Background: Apoptosis deficiency causes necrotic cell death that activates the innate immune response in mammals.
Results: Apoptosis-deficient Drosophila exhibited constitutive Toll pathway activation mediated by serine protease Persephone.
Conclusion: Apoptosis-deficient Drosophila produces damage-associated molecular patterns (DAMPs) leading to canonical Toll signaling through a Persephone-mediated proteolytic cascade that cleaves the Toll ligand Spätzle.
Significance: DAMP-mediated Toll-like receptor signaling is conserved in invertebrates.
Keywords: Apoptosis, Caspase, Drosophila, Innate Immunity, Necrosis (Necrotic Death), Serine Protease, Stress Response, Toll Receptors, Damage-associated Molecular Patterns
Abstract
Apoptosis is an evolutionarily conserved mechanism that removes damaged or unwanted cells, effectively maintaining cellular homeostasis. It has long been suggested that a deficiency in this type of naturally occurring cell death could potentially lead to necrosis, resulting in the release of endogenous immunogenic molecules such as damage-associated molecular patterns (DAMPs) and a noninfectious inflammatory response. However, the details about how danger signals from apoptosis-deficient cells are detected and translated to an immune response are largely unknown. In this study, we found that Drosophila mutants deficient for Dronc, the key initiator caspase required for apoptosis, produced the active form of the endogenous Toll ligand Spätzle (Spz). We speculated that, as a system for sensing potential DAMPs in the hemolymph, the dronc mutants constitutively activate a proteolytic cascade that leads to Spz proteolytic processing. We demonstrated that Toll signaling activation required the action of Persephone, a CLIP domain serine protease that usually reacts to microbial proteolytic activities. Our findings show that the Persephone proteolytic cascade plays a crucial role in mediating DAMP-induced systemic responses in apoptosis-deficient Drosophila mutants.
Introduction
Inflammatory responses are activated by determinants that are inherent to infectious agents, known as pathogen-associated molecular patterns, as well as by endogenous factors produced by host cells. These latter signals, analogically termed damage- (or danger)-associated molecular patterns (DAMPs),3 are inaccessible to the immune system but can be released by severely stressed and damaged cells as in the case of pathogenic infection (1), traumatic injury (2), or spontaneous tumor formation (3).
Physiological responses to DAMPs are functionally governed by the innate immune system, which is evolutionarily conserved and plays a key role in homeostasis. Among the cellular receptors that sense these danger signals, the Toll-like receptor family is reported to be responsive to a variety of DAMPs. DAMP release is associated with cellular damage, in particular the loss of membrane integrity or rupture of the plasma membrane, a hallmark of the necrotic form of cell death. In eukaryote cells, an intracellular family of proteases known as caspases functions to orchestrate apoptotic signaling, leading to the removal of unwanted cells without stimulating the immune system. However, necrotic cell death can arise from apoptotic pathway suppression because of caspase deficiency or pharmacological inhibitors (4, 5).
Dronc, the Drosophila ortholog of caspase-9, is thought to be crucial in initiating apoptotic caspase cascades during development as well as in response to stress (6, 7). Animals lacking zygotic dronc arrest as early to mid pupae and exhibit a wide range of defects, including impaired apoptosis, random formation of melanotic clots, and severe blood cell hyperplasia (8–11).
In the current study, we demonstrated that the hemolymph of apoptosis-deficient Drosophila dronc mutants contains DAMPs that activate the Toll innate immune pathway. We provide substantial evidence that the extracellular protease Persephone is indispensable for sensing the DAMPs derived from apoptosis-deficient cells, indicating that Toll-like receptor-mediated DAMP signaling is conserved in Drosophila.
EXPERIMENTAL PROCEDURES
Drosophila Strains
Drosophila stocks were raised on standard cornmeal-agar media at 25 °C and 60–64% humidity, in either vials or bottles, unless otherwise indicated. Fly stocks used for overexpression experiments were generated through transgene combination or homologous recombination, using standard crosses. The genetic deletion lines droncΔA8 and driceΔ2C8 were described previously (8). The following strains were obtained from the Bloomington Drosophila Stock Center: w1118, Act5C-Gal4, and w;;His2Av-mRFP, FRT80B/TM6B, Tb. The tissue-specific Gal4 driver for hemocytes (pxn-Gal4) was described previously (12).
The stock lines spzrm7, dMyd88Kra1, Tl10b, and Drs-GFP were described previously and were kindly provided by Dr. Shoichiro Kurata with the authors' permission. Dronc Nc51 (9) was a generous gift from Dr. John M. Abrams; UAS-Droncwt-FLAG was from Dr. Sally Kornbluth (13); UAS-DroncC318A was from Dr. Pascal Meier (6); UASp-Spz-HA was from Dr. David Stein (14); and pxn-Gal4, UAS-GFP was from Dr. Michael J. Galko. dark82 was obtained by the courtesy of Dr. John M. Abrams (15). psh1 was a kind gift from Dr. Jean-Marc Reichhart (16).
Drosophila Culture
To prepare germ-free animals, wild-type and droncΔA8 mutant embryos were collected on the surface of grape juice agar and were subjected to consecutive washing with 70% ethanol, 70% ethanol, 2.7% sodium hypochlorite solution (Purelox-X), 70% ethanol, 70% ethanol, and 1× PBS buffer in a clean cabinet. Subsequently, the dechorionated embryos were maintained in sterile food at 25 °C (method adopted from Ref. 17). The removal of bacteria was confirmed by plating assay on LB medium as follows (37 °C, 24 h). The larvae undergo a primary wash with 70% ethanol to remove the microbes attached to the body surface, followed by a hand-prepared homogenation in sterile PBS solution. The total homogenates corresponding to contents in one individual third instar larva were inoculated on LB plate. No bacterial colonies were detected in germ-free condition.
Quantitative Reverse Transcription-PCR
A sample batch containing five or six wandering third instar larvae or the dissected fat bodies from eight or nine individual larvae was homogenized with a Multi-Beads Shocker (Yasui Kikai) set to 1500 rpm, 15 s, and three cycles. The homogenate was used for RNA extraction with TRIzol reagent according to the manufacturer's instructions. The cDNA was synthesized with the Takara PrimeScript RT reagent kit with gDNA eraser using 1 μg of total RNA. The cDNA products (5 μl of a 10-fold diluted solution) were then used for PCR amplification with Takara Premix Ex Taq II (Tli RNaseH Plus) in a LightCycler 480 system (Roche Applied Science). The quantitative PCR cycle was as follows: denaturing at 95 °C for 30 s → PCR cycles at 95 °C for 5 s and at 55 °C for 25 s for 45 cycles → melting curve at 95 °C for 5 s, at 65 °C for 1 min, and at 97 °C with acquisition every 5 s → cooling down at 40 °C for 10 s. The housekeeping genes RNA polymeraseII and rp49 were used as internal controls interchangeably. For each set of data, the value of one wild-type sample was used as a calibrator, and the relative ratios of the other genotypes versus the calibrator were calculated accordingly. The results were summarized from three or more independent tests. The primers used are summarized in supplemental Table S1.
Mosaic Clone Analysis
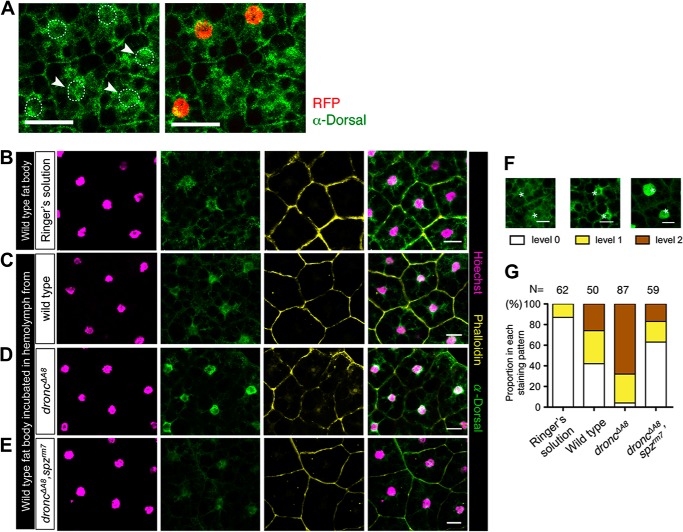
To generate dronc null clones in the larval fat bodies, embryos at 6–8 h after egg laying, with the genotype hs-Flp;; His2Av-RFP, FRT80B/droncΔA8, FRT80B, were subjected to a 30-min heat shock pulse at 37 °C to induce FLP-mediated recombination between cis-acting allelic FRT sites. The heat-treated larvae were kept at 21 °C until they reached the wandering third instar stage. In the dissected larval fat body, cell clones were recognized by loss of the His2Av-RFP allele, which directs nuclei-localized red fluorescence protein (RFP) expression. Both the droncΔA8 mosaic clone and the control fat body clone were subjected to immunohistochemistry staining to examine the localization of Dorsal.
Immunohistochemical Staining and GFP Fluorescence Analysis
Wandering third instar larvae were dissected to collect fat bodies or blood cells. The samples were fixed with 4% paraformaldehyde for 22.5 min at room temperature, followed by five consecutive 10-min washes in PBST (PBS + 0.1% Triton X-100). The fixed samples were blocked for 30 min in 5% donkey serum, followed by overnight incubation at 4 °C with primary antibodies: mouse monoclonal anti-Dorsal (1:200, clone 7A4 (18); Hybridoma Bank), or rabbit polyclonal anti-tRFP antibody (1:250; Evrogen AB232). Washed samples were incubated for 1–2 h at room temperature with the corresponding secondary antibody: Alexa Fluor goat anti-mouse IgG (1:500; Invitrogen) or donkey anti-rabbit-cy3 (1:500; Jackson). F-actin and nuclear DNA were labeled with rhodamine-conjugated phalloidin (1:500) and Höechst 33342 (4 μm), respectively. Samples were mounted in mounting buffer and analyzed by confocal microscopy (TCS SP5; Leica). Images were captured after maximum projection processing of multiple confocal scanning planes. To examine the GFP fluorescence emitted from the larval fat body (Drs-GFP reporter) or hemocytes (pxn-Gal4, UAS-GFP), the larvae were washed with PBS and submerged in ice-chilled PBS, and then the intact larvae were analyzed under a Leica stereomicroscope equipped with GFP-compatible filters.
Western Blotting of Larval Hemolymph
Wandering third instar larvae were collected from control (pxn-Gal4, UASp-spz-HA) and mutant (pxn-Gal4, UASp-spz-HA; droncΔA8) groups; washed successively in PBS, 70% ethanol, and PBS; and placed on ice until dissection. The dissection was performed by ripping off a strip of the larval skin longitudinally starting from the posterior dorsal end without compromising internal tissues. For both genotypes, hemolymph containing an adequate number of blood cells from five or six larvae was bled into 10 μl of PBS, followed by centrifugation at 5000 × g and 4 °C for 30 min. The supernatant was then further centrifuged at 8000 × g for 10 min to obtain the hemolymph aqueous fraction. The samples were separated on 15% SDS-polyacrylamide gels under denaturing conditions and then semi-dry transferred to an Immobilon-P membrane (Millipore) in transfer buffer (25 mm Tris, 192 mm glycine, and 20% methanol). After blocking for 40 min in 4% skim milk/TBST (w/v) at room temperature, the membrane was incubated with mouse monoclonal anti-HA tag (clone 12CA5, 1:1000; Roche Applied Science) at 4 °C overnight and then incubated with horseradish peroxidase-conjugated anti-mouse IgG antibody (1:1000; Promega) for 1 h at room temperature. The blots were visualized using Immobilon Western Chemiluminescence (Millipore). Western blotting images were captured using an LAS4000 MINI (GE Healthcare ImageQuant) and were processed using Photoshop software.
Fat Body ex Vivo Culture Assays
Isolated wild-type fat bodies were incubated in Ringer's solution, with or without hemolymph extracts from flies of various genotypes. Each fat body explant was cultured with hemolymph obtained from four individual donor larvae. The co-incubation was performed for ∼7 h in a humid incubator. The fat body explants were then subjected to Dorsal immunostaining analysis to measure the extent of immune activation, and the images were captured with a confocal microscope (TCS SP5; Leica). After recovering the images, we scored the results by classifying the degree of Dorsal activation in the fat body explants according to three distinctive Dorsal staining patterns (see Fig. 3F); fat body cells with minimal, medium, or intense Dorsal staining in the nuclei were designated as levels 0, 1, and 2, respectively. Subsequently, the percentage of cells associated with each of the three classes was calculated for each of the genotypes evaluated (see Fig. 3G).
FIGURE 3.
Dorsal activation is triggered by extracellular stimuli. A, dorsal intracellular distribution in droncΔA8 mosaic clones. RFP negative cells are homozygous droncΔA8 mutant cells. Nuclear RFP (red) and Dorsal protein (green) were examined by immunohistochemical staining. The larval genotype was hs-Flp;;His2Av-RFP,FRT80B/droncΔA8,FRT80B. Scale bar, 50 μm. B–E, immunohistochemistry staining of fat body explants incubated with Ringer's solution (B), hemolymph from wild-type (w1118, C), droncΔA8 mutants (D), or droncΔA8 spzrm7 double mutants (E). The distribution of DNA (magenta), Dorsal (green), and F-actin (yellow) is shown as maximal projection confocal images. An image overlay illustrates Dorsal nuclear translocation. Scale bar, 20 μm. F, classification of Dorsal distribution in fat body explants, based on Dorsal nuclear staining patterns. Scale bar, 20 μm. G, for each culture condition, the percentage of cells associated with each Dorsal staining pattern was calculated. The total cell number counted for each treatment is indicated as N. N was normalized to 100%.
Statistical Tests
Experimental differences were compared between experimental and control groups, and p values were obtained using the two-tailed Student's t test. The one-way analysis of variance test was used to compare multiple sets of samples.
RESULTS
Dronc Deficiency Leads to Drosomycin Induction in the Fat Body through Dorsal Activation
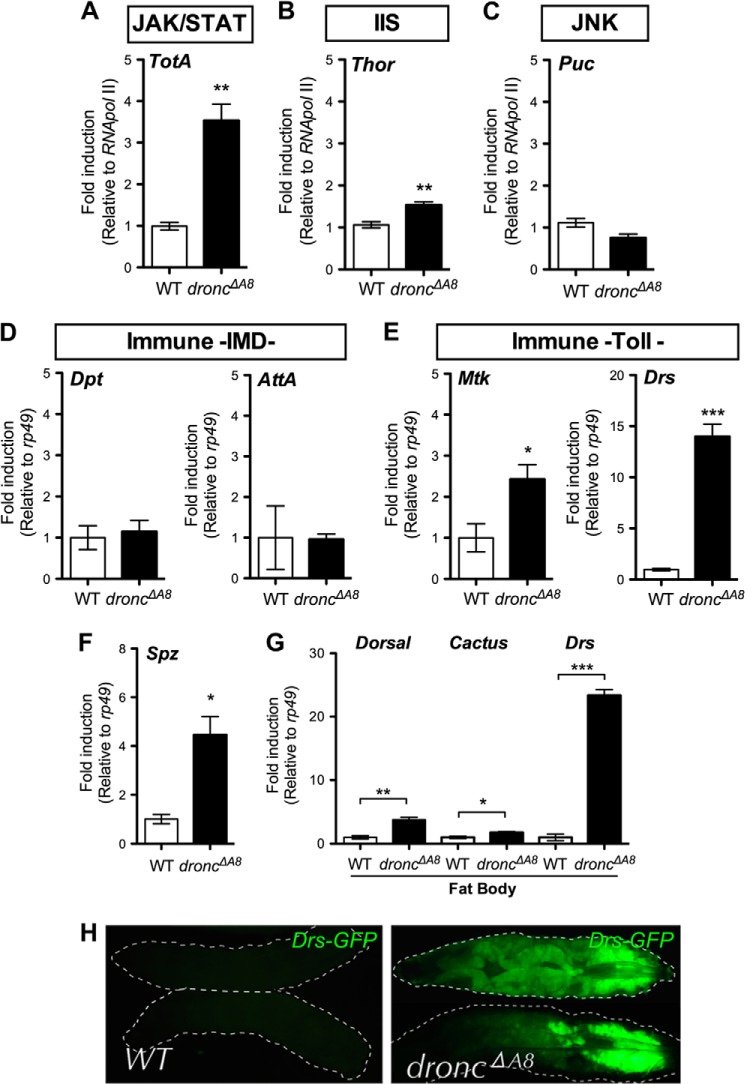
In Drosophila, Dronc is the initiator caspase activated at the onset of apoptosis, and loss of this protein blocks most, if not all of the apoptosis occurring during development and in response to stress (8–11). To study the stress-responsive pathway that is triggered in the dronc null mutant (droncΔA8), we measured the expression level of a number of stress signal target genes using quantitative RT-PCR in L3 larvae. The dronc mutants exhibited elevated levels of transcripts for Turandot A (TotA) and Thor, the obligate downstream target genes for the JAK/STAT pathway, and the insulin receptor cascade, respectively (Fig. 1, A and B) (19–21). Turandot proteins are involved in the physical protection and repair of damaged tissue upon stimulation by mechanical, thermal, or infectious stress (22), whereas the expression of the translational initiation factor Thor is induced in response to wounding (21), starvation (20), or oxidative stress (23) through the activation of dFOXO. In contrast, expression of the transcript encoding the phosphatase Puckered (Puc), a negative regulator of the JNK pathway (24), was unchanged in the mutant larvae (Fig. 1C), suggesting that JNK signaling may not be important for responding to the physiological stress associated with caspase deficiency.
FIGURE 1.
droncΔA8 mutants exhibit elevated stress responses and constitutive Toll pathway signaling. A–E, transcript levels of genes for stress response and innate immunity were examined using quantitative RT-PCR with total RNA from wild-type and droncΔA8 L3 larvae. F, transcripts encoding extracellular components Spz were significantly induced in the whole body of droncΔA8 flies. G, transcripts encoding the Dorsal/NF-κB, Cactus/IκB, and Drs were induced in the droncΔA8 fat body. H, expression of the Drs-GFP fluorescent reporter was induced predominantly in the droncΔA8 fat body. The genotype was WT, Drs-GFP, Dpt-lacZ. droncΔA8, Drs-GFP, Dpt-lacZ;; droncΔA8/droncΔA8. Statistics are results of Student's t test. *, p < 0.05; **, p < 0.01; ***, p < 0.0001.
It was previously shown that the Thor promoter contains a canonical NF-κB recognition sequence and that Thor plays critical roles in host immune defense (21). We next assessed the activation of immune pathways by quantifying the expression of antimicrobial peptides, an array of immune effectors predominantly regulated by the two central immune pathways in Drosophila. Diptericin (Dpt) and Attacin A (AttA) are induced via Imd signaling, whereas Metchnikowin (Mtk) and Drosomycin (Drs) are preferentially induced following the activation of Toll signaling (25). Quantitative RT-PCR demonstrated that Mtk was induced in droncΔA8 mutants, whereas there were no changes in Dpt or AttA expression compared with the controls (Fig. 1, compare D and E). Remarkably, a strong induction of Drs (>10-fold) was observed in the mutant larvae (Fig. 1E), suggesting appreciable immune activation through the Toll signaling pathway.
Further analysis indicated the concurrent up-regulation of Spätzle transcripts (Fig. 1F), as well as an enrichment of transcripts encoding the Rel inhibitor Cactus and the NF-κB-like protein Dorsal in the fat body cells (the insect counterpart of the liver and white adipose tissue in mammals) (Fig. 1G). The increased expression of the transcript encoding Cactus suggested that chronic Toll activation in the droncΔA8 mutant might induce a negative feedback response. The profound elevation of Drs in the fat body cells indicated that this tissue is the primary site of Toll pathway activation (Fig. 1G). This possibility was confirmed by the observation of an intense GFP signal in the fat body of dronc mutants carrying the Drs-GFP, Dipt-lacZ (DD) reporter transgene (25, 26) (Fig. 1H).
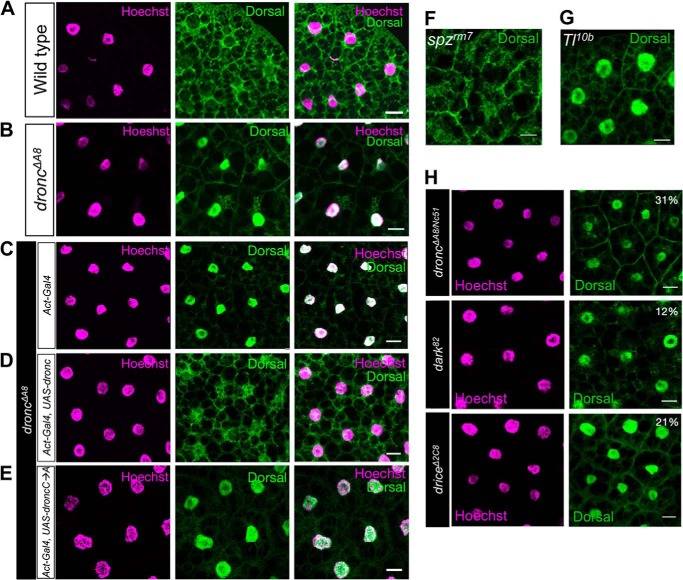
These observations led us to examine the intracellular localization of Dorsal. In wild-type fat body cells, Dorsal protein was distributed evenly between the nuclei and cytosol (Fig. 2A), indicating a lack of Toll signaling activation, analogous to spzrm7, the loss of function mutant for the Toll ligand Spätzle (Fig. 2F). However, in droncΔA8 fat body cells, the nuclear translocation of Dorsal was observed (Fig. 2B, penetrance: 53%), analogous to Toll10b, a constitutively active mutant of the Toll receptor (Fig. 2G).
FIGURE 2.
Intracellular distribution of Dorsal in fat body cells. A and B, immunohistochemistry staining revealed a substantial nuclear accumulation of Dorsal protein in droncΔA8 fat body cells. Images show the maximal projection of multiple confocal images. C–E, the enzymatic activity of Dronc plays an essential role in repressing spontaneous activation of the Toll/Dorsal cascade. The details are described in the text. The genotypes were Act5c-Gal4/+; droncΔA8/droncΔA8 (C); Act5c-Gal4/UAS-droncwt-FLAG; droncΔA8/droncΔA8 (D); and Act5c-Gal4/UAS-droncC318A; droncΔA8/droncΔA8 (E). F and G, immunohistochemical staining of Dorsal; spzrm7 and Toll10b (Tl10b) mutants were used as negative and positive controls, respectively. H, dorsal nuclear translocation in the fat bodies of other apoptosis-deficient mutants was observed to various extents. The penetrance of Dorsal nuclear staining among the tested larvae is indicated as a percentage in the top right corner of each panel. Scale bar, 20 μm.
The accumulation of Dorsal in the nuclei of dronc mutants was readily reversed by the ubiquitous expression of wild-type dronc (Act5C > droncwt; Fig. 2, C and D). In contrast, droncC318A, which encodes a catalytically inactive Dronc protein that exhibits impaired autoprocessing and enzymatic activity (6, 7), failed to rescue the dronc mutant phenotype when expressed as a transgene (Fig. 2E). Taken together, we conclude that defective Dronc activity leads to a systemic activation of Toll signaling in vivo.
The association of the Dorsal nuclear translocation phenotype with apoptotic deficiency was further confirmed by the observation of the same phenotype in mutants of dark (the Drosophila ortholog for apaf-1) (penetrance, 12%) and drICE (the predominant effector caspase in Drosophila) (penetrance, 21%) (8, 15) and in a transheterozygous dronc mutant (droncΔA8/Nc51) (penetrance, 31%) (Fig. 2H) (9). Thus, impaired apoptotic signaling results in spontaneous Toll signaling in the fat body cells.
Humoral Factors in the Hemolymph from droncΔA8 Mutants Sustain the Activation of Dorsal in the Fat Body
The fact that larval fat body cells do not undergo programmed cell death via apoptosis (27) prompted us to investigate the cell autonomy of the dronc deficiency-induced Dorsal activation in fat body cells. To this end, we generated a genetic mosaic for droncΔA8 in an otherwise heterozygous background based on a ubiquitously induced recombination and examined Dorsal localization in the mosaic fat bodies. In droncΔA8 mosaic clones, Dorsal nuclear translocation could not be detected (Fig. 3A), suggesting that dronc deficiency does not directly lead to Dorsal activation cell autonomously but may exert its effect via hemolymph-borne soluble factors.
To explore this possibility, we set up a fat body ex vivo culture system to examine whether hemolymph from droncΔA8 mutants contains DAMPs that could lead to Dorsal activation in wild-type fat body explants. In controls in which Ringer's solution was used in the co-culture, Dorsal protein was predominantly found in the cytoplasm (Fig. 3B), and most cells exhibited staining at level 0 (Fig. 3, F and G; see also “Experimental Procedures”), suggesting that the ex vivo culture procedure itself does not lead to immune activation in the isolated fat body. Addition of wild-type hemolymph to the co-culture system induced a moderate level of Dorsal nuclear translocation (Fig. 3C), possibly because of factors released from tissue damage occurring during the course of hemolymph extraction. Thus, wild-type hemolymph induced level 2 Dorsal activation in one-fourth of the fat body cells (Fig. 3G). As seen in Fig. 3D, the addition of droncΔA8 hemolymph resulted in a profound increase in the level of Dorsal nuclear translocation. Taken together, these findings support the idea that the constitutive Dorsal activation observed in droncΔA8 mutant fat bodies was due to humoral DAMPs circulating in the hemolymph.
In Drosophila, Toll receptor activation at the cell surface requires interaction with its cytokine-like ligand Spätzle (Spz) (28). Remarkably, genetic depletion of functional spz from the droncΔA8 hemolymph (droncΔA8 spzrm7) abolished its ability to enhance Dorsal activation in fat body explants and restored the staining pattern to a level where most cells exhibited cytoplasmic Dorsal localization (Fig. 3, E and G). Taken together, these data suggest that the Toll/Dorsal signaling observed in droncΔA8 fat bodies is mediated by DAMPs in the hemolymph.
Toll Activation Is Induced by Host Factors
The Toll signaling pathway is activated following the detection by Toll receptors of microbial determinants, predominantly found in fungi and Gram-positive bacteria (29, 30). However, in germ-free droncΔA8 mutants that were not exposed to microbial components, Drs was induced, whereas Dpt remained unaffected (Fig. 4). This finding indicated that in droncΔA8 mutant larvae, the Toll ligand Spz is probably activated by host factors, rather than by microbial determinants, leading to activation of the canonical Toll signaling pathway. These immunogenic host factors are likely to be DAMPs that are up-regulated in the apoptosis-deficient mutant.
FIGURE 4.
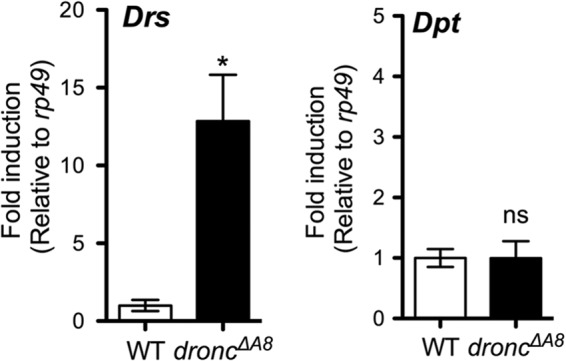
Drosomycin induction in droncΔA8 mutants cultured under germ-free conditions. Quantitative RT-PCR analysis showed a sustained elevation of Drs in germ-free droncΔA8 mutants, whereas Dpt was not induced under either condition. Statistics are results of Student's t test. *, p < 0.05. The data show means ± S.E.
Hyperactivation of Toll Signaling in Circulating Hemocytes
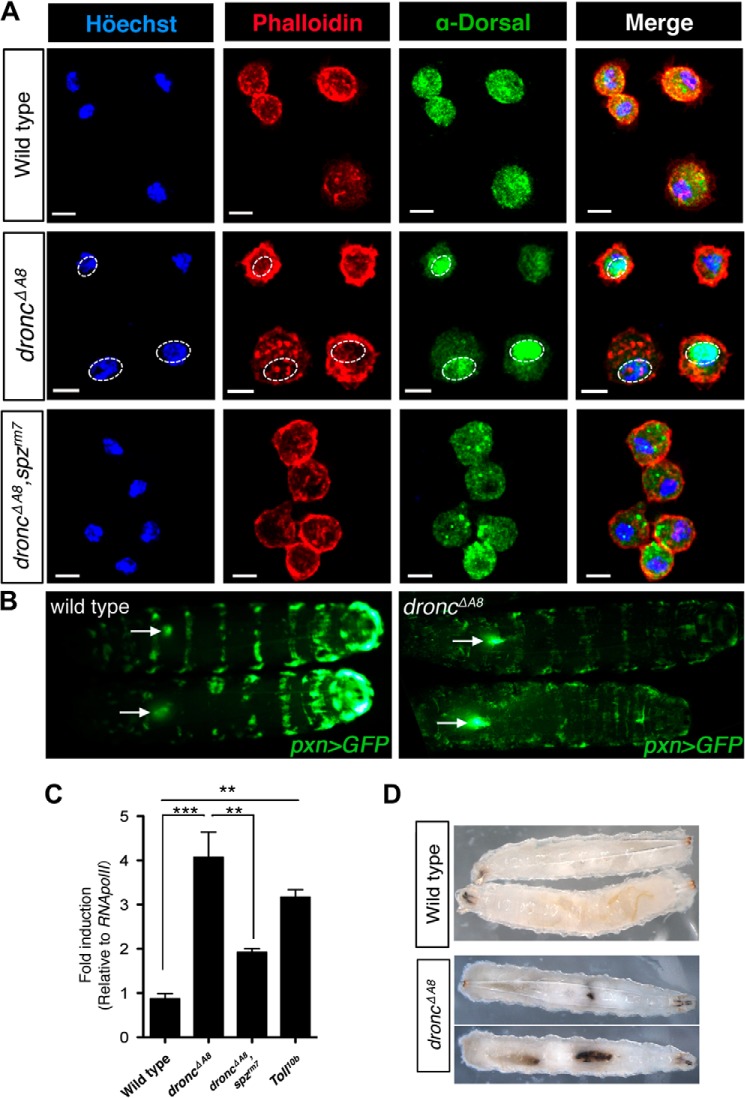
In addition to initiating a systemic immune response, Spz also functions pleiotropically, regulating hematopoietic development and proliferation, as well as malignancy in vivo, probably through engaging the Toll receptor in hematopoietic tissues (31). Consistent with this scenario, we detected robust Dorsal nuclear translocation in the circulating hemocytes of droncΔA8 mutants, which was comparable to that seen in fat bodies and was also dependent on functional Spz (Fig. 5A). Hyperactivation of the Drosophila Toll pathway could cause hematopoietic phenotypes previously associated with dysregulated Toll signaling, including lymph gland hypertrophy, blood cell hyperproliferation, and the spontaneous formation of melanotic clots (32, 33). These defects were nicely corroborated in the droncΔA8 mutants (Fig. 5, B–D). Interestingly, increased hemocyte number assessed by hemese expression (Fig. 5C) and spontaneous melanization were observed in the dronc mutants (Fig. 5D), as well as in another apoptosis-deficient mutant (dark), in previous reports (9, 11, 34). In Drosophila, circulating hemocytes function as sentinel cells, sensing diffusive signals from tissue damage and assisting in tissue repair and in replenishing hematopoietic tissue populations upon stimulation (35–37). The Toll signaling activation in hematopoietic cells in the droncΔA8 mutant may function as an adaptive mechanism in response to extensive tissue damage caused by apoptotic deficiency.
FIGURE 5.
droncΔA8 mutants exhibit hematopoietic disorders and random formation of melanotic clots. A, Spätzle mediates Dorsal nuclear translocation in circulating hemocytes from droncΔA8 mutants. The nuclei (cyan) and cell periphery (red) were visualized by staining with Höechst 33342 and rhodamine-conjugated phalloidin, respectively. Immunohistochemical staining showed the nuclear accumulation of Dorsal (green) in droncΔA8 mutants, whereas the loss of spz abrogated this phenotype. The genotype for each row is indicated at left. Scale bar, 5 μm. B, visualization of lymph gland hypertrophy. Images show GFP fluorescence from the dorsal side. The arrows point to lymph glands containing GFP-expressing hemocytes, with the arrowheads oriented toward the posterior end of the larvae. The genotypes were pxn-Gal4, UAS-GFP for wild type and pxn-Gal4, UAS-GFP; droncΔA8/droncΔA8 for droncΔA8 mutants. C, quantitative RT-PCR analysis of hemese, a pan-hemocyte marker in Drosophila (32, 57) indicated that droncΔA8 mutants have increased total blood cell numbers, comparable to the level observed in Tl10b mutants. The data show means ± S.E. Statistics are results of one-way analysis of variance test. **, p < 0.01; ***, p < 0.0001. D, induction of a melanotic tumor in droncΔA8 flies.
The CLIP Domain Serine Protease Persephone Acts Upstream to Facilitate Spätzle Cleavage in droncΔA8 Hemolymph
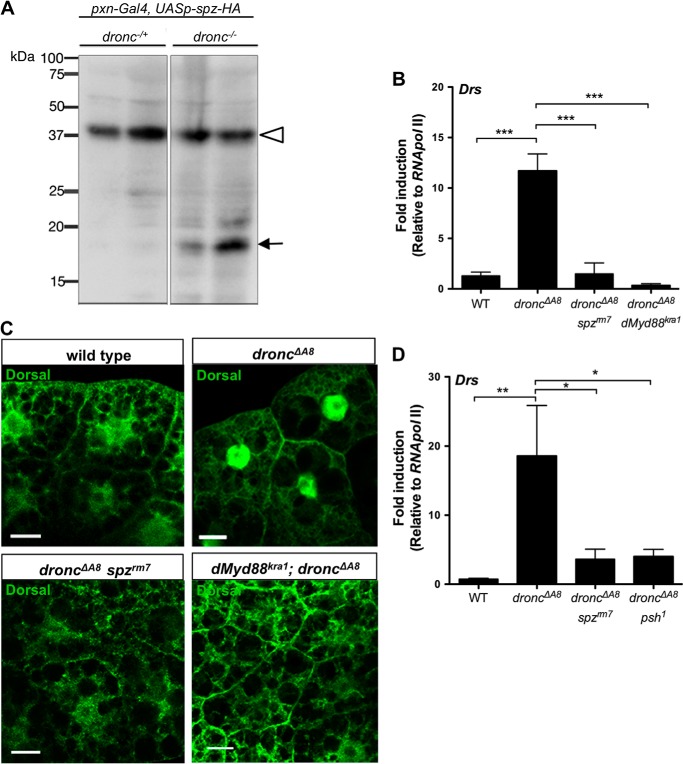
The endogenous cytokine-like factor Spz is secreted as an inactive full-length protein and undergoes intramolecular cleavage to produce the C-terminal 106-amino acid fragment (Spz C-106) that binds to Toll and initiates signaling (28). The non-cell autonomous activation of Dorsal in the fat body prompted us to explore whether Spz C-106 is produced in droncΔA8 mutants. Hemocyte-borne Spz is reported to be both necessary and sufficient to induce the activation of NF-κB factors in fat bodies (38). We overexpressed HA-tagged pro-Spz (14) using a hemocyte-specific driver (pxn-GAL4) (12) in the droncΔA8 background so the tagged form of Spz would be secreted from these circulating blood cells. Indeed, Western blots of hemolymph extracted from the transgenic animals demonstrated the presence of the active form of Spz (Spz C106-HA, 19kDa) in the droncΔA8 mutants (Fig. 6A). This result provided additional evidence that the Dorsal activation in fat body cells was initiated by Toll receptor binding of its soluble ligand Spz, which undergoes intramolecular cleavage.
FIGURE 6.
Spz and Psh are indispensable for Toll signaling activation in the fat bodies of droncΔA8 mutants. A, detection of the proteolytically processed form of Spz-HA in droncΔA8 hemolymph extracts by Western blot analysis. Duplicated samples of the same genotype were shown. The genotypes are pxn-Gal4/UASp-spz-HA; droncΔA8/+ for dronc+/− and pxn-Gal4/UASp-spz-HA; droncΔA8/droncΔA8 for dronc−/−. B and C, impairment of either spz or dMyd88 abolished the spontaneous Toll/Dorsal activation in droncΔA8, shown by quantitative RT-PCR analysis (B) and immunohistochemical staining (C). Scale bar, 20 μm. D, genetic analysis indicated that the serine protease Psh was required for Toll receptor activation. The data show means ± S.E. Statistics are results of one-way analysis of variance test. *, p < 0.05; **, p < 0.01.
After Toll receptor ligation at the cell surface, intracellular immune signaling is initiated by recruitment of the intracellular adaptor dMyd88 (39). We next investigated whether dMyd88 is required to relay the signal for Drs induction in droncΔA8 mutants. In both the double-mutant lines droncΔA8 spzrm7 and droncΔA8 dMyd88Kra1, the Toll signaling activation was completely abrogated, as indicated by the absence of both Drs induction and Dorsal nuclear translocation (Fig. 6, B and C), the two hallmarks of autoimmune activation observed in droncΔA8 flies. Our genetic analysis clearly demonstrated that in droncΔA8, the canonical Toll signaling modules mediate Dorsal activation in the fat body.
Persephone (Psh) is an extracellular CLIP domain serine protease that activates Spz, through a proteolytic cascade that is partly independent of proteoglycan sensing (40). The proteolytic maturation of Psh can be triggered by virulent proteases secreted by fungi or Gram-positive bacteria (40, 41). To test whether Psh was involved in the DAMP-mediated Toll activation, we replaced the wild-type psh allele in droncΔA8 with a loss of function allele psh1, which encodes a Psh protein with impaired catalytic activity (16), and assessed Drs expression in the double mutants. Analysis by qRT-PCR showed that, like the spz impairment (spzrm7), the loss of Psh function greatly reduced Drs production in the droncΔA8 background (Fig. 6D). In conclusion, our genetic analysis suggested that in droncΔA8 mutants, endogenous, hemolymph-borne DAMPs induce systemic Toll immune signaling by activating the Psh protease, which triggers the proteolytic cleavage of the Toll ligand, Spz.
DISCUSSION
In the current study, we present evidence that the Toll signaling pathway is systemically and constitutively activated in apoptosis-deficient Drosophila mutant larvae. We further demonstrated that the activation of this immune pathway was independent of microbial challenge and was due to endogenous humoral factors found in the hemolymph, where the endogenous ligand Spz is processed to its active form through a proteolytic cascade. Our findings also revealed that Persephone, the Drosophila hemolymph serine protease, was crucial in mediating inflammatory responses to endogenous molecules, demonstrating its involvement in a danger-sensing mechanism.
In Drosophila, several forms of stress, including mechanical injury (42), temperature shift (43), starvation (44), and aging (45, 46) have been shown to trigger immune system activation in the absence of a pathogen. The present study using Drosophila third instar larvae supports the scenario that Toll signaling is a key mechanism in the host defense system that responds to endogenous inflammatory factors, consistent with the dedicated role of Toll-like receptors in vertebrate “danger” sensing (1, 47, 48). A number of previous studies that focused on nonmicrobial signal-activated host defense in Drosophila showed that the Imd/Relish cascade is activated by endogenous inflammatory agents, such as undigested DNA (49, 50) and the neuro-peptide bursicon (51), as well as undefined humoral factors released from the intestine after tissue damage (52). The observation of different signaling pathways that are activated in response to various forms of stress, and in the dronc mutant, indicate that specific detection mechanisms are used to discriminate among various forms of threat.
Is there any physiological role of Toll-mediated immune response in apoptosis-deficient organisms? In our preliminary observation, dronc−/− larvae lacking spz showed the lack of melanotic tumor. However, the mutant larvae reached the lethal point approximately at the same stage, irrespective of their inability to activate the Toll signaling. We still believe that this prominent immune pathway could benefit the animals in a way that prepares them for upcoming tissue damage, for instance, by increasing the hemocytes whose function is crucial in sequestering large debris and relaying signals that are required in tissue repair. Melanization has been shown under certain circumstances to enhance the effectiveness of defense signaling upon a life-threatening stimulus (53). Thus, we think that the activation of Toll would have some beneficial roles in apoptosis-deficient organisms.
Drosophila Toll activation involves the activation of two distinct, but parallel pathways, namely a pattern recognition receptor-dependent pathway and a Psh-dependent pathway (40), that converge on the proteolytic cleavage of Spz (54). The pattern recognition receptor-dependent pathway consists of a set of circulating receptors, including peptidoglycan recognition proteins and Gram-negative bacteria-binding proteins, capable of discriminating between bacterial and fungal determinants (55), the recognition of which triggers a subsequent proteolytic cascade leading to Spz activation. The parallel pathway is activated when Psh senses virulence factors secreted from filamentous fungi (41) or Gram-positive bacteria (40). Although the molecular mechanism of how these enzymes are detected by Psh is currently unknown, Psh has been implicated as a key factor in the “danger signal-dependent extracellular pathway” ever since the recognition of its ability to detect abnormal protease activity (40). Our findings extend this theory by showing that Psh is responsible for endogenous DAMP-mediated systemic activation of Toll signaling. The activation of innate immunity-regulated genes including Drs and Mtk was previously observed following serine protease activation in response to wound stimulation (56). Serine protease(s) released from necrotic cells may act to initiate the Psh-dependent Toll signaling pathway.
Acknowledgments
We are grateful to W.-J. Lee for instructions on the method for generating germ-free Drosophila and to S. Kurata, J. M. Abrams, S. Kornbluth, P. Meier, D. Stein, M. J. Galko, J. M. Reichhart, B. Lemaitre, the Vienna Drosophila RNAi Center, and the Bloomington Drosophila Stock Center for Drosophila stocks and antibodies. We thank T. Chihara and Y. Yamaguchi for active discussions.
This work was supported by grants from the Japanese Ministry of Education, Science, Sports, Culture, and Technology (to M. M. and E. K.) and the Uehara Memorial Foundation (E. K.).

This article contains supplemental Table S1.
- DAMP
- damage-associated molecular pattern
- RFP
- red fluorescence protein.
REFERENCES
- 1. Gallucci S., Matzinger P. (2001) Danger signals. SOS to the immune system. Curr. Opin. Immunol. 13, 114–119 [DOI] [PubMed] [Google Scholar]
- 2. Cai C. L., Martin J. C., Sun Y., Cui L., Wang L., Ouyang K., Yang L., Bu L., Liang X., Zhang X., Stallcup W. B., Denton C. P., McCulloch A., Chen J., Evans S. M. (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coussens L. M., Werb Z. (2002) Inflammation and cancer. Nature 420, 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silva M. T., do Vale A., dos Santos N. M. (2008) Secondary necrosis in multicellular animals. An outcome of apoptosis with pathogenic implications. Apoptosis 13, 463–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chautan M., Chazal G., Cecconi F., Gruss P., Golstein P. (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9, 967–970 [DOI] [PubMed] [Google Scholar]
- 6. Meier P., Silke J., Leevers S. J., Evan G. I. (2000) The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 19, 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorstyn L., Colussi P. A., Quinn L. M., Richardson H., Kumar S. (1999) DRONC, an ecdysone-inducible Drosophila caspase. Proc. Natl. Acad. Sci. U.S.A. 96, 4307–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kondo S., Senoo-Matsuda N., Hiromi Y., Miura M. (2006) DRONC coordinates cell death and compensatory proliferation. Mol. Cell. Biol. 26, 7258–7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chew S. K., Akdemir F., Chen P., Lu W. J., Mills K., Daish T., Kumar S., Rodriguez A., Abrams J. M. (2004) The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev. Cell 7, 897–907 [DOI] [PubMed] [Google Scholar]
- 10. Xu D., Li Y., Arcaro M., Lackey M., Bergmann A. (2005) The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development 132, 2125–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daish T. J., Mills K., Kumar S. (2004) Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev. Cell 7, 909–915 [DOI] [PubMed] [Google Scholar]
- 12. Stramer B., Wood W., Galko M. J., Redd M. J., Jacinto A., Parkhurst S. M., Martin P. (2005) Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168, 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang C. S., Thomenius M. J., Gan E. C., Tang W., Freel C. D., Merritt T. J., Nutt L. K., Kornbluth S. (2010) Metabolic regulation of Drosophila apoptosis through inhibitory phosphorylation of Dronc. EMBO J. 29, 3196–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho Y. S., Stevens L. M., Stein D. (2010) Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr. Biol. 20, 1133–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akdemir F., Farkas R., Chen P., Juhasz G., Medved'ová L., Sass M., Wang L., Wang X., Chittaranjan S., Gorski S. M., Rodriguez A., Abrams J. M. (2006) Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development 133, 1457–1465 [DOI] [PubMed] [Google Scholar]
- 16. Ligoxygakis P., Pelte N., Hoffmann J. A., Reichhart J. M. (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297, 114–116 [DOI] [PubMed] [Google Scholar]
- 17. Ryu J. H., Kim S. H., Lee H. Y., Bai J. Y., Nam Y. D., Bae J. W., Lee D. G., Shin S. C., Ha E. M., Lee W. J. (2008) Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 [DOI] [PubMed] [Google Scholar]
- 18. Whalen A. M., Steward R. (1993) Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J. Cell Biol. 123, 523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ekengren S., Tryselius Y., Dushay M. S., Liu G., Steiner H., Hultmark D. (2001) A humoral stress response in Drosophila. Curr. Biol. 11, 714–718 [DOI] [PubMed] [Google Scholar]
- 20. Tettweiler G., Miron M., Jenkins M., Sonenberg N., Lasko P. F. (2005) Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 19, 1840–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernal A., Kimbrell D. A. (2000) Drosophila Thor participates in host immune defense and connects a translational regulator with innate immunity. Proc. Natl. Acad. Sci. U.S.A. 97, 6019–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agaisse H., Perrimon N. (2004) The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 198, 72–82 [DOI] [PubMed] [Google Scholar]
- 23. Wang M. C., Bohmann D., Jasper H. (2005) JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 [DOI] [PubMed] [Google Scholar]
- 24. Martín-Blanco E., Gampel A., Ring J., Virdee K., Kirov N., Tolkovsky A. M., Martinez-Arias A. (1998) puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrandon D., Imler J. L., Hetru C., Hoffmann J. A. (2007) The Drosophila systemic immune response. Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 7, 862–874 [DOI] [PubMed] [Google Scholar]
- 26. Ferrandon D., Jung A. C., Criqui M., Lemaitre B., Uttenweiler-Joseph S., Michaut L., Reichhart J., Hoffmann J. A. (1998) A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 17, 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelliot A., Bond N., Hoshizaki D. K. (2006) Fat-body remodeling in Drosophila melanogaster. Genesis 44, 396–400 [DOI] [PubMed] [Google Scholar]
- 28. Weber A. N., Tauszig-Delamasure S., Hoffmann J. A., Lelièvre E., Gascan H., Ray K. P., Morse M. A., Imler J. L., Gay N. J. (2003) Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat. Immunol. 4, 794–800 [DOI] [PubMed] [Google Scholar]
- 29. Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- 30. Hoffmann J. A., Reichhart J. M. (2002) Drosophila innate immunity. An evolutionary perspective. Nat. Immunol. 3, 121–126 [DOI] [PubMed] [Google Scholar]
- 31. Lemaitre B., Meister M., Govind S., Georgel P., Steward R., Reichhart J. M., Hoffmann J. A. (1995) Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J. 14, 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I., Hultmark D. (2004) A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. U.S.A. 101, 14192–14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qiu P., Pan P. C., Govind S. (1998) A role for the Drosophila Toll/cactus pathway in larval hematopoiesis. Development 125, 1909–1920 [DOI] [PubMed] [Google Scholar]
- 34. Rodriguez A., Oliver H., Zou H., Chen P., Wang X., Abrams J. M. (1999) Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat. Cell Biol. 1, 272–279 [DOI] [PubMed] [Google Scholar]
- 35. Márkus R., Laurinyecz B., Kurucz E., Honti V., Bajusz I., Sipos B., Somogyi K., Kronhamn J., Hultmark D., Andó I. (2009) Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 106, 4805–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shim J., Mukherjee T., Banerjee U. (2012) Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 14, 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pastor-Pareja J. C., Wu M., Xu T. (2008) An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Model. Mech. 1, 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shia A. K., Glittenberg M., Thompson G., Weber A. N., Reichhart J. M., Ligoxygakis P. (2009) Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J. Cell Sci. 122, 4505–4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tauszig-Delamasure S., Bilak H., Capovilla M., Hoffmann J. A., Imler J. L. (2002) Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat. Immunol. 3, 91–97 [DOI] [PubMed] [Google Scholar]
- 40. El Chamy L., Leclerc V., Caldelari I., Reichhart J. M. (2008) Sensing of “danger signals” and pathogen-associated molecular patterns defines binary signaling pathways “upstream” of Toll. Nat. Immunol. 9, 1165–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gottar M., Gobert V., Matskevich A. A., Reichhart J. M., Wang C., Butt T. M., Belvin M., Hoffmann J. A., Ferrandon D. (2006) Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127, 1425–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Márkus R., Kurucz E., Rus F., Andó I. (2005) Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett 101, 108–111 [DOI] [PubMed] [Google Scholar]
- 43. Tsuzuki S., Ochiai M., Matsumoto H., Kurata S., Ohnishi A., Hayakawa Y. (2012) Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Sci. Rep. 2, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Becker T., Loch G., Beyer M., Zinke I., Aschenbrenner A. C., Carrera P., Inhester T., Schultze J. L., Hoch M. (2010) FOXO-dependent regulation of innate immune homeostasis. Nature 463, 369–373 [DOI] [PubMed] [Google Scholar]
- 45. Zerofsky M., Harel E., Silverman N., Tatar M. (2005) Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103–108 [DOI] [PubMed] [Google Scholar]
- 46. Ramsden S., Cheung Y. Y., Seroude L. (2008) Functional analysis of the Drosophila immune response during aging. Aging Cell 7, 225–236 [DOI] [PubMed] [Google Scholar]
- 47. Matzinger P. (2002) The danger model. A renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 48. Piccinini A. M., Midwood K. S. (2010) DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seong C. S., Varela-Ramirez A., Aguilera R. J. (2006) DNase II deficiency impairs innate immune function in Drosophila. Cell. Immunol. 240, 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mukae N., Yokoyama H., Yokokura T., Sakoyama Y., Nagata S. (2002) Activation of the innate immunity in Drosophila by endogenous chromosomal DNA that escaped apoptotic degradation. Genes Dev. 16, 2662–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. An S., Dong S., Wang Q., Li S., Gilbert L. I., Stanley D., Song Q. (2012) Insect neuropeptide bursicon homodimers induce innate immune and stress genes during molting by activating the NF-κB transcription factor Relish. PLoS One 7, e34510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu S. C., Liao C. W., Pan R. L., Juang J. L. (2012) Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 11, 410–417 [DOI] [PubMed] [Google Scholar]
- 53. Tang H., Kambris Z., Lemaitre B., Hashimoto C. (2006) Two proteases defining a melanization cascade in the immune system of Drosophila. J. Biol. Chem. 281, 28097–28104 [DOI] [PubMed] [Google Scholar]
- 54. Jang I. H., Chosa N., Kim S. H., Nam H. J., Lemaitre B., Ochiai M., Kambris Z., Brun S., Hashimoto C., Ashida M., Brey P. T., Lee W. J. (2006) A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell 10, 45–55 [DOI] [PubMed] [Google Scholar]
- 55. Michel T., Reichhart J. M., Hoffmann J. A., Royet J. (2001) Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414, 756–759 [DOI] [PubMed] [Google Scholar]
- 56. Patterson R. A., Juarez M. T., Hermann A., Sasik R., Hardiman G., McGinnis W. (2013) Serine proteolytic pathway activation reveals an expanded ensemble of wound response genes in Drosophila. PLoS One 8, e61773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurucz E., Zettervall C. J., Sinka R., Vilmos P., Pivarcsi A., Ekengren S., Hegedüs Z., Ando I., Hultmark D. (2003) Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 100, 2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]