acta 1799, 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai L., Fritz D., Stefanovic L., Stefanovic B. (2010) Nonmuscle myosin-dependent synthesis of type I collagen. J. Mol. Biol. 401, 564–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Challa A. A., Stefanovic B. (2011) A novel role of vimentin filaments: binding and stabilization of collagen mRNAs. Mol. Cell Biol. 31, 3773–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manojlovic Z., Blackmon J., Stefanovic B. (2013) Tacrolimus (FK506) prevents early stages of ethanol induced hepatic fibrosis by targeting LARP6 dependent mechanism of collagen synthesis. PLoS One 8, e65897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manojlovic Z., Stefanovic B. (2012) A novel role of RNA helicase A in regulation of translation of type I collagen mRNAs. Rna 18, 321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee R. T., Libby P. (1997) The unstable atheroma. Arterioscler. Thromb. Vasc. Biol. 17, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 15. Rekhter M. D. (1999) Collagen synthesis in atherosclerosis: too much and not enough. Cardiovasc. Res. 41, 376–384 [DOI] [PubMed] [Google Scholar]
- 16. Adiguzel E., Ahmad P. J., Franco C., Bendeck M. P. (2009) Collagens in the progression and complications of atherosclerosis. Vasc. Med. 14, 73–89 [DOI] [PubMed] [Google Scholar]
- 17. Fuster V., Moreno P. R., Fayad Z. A., Corti R., Badimon J. J. (2005) Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 46, 937–954 [DOI] [PubMed] [Google Scholar]
- 18. Libby P., Ridker P. M., Hansson G. K. (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 [DOI] [PubMed] [Google Scholar]
- 19. Sukhanov S., Higashi Y., Shai S. Y., Blackstock C., Galvez S., Vaughn C., Titterington J., Delafontaine P. (2011) Differential requirement for nitric oxide in IGF-1-induced anti-apoptotic, anti-oxidant and anti-atherosclerotic effects. FEBS Lett. 585, 3065–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sukhanov S., Higashi Y., Shai S. Y., Vaughn C., Mohler J., Li Y., Song Y. H., Titterington J., Delafontaine P. (2007) IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27, 2684–2690 [DOI] [PubMed] [Google Scholar]
- 21. Shai S. Y., Sukhanov S., Higashi Y., Vaughn C., Kelly J., Delafontaine P. (2010) Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler. Thromb. Vasc. Biol. 30, 1916–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J., Niu W., Nikiforov Y., Naito S., Chernausek S., Witte D., LeRoith D., Strauch A., Fagin J. A. (1997) Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J. Clin. Invest. 100, 1425–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida T., Galvez S., Tiwari S., Rezk B. M., Semprun-Prieto L., Higashi Y., Sukhanov S., Yablonka-Reuveni Z., Delafontaine P. (2013) Angiotensin II Inhibits Satellite Cell Proliferation and Prevents Skeletal Muscle Regeneration. J. Biol. Chem. 288, 23823–23832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao X., Zhang L. K., Zhang C. Y., Zeng X. J., Yan H., Jin H. F., Tang C. S., Du J. B. (2008) Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens Res. 31, 1619–1630 [DOI] [PubMed] [Google Scholar]
- 25. Higashi Y., Pandey A., Goodwin B., Delafontaine P. (2013) Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1. Biochim. Biophys. Acta 1832, 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peritz T., Zeng F., Kannanayakal T. J., Kilk K., Eiriksdóttir E., Langel U., Eberwine J. (2006) Immunoprecipitation of mRNA-protein complexes. Nat. Protoc. 1, 577–580 [DOI] [PubMed] [Google Scholar]
- 27. Tenenbaum S. A., Lager P. J., Carson C. C., Keene J. D. (2002) Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods 26, 191–198 [DOI] [PubMed] [Google Scholar]
- 28. Stefanovic B., Schnabl B., Brenner D. A. (2002) Inhibition of collagen alpha 1(I) expression by the 5′ stem-loop as a molecular decoy. J. Biol. Chem. 277, 18229–18237 [DOI] [PubMed] [Google Scholar]
- 29. Grimm C., Stefanovic B., Schümperli D. (1993) The low abundance of U7 snRNA is partly determined by its Sm binding site. EMBO J. 12, 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rich L., Whittaker P. (2005) Collagen and Picrosirius red staining: A Polarized Light Assessment of Fibrillar Hue and Spacial Distribution. Brazilian J. Morph. Sci. 22, 97–104 [Google Scholar]
- 31. Rajavashisth T. B., Xu X. P., Jovinge S., Meisel S., Xu X. O., Chai N. N., Fishbein M. C., Kaul S., Cercek B., Sharifi B., Shah P. K. (1999) Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation 99, 3103–3109 [DOI] [PubMed] [Google Scholar]
- 32. Dayan D., Hiss Y., Hirshberg A., Bubis J. J., Wolman M. (1989) Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 93, 27–29 [DOI] [PubMed] [Google Scholar]
- 33. Ovchinnikova O., Robertson A. K., Wågsäter D., Folco E. J., Hyry M., Myllyharju J., Eriksson P., Libby P., Hansson G. K. (2009) T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe(-/-) mice. Am. J. Pathol. 174, 693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wan X., Harkavy B., Shen N., Grohar P., Helman L. J. (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26, 1932–1940 [DOI] [PubMed] [Google Scholar]
- 35. Wei Y., Gong J., Thimmulappa R. K., Kosmider B., Biswal S., Duh E. J. (2013) Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc. Natl. Acad. Sci. U.S.A. 110, E3910–E3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gherzi R., Trabucchi M., Ponassi M., Gallouzi I. E., Rosenfeld M. G., Briata P. (2010) Akt2-mediated phosphorylation of Pitx2 controls Ccnd1 mRNA decay during muscle cell differentiation. Cell Death Differ. 17, 975–983 [DOI] [PubMed] [Google Scholar]
- 37. Garnero P., Schott A. M., Prockop D., Chevrel G. (2009) Bone turnover and type I collagen C-telopeptide isomerization in adult osteogenesis imperfecta: associations with collagen gene mutations. Bone 44, 461–466 [DOI] [PubMed] [Google Scholar]
- 38. Chung H. J., Steplewski A., Chung K. Y., Uitto J., Fertala A. (2008) Collagen fibril formation. A new target to limit fibrosis. J. Biol. Chem. 283, 25879–25886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Canty E. G., Kadler K. E. (2005) Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 118, 1341–1353 [DOI] [PubMed] [Google Scholar]
- 40. Deguchi J. O., Aikawa E., Libby P., Vachon J. R., Inada M., Krane S. M., Whittaker P., Aikawa M. (2005) Matrix metalloproteinase-13/collagenase-3 deletion promotes collagen accumulation and organization in mouse atherosclerotic plaques. Circulation 112, 2708–2715 [DOI] [PubMed] [Google Scholar]
- 41. Beck K., Boswell B. A., Ridgway C. C., Bächinger H. P. (1996) Triple helix formation of procollagen type I can occur at the rough endoplasmic reticulum membrane. J. Biol. Chem. 271, 21566–21573 [DOI] [PubMed] [Google Scholar]
- 42. Valavanis C., Wang Z., Sun D., Vaine M., Schwartz L. M. (2007) Acheron, a novel member of the Lupus Antigen family, is induced during the programmed cell death of skeletal muscles in the moth Manduca sexta. Gene 393, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun R., Chen W., Zhao X., Li T., Song Q. (2011) Acheron regulates vascular endothelial proliferation and angiogenesis together with Id1 during wound healing. Cell Biochem. Funct. 29, 636–640 [DOI] [PubMed] [Google Scholar]
- 44. Shao R., Scully S. J., Jr., Yan W., Bentley B., Mueller J., Brown C., Bigelow C., Schwartz L. M. (2012) The novel lupus antigen related protein acheron enhances the development of human breast cancer. Int. J. Cancer 130, 544–554 [DOI] [PMC free article] [PubMed] [Google Scholar]