Background: Meprin metalloproteinases are implicated in the pathogenesis of inflammatory diseases where the cytokine IL-6 plays a role.
Results: Meprins cleave and decrease the biological activity of IL-6.
Conclusion: IL-6 activity at inflammatory sites is regulated in part by meprins.
Significance: Decreasing IL-6 bioactivity is an underlying mechanism for meprin regulation of inflammation.
Keywords: Cell Culture, Cytokine, Inflammation, Interleukin, Metalloprotease, Protease, Meprins
Abstract
Meprins have been implicated in the pathogenesis of several inflammatory diseases, including inflammatory bowel disease, in which the cytokine IL-6 is a prominent effector molecule. Because IL-6 levels are elevated markedly in meprin α and α/β knockout mice in an experimental model of inflammatory bowel disease, the interaction between meprins and IL-6 was studied. The results demonstrate that rodent and human meprin A and B cleave IL-6 to a smaller product and, subsequently, are capable of extensive degradation of the cytokine. Analysis of the limited degradation product formed by meprin A indicated that three to five amino acids are removed from the C terminus of the cytokine. Meprin A and meprin B cleaved IL-6 with micromolar affinities (Km of 4.7 and 12.0 μm, respectively) and with high efficiencies (kcat/Km of 0.2 and 2.5 (m−1/s−1) × 106, respectively). These efficiency constants are among the highest for known meprin substrates. Madin-Darby canine kidney cells transiently transfected with meprin α or meprin β constructs also cleave exogenous IL-6. Both human and murine IL-6 cleaved by meprin A or B are inactivated, as demonstrated by their decreased capability to stimulate proliferation of B9 cells. These results are consistent with the proposition that one function of meprin metalloproteases is to modulate inflammation by inactivating IL-6.
Introduction
IL-6 is a strong proinflammatory cytokine, although it can also act as an anti-inflammatory mediator in some contexts (1). This cytokine is an important inducer of acute phase proteins by the liver to modulate the immune response and to keep microbe growth under control at the site of injury (2). IL-6 is a polypeptide containing four α-helices configured in an up-down-up-down orientation (3). IL-6 is ∼21–28 kDa, depending on posttranslational modifications. This cytokine is expressed during the course of the inflammatory response by a variety of cell types, such as epithelial cells and monocytes (4, 5). IL-6 is required for effective clearance of pathogens such as Listeria monocytogenes and Streptococcus pneumoniae along with being a requirement for proper wound resolution and closure (6–8). However, excessive and uncontrolled levels of IL-6 exacerbate several inflammatory diseases, such as inflammatory bowel disease (IBD)3 (9).
Meprin metalloproteases have also been linked to the pathogenesis of inflammatory disorders such as acute renal failure, urinary tract infections, and IBD (10–12). A polymorphism in the meprin α gene has been linked to IBD in humans. Patients with this polymorphism, located in the 3′ UTR region of the meprin α gene, show decreased meprin α expression compared with those with the wild-type meprin α gene (13). Wild-type and meprin α KO mice, subjected to experimental IBD where inflammation was induced by oral dextran sulfate sodium administration, showed markedly different cytokine profiles after the induction of inflammation. The levels of cytokines such as IL-1, IL-18, and IL-6 were increased significantly in the serum and colon of the meprin α KO mice. ProIL-1 and proIL-18 are known meprin substrates and are activated by meprins in vitro (14–16). However IL-6, secreted as an active cytokine, does not require proteolytic activation. Furthermore, when meprin α/β null mice were subjected to dextran sulfate sodium-induced IBD, the only cytokine that increased, of 16 measured in the colons of both wild-type and double-meprin null mice, was IL-6 (11). These animal studies indicate that meprins modulate IL-6 levels at inflammatory sites. This study was initiated to determine whether meprins were capable of directly degrading IL-6.
Meprins are zinc-dependent proteases comprised of evolutionarily related meprin α and β multidomain subunits. These subunits both contain active protease domains of the “astacin family” and are ∼40% identical in their primary amino acid sequences (17, 18). The subunits form homomeric and heteromeric isoforms. Both secreted and membrane-bound forms are present at inflammatory sites. Meprins are capable of cleaving a wide variety of substrates, including extracellular matrix proteins, small bioactive peptides, the tight junction protein occludin, and intracellular proteins such as villin and actin (18–21). Given that meprins have been implicated in the inflammatory response, finding physiologically relevant meprin substrates is of great interest. In that regard, several cytokines (such as the aforementioned IL-1 and IL-18 along with osteopontin; MCP-1; MIP-1; regulated on activation, normal T Expressed and secreted (RANTES); VEGF-A; and pro-kallikrein 7) have also been identified as meprin substrates in vitro (14, 15, 22–24). Meprins are relatively nonspecific proteases, although studies with peptide libraries have shown that murine meprin α prefers to cleave after small and aromatic amino acids and that meprin β prefers to cleave after acidic amino acids (22).
Secreted and membrane-bound forms of meprin exist in vivo because of cleavage of the “I” domain of the meprin α subunit intracellularly in the endoplasmic reticulum during maturation. This causes the meprin α subunit to be released from the surface of the cell. Meprin β lacks the I domain. Thus, this subunit is bound to the cell surface by its transmembrane domain (25, 26). Several cell types, such as the epithelial cells of the human colon, only express the meprin α subunit. The secreted meprin α forms large-order homo-oligomers called homomeric meprin A. Kidney brush-border cells in humans, rats, and most inbred mice (such as C57BL6) express both meprin α and meprin β. The resulting meprin isoform is a heterotetramer of α and β subunits called heteromeric meprin A. Some inbred mouse strains (C3H/He) do not express meprin α in their adult kidney proximal tubule cells but do express the meprin β subunit. This isoform of meprin, meprin B, exists as a dimer of meprin β subunits anchored to the cell surface (17, 18).
The cytokine data obtained from the meprin null mice subjected to experimental IBD led to the hypothesis that meprins play a role in modulating inflammation by cleaving IL-6, thus decreasing the activity of this cytokine and keeping the resulting inflammation under control. To test this hypothesis in this study, recombinant meprins were incubated with recombinant human and mouse IL-6, and the fragmentation and activity of the cytokine were assessed. Both isoforms of IL-6 were studied because, although human and mouse IL-6 have the same tertiary structure, they only share 42% sequence similarity and have different glycosylation patterns. Furthermore, there are several biological differences between human and murine IL-6. For example, mouse IL-6 is recognized by both human and mouse IL-6 receptors but not vice versa (27, 28). Both human and murine forms of meprins were used in this study because of the differences in the enzymatic activities of these species (29).
MATERIALS AND METHODS
Reagents
Human IL-6 expressed in a eukaryotic cell line was procured from the NCI, National Institutes of Health Preclinical Repository. Additional human IL-6 and murine IL-6, both expressed in and purified from Escherichia coli, were purchased from PeproTech. To identify different forms of IL-6, the human IL-6 from the NCI will be referred to as rhIL-6, whereas the human IL-6 from PeproTech will be referred to as hIL-6 hereafter. The polyclonal antibody used to visualize human IL-6 on immunoblots was purchased from Thermo Scientific Pierce. Horseradish peroxidase-conjugated secondary antibody was purchased from GE Healthcare. Materials for buffers and other reagents were purchased from Sigma except where indicated. Homomeric, recombinant human meprin A and B were provided by Dr. Christoph Becker-Pauly (University of Mainz, Mainz, Germany; currently at Christian Albrechts University, Kiel, Germany).
Expression, Purification, and Activation of Recombinant Mouse Meprin A and Rat Meprin B
Constructs for mouse meprin α (secreted, homomeric mouse meprin A) and rat meprin β (homomeric rat meprin B) were transfected into HEK 293 cells for stable expression of meprin A and B. The DNA constructs for the mouse meprin α protein (1–615) and for the rat meprin β protein (1–648) were both tagged at the C terminus with a 6× histidine tag for ease of purification (30, 31). The truncation of the rat meprin B protein at the EGF-transmembrane border allowed for the secretion of rat meprin B into the cell culture medium (31). This medium was then collected, and the secreted mouse meprin A and rat meprin B proteins were purified by metal-chelating chromatography. The purified meprin A and meprin B proteases were activated by incubation with trypsin at 37 °C in a 6:1 molar ratio for 45 min for meprin A and 1 h for meprin B. Trypsin was removed from the purified meprin preparations by passing the activated proteases through a Sephadex G-25 column (Sigma). No residual trypsin activity was detected in the activated meprin preparations (20). To stop the activity of meprins prior to sample loading onto SDS-PAGE or for control experiments, 50 μl of actinonin was used to inhibit both meprin A and meprin B (32). Meprins were flash-frozen in liquid nitrogen and stored at −80 °C.
Cleavage of Human and Murine IL-6 by Meprins
IL-6 (1 μm) and homomeric meprin A or meprin B (0.1 μm) were incubated together in 20 mm Tris, 50 mm NaCl (50-μl total volume) (pH 7.5) for up to 20 h at 37 °C. Prior to loading meprin/IL-6 samples on SDS-PAGE gels, the meprin reaction was stopped by addition of 50 μm actinonin and by boiling in sample buffer containing β-mercaptoethanol and SDS. The meprin and IL-6 incubations were separated on 15% SDS-PAGE gels with the proteins visualized by SimplyBlueTM SafeStain (Coomassie Blue dye, Invitrogen). Protein band quantitation in both the Coomassie-stained gels and Western blot analyses was done with ImageJ, with densitometric values given as percentage values.
Determination of Kinetic Values of Meprin Cleavage of Human IL-6
The rate of disappearance of the substrate band of rhIL-6 after incubation with mouse meprin A and rat meprin B was determined by laser densitometry. Meprin A or B (3 nm for meprin A and 0.2 nm for meprin B) were incubated with increasing amounts of rhIL-6 (2–10 μm for meprin A and 2–22 μm for meprin B) for varying times (0–10 min). The meprin/IL-6 interactions were stopped with the addition of 50 μm actinonin, and then the mixture was boiled in SDS-containing sample buffer. Protein products were separated on 15% SDS-PAGE. The rhIL-6 band was visualized by KryptonTM protein stain (Thermo Scientific), and the rate of rhIL-6 band disappearance was quantitated using a Typhoon 9400 variable mode imager (Amersham Biosciences) with ImageQuant version 5.2 (Molecular Dynamics) software. The rate of substrate disappearance was plotted against the substrate concentration using GraphPad 5 Prism. The apparent Km and Vmax values were derived from fitting the velocity/substrate curve to the Michaelis-Menten equation using nonlinear regression analysis. Velocity values were calculated in duplicate and are an average of two experiments with five velocity values determined for the substrate concentrations listed previously. The kcat values were calculated by dividing the Vmax (micromolar/minute) by the meprin concentration via GraphPad 5 Prism. The kcat value was calculated with the assumption that the meprins are 100% activated after limited trypsin digestion because no latent form of meprins was detected via protein staining or Western blotting after separation 8% SDS-PAGE. This kcat value, now in units of seconds, was then divided by the Km value to calculate the catalytic efficiency constant kcat/Km.
Identification of the Meprin A Cleavage Site on IL-6
Recombinant mouse meprin A (0.1 μm) was incubated with rhIL-6 (1 μm) for 1 h, after which the reaction was inhibited with 50 μm actinonin and by boiling in sample buffer with SDS and β-mercaptoethanol. Proteins were separated on 15% SDS-PAGE and visualized with Coomassie Blue staining with SimplyBlueTM SafeStain (Invitrogen). The resultant IL-6 product band was excised from the SDS-PAGE gel. Uncleaved and homomeric mouse meprin A-cleaved rhIL-6 protein bands were processed for MS analysis as described previously (20). Briefly, protein bands were excised from SDS-PAGE gels and destained. After drying, gel slices were rehydrated, and proteins were digested with trypsin. Peptides in the gel slices were extracted with acetonitrile and TFA. Peptides were eluted from C18 Zip Tips, placed on MALDI-time-of-flight plates, and analyzed via MS. The peptides present in the uncleaved and meprin A-cleaved rhIL-6 samples were identified by ProteinPilot software. Endoproteinase Glu-C was also used in an attempt to further elaborate the meprin cleavage site on IL-6. The same digestion and peptide retrieval and analysis protocol as for trypsin was followed.
Analyzing Interaction of IL-6 and Meprins with Madin-Darby Canine Kidney (MDCK) Cell System
MDCK cells were maintained in MEM (Invitrogen). MDCK cells were transfected with constructs of full-length rat meprin α and full-length rat meprin β, as described previously (16). In brief, MDCK cells at 30% confluency in 24-well plates were transfected with rat meprin α and β constructs using Lipofectamine 2000 (Invitrogen). After 36 h, the MDCK-transfected expressed meprins were activated using limited trypsin digestion. That is, 10 μl of trypsin (1 mg/ml) was added to 1 ml of serum-free MEM and incubated with the transfected MDCK cells for 30 min at 37 °C. The cells were washed with serum-free MEM, and any residual trypsin activity was inhibited by incubating the cells with 10 μl of 2 mg/ml soybean trypsin inhibitor (in water) in 1 ml of serum-free MEM for 1 h. The MDCK cells were washed again with serum-free MEM, and then 1 μg of rhIL-6 was added to the MDCK cells and allowed to incubate for 24 h. The culture medium was then isolated, separated on 15% SDS-PAGE, transferred to nitrocellulose via a Trans-Blot S.D. semi-dry transfer cell (Bio-Rad), and then probed with the polyclonal antibody for human IL-6.
Determining the Activity of Meprin-cleaved IL-6 with a B9 Cell Proliferation Assay
The biological activities of treated and untreated IL-6 were measured as a function of B9 cell proliferation. The B9 mouse cell line was provided by Dr. Tom Scott (Clemson University) (33). These cells were maintained in RPMI 1640 medium (Invitrogen) containing 5% FBS, 1/100 antibiotic/antimycotic (Invitrogen), 50 μm β-mercaptoethanol, and 2 ng/ml murine IL-6. B9 cells were subcultured 1:10 every 3 or 4 days. Prior to treatment with IL-6 or meprin-cleaved IL-6, B9 cells were washed twice with serum-free and IL-6 free RPMI media to remove any residual IL-6 remaining from normal culturing/passaging. The B9 cells were then cultured in 96-well plates at a concentration of 20,000 cells/well in a final concentration of 100 μl (200 cells/μl) and incubated with uncleaved or meprin A- or meprin B-cleaved IL-6 (concentration of 0.5 ng/ml IL-6) for 72 h. B9 cells were treated with IL-6 that was incubated with meprin A or meprin B for several time points, and then further meprin digestion was inhibited with 50 μm actinonin prior to addition to the B9 cells. Cell growth was measured 72 h after cytokine treatment by adding 20 μl of CellTiter 96® reagent (Promega) to each well and then incubating the plates for an additional 1–2 h at 37 °C. The absorbance at 490 nm for each well was read with a UV/visible plate reader (Spectra Max 190, Molecular Devices). The proliferation of B9 cells was expressed as the difference between the wavelength 490-nm value of B9 cells not treated with IL-6 and B9 cells treated with uncleaved human and murine IL-6. The activity of meprin-treated IL-6 preparations was reported as the ratio of B9 cell proliferation for cultures not treated with IL-6 to those incubated with IL-6, with the former as the denominator. For tabulation of the results, these ratios were converted to percentages, with this ratio of proliferation between B9 cells not treated with IL-6 and those treated with uncleaved IL-6 referred to as “100% activity.” Experiments were done in triplicate, with each experimental set value comprised of the 490-nm wavelength values for 6 wells.
RESULTS
Homomeric Meprin A and B Cleave Murine IL-6
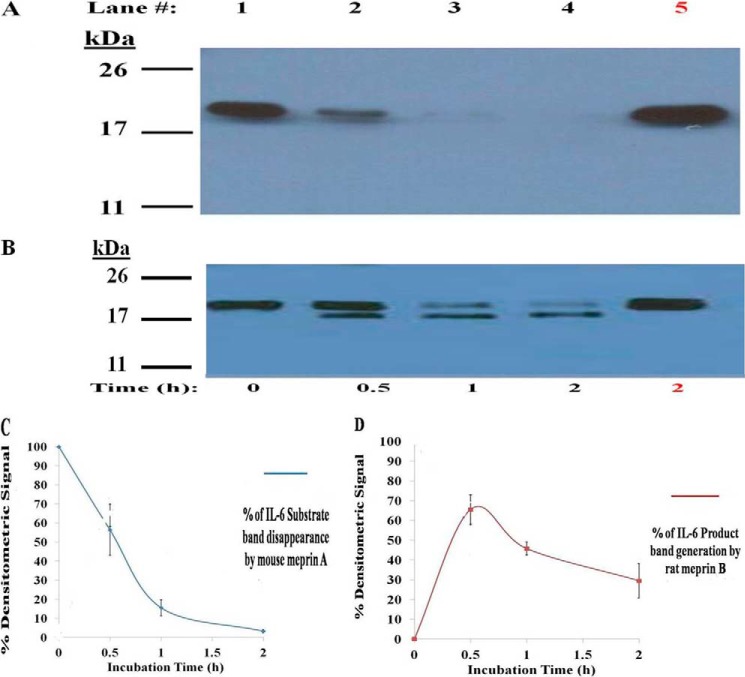
To determine whether IL-6 is a substrate of the meprins, mouse meprin A or rat meprin B was incubated with murine IL-6 (mIL-6), and the resulting products were separated by SDS-PAGE (Fig. 1, A and B). Fig. 1 shows the results of the Western blot analysis. Similar results were observed with Coomassie Blue staining (not shown). Mouse meprin A degraded murine IL-6. Immunoblotting did not detect any meprin A-derived product of murine IL-6. It is likely that limited degradation products were degraded extensively under these conditions. Rat meprin B cleaved murine IL-6 (∼22 kDa) to a smaller product (∼ 21 kDa), and this product was degraded further with time. The meprin B-generated product of murine IL-6 was detected after 30 min of incubation. The disappearance of the mouse IL-6 substrate signal after meprin A incubation and the appearance of meprin B-generated murine IL-6 product have been plotted in Fig. 1, C and D. Approximately 50 and 15% of the mIL-6 signal remained after 0.5 and 1 h of incubation with mouse meprin A. After 2 h of incubation with mouse meprin A, less than 10% of the initial mIL-6 signal remained (Fig. 1C). The fragmentation pattern of mIL-6 generated by rat meprin B was different from the pattern generated by mouse meprin A. Meprin B converted ∼65% of murine IL-6 to a smaller product (∼ 21 kDa) after 0.5 h. Upon further incubation with meprin B, the signal of this 21-kDa product decreased linearly with time, with only ∼30% of the 21-kDa murine IL-6 product remaining after 4 h of incubation.
FIGURE 1.
Cleavage of murine IL-6 by rodent meprin A and meprin B. Homomeric, recombinant mouse meprin A and rat meprin B (0.1 μm) were incubated with murine IL-6 (1 μm) as described under “Materials and Methods.” Both meprin A (A) and meprin B (B) proteolytically cleave murine IL-6 to a smaller product, discernable on 15% SDS-PAGE visualized by Western blotting, upon increasing time of incubation (lanes 1–4). Meprin cleavage of murine IL-6 was prevented when meprin A and meprin B were inhibited by 50 μm actinonin (lane 5). The densitometric loss of the murine IL-6 signal caused by meprin A and densitometric gain of meprin B-generated mIL-6 product from three representative Western blots are plotted (C and D).
Homomeric Meprin A and B Cleave Human IL-6
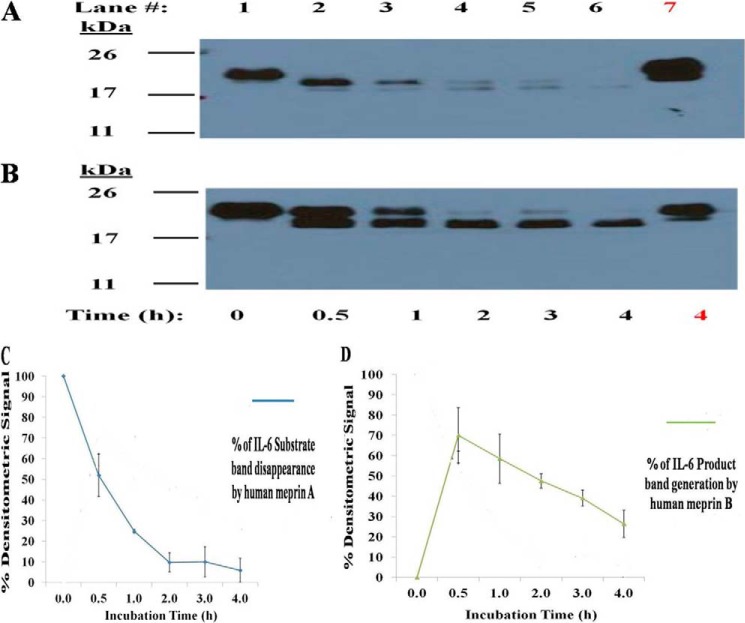
The ability of human meprins to cleave human IL-6 was also tested (Fig. 2). Human meprin A and meprin B cleaved human IL-6 from a 22-kDa substrate to a 21-kDa substrate (Fig. 2, A and B). Similar results of human IL-6 fragmentation by human meprin A and B was seen with both Coomassie staining and immunoblotting (data not shown). After 0.5 h of incubation with human meprin A, ∼50% of hIL-6 substrate remained. After 1 and 2 h of incubation with meprin A, the amount of hIL-6 decreased to ∼20% and <5% of the original signal (Fig. 2C). Similar to what was observed with rat meprin B cleavage of mIL-6, human meprin B cleaves hIL-6 to a slightly smaller product (∼21 kDa). After 0.5 h of incubation, the meprin B product accumulation signal was ∼65% of the signal of uncleaved hIL-6. After 1 and 2 h of incubation, the amount of the meprin-B-generated hIL-6 product decreased to 50 and 35%, respectively (Fig. 2C).
FIGURE 2.
Cleavage of human IL-6 by human meprin A and meprin B. The homomeric isoforms of meprin A and meprin B (0.1 μm) were incubated with human IL-6 (1 μm) as described under “Materials and Methods.” Fragmentation of IL-6 by the meprins was visualized by Western blotting. Hydrolysis of IL-6 by both meprin A and meprin B with 2 h of incubation was inhibited by adding 50 μm actinonin to the meprin/IL-6 reaction mixture (A and B, lane 5). The densitometric loss of the murine IL-6 signal caused by meprin A and densitometric gain of meprin B-generated mIL-6 product from three representative Western blots are plotted (C and D).
Homomeric Meprin A and B Cleave Human IL-6 with High Affinity
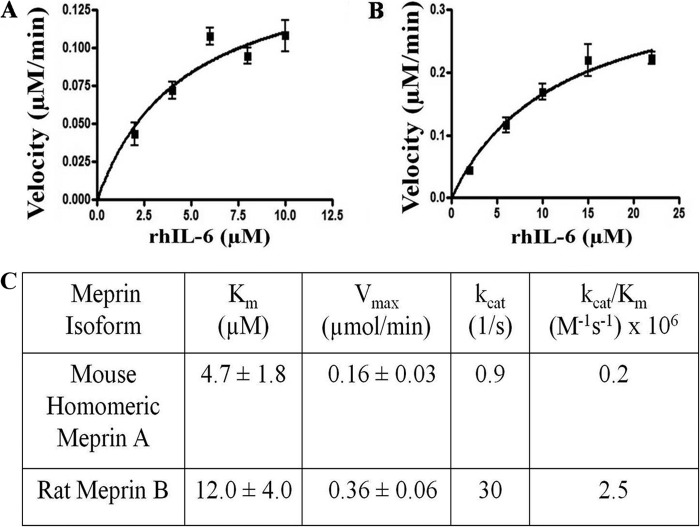
A series of additional studies demonstrated that assays with human IL-6 substrates and murine meprins resulted in similar products as homologous assays. Accordingly, the affinity of human IL-6 as a meprin substrate was determined using the readily available isoform of recombinant human IL-6 (rhIL-6, procured from NCI, National Institutes of Health) and the plentiful stocks of homomeric mouse meprin A and homomeric rat meprin B. For kinetic studies, lower concentrations of meprins (3 nm and 0.2 nm for meprin A and B, respectively) were used than in the previous studies. The rate of rhIL-6 substrate band disappearance after incubation with mouse meprin A, and rat meprin B was determined by densitometric analysis of fluorescently stained SDS-PAGE gels. The velocity plots of meprin A and meprin B cleavage of rhIL-6 are shown in Fig. 3.
FIGURE 3.
Velocity curves and kinetic values for meprin A and B cleavage of human interleukin 6. The velocity of recombinant homomeric mouse meprin A (A) and homomeric rat meprin B (B) cleavage of rhIL-6 as plotted by GraphPad Prism 5 and described under “Materials and Methods” (n = 2 for each point, with each velocity value determined in duplicate). Meprin A (3 nm) and meprin B (0.2 nm) were incubated with rhIL-6 with varying amounts of substrate. Increasing amounts of rhIL-6 (2–10 μm for meprin A and 2–22 μm for meprin B) were incubated with meprin A and meprin B in 20 mm Tris and 50 mm NaCl (pH 7.50) for varying times (0–10 min). The Km and Vmax values were calculated by using nonlinear regression analysis with GraphPad 5 (n = 2). The kcat (apparent) and kcat/Km (apparent) values were calculated as described under “Materials and Methods.”
Meprin A had a significantly lower Km (∼3-fold lower) for rhIL-6 than meprin B. Two more kinetic (apparent) values were calculated from the values derived for the Km and Vmax parameters: the enzyme turnover constant (kcat) and the catalytic enzyme efficiency (kcat/Km) values (Fig. 3C). The kcat value for meprin B was 33-fold higher than for meprin A, indicating that meprin B cleaves rhIL-6 at a faster rate even though rhIL-6 has a greater affinity for meprin A. Overall, rat meprin B cleaves rhIL-6 ∼12-fold more efficiently than mouse meprin A.
Meprin A Cleaves Human IL-6 at its C terminus
From the fragmentation gels of IL-6 incubation with meprins, it was clear that meprin initially removes a small segment of the cytokine. No additional smaller products or fragments of IL-6 generated by meprins, other than what was observed in the SDS-PAGE gels shown in Figs. 1 and 2, have been detected either by protein staining or immunoblotting. To identify the site of meprin A cleavage on IL-6, the meprin A-cleaved IL-6 product separated by SDS-PAGE was excised and analyzed via MS as described under “Materials and Methods.” Peptide maps for both uncleaved rhIL-6 and meprin A-cleaved rhIL-6 were generated using MS/MS. As seen in Fig. 4, the peptide sequences identified for rhIL-6 and the meprin-A rhIL-6 product are identical except for the C-terminal peptides. The difference in the last peptide was that three to five amino acids are present in the uncleaved rhIL-6 but missing from the meprin-cleaved rhIL-6. Potential meprin cleavage locations at the C terminus of IL-6 are Arg-Ala, Ala-Leu, and Leu-Arg. The rhIL-6 peptide maps generated by Glu-C were inconclusive at the C-terminal end, but IL-6 peptides at the N-terminal end were all intact (data not shown). This confirms that meprin cleaves IL-6 at the C-terminal end.
FIGURE 4.

Determination of the cleavage site on rhIL-6 by mouse meprin A. The full primary sequence of human IL-6 is shown in A (uncleaved IL-6) and B (meprin A-cleaved IL-6). The sequences identified by MS analysis are shown in green (80% confidence). Comparing the two forms of IL-6 in A and B, all peptides identified were identical, except the C-terminal peptide in the meprin-A-cleaved IL-6.
Meprin A and B Cleavage of Human and Murine IL-6 Decreases the Activity of This Cytokine
To determine whether meprin cleavage of both human and mouse IL-6 decreases biologic activity, B9 cells, a mouse hybridoma cell line that requires IL-6 for proliferation, were used to compare the activity of uncleaved and meprin-cleaved IL-6. Cell proliferation of B9 cells incubated with uncleaved IL-6 was considered as 100% activity, with the proliferation of the B9 cells incubated with meprin-cleaved IL-6 plotted as a percentage compared with the 100% activity value. Overall, meprin A and B cleavage of both human and murine IL-6 (PeproTech) decreased the activity of these cytokines (Figs. 5 and 6). Human meprin A decreased the activity of human IL-6 to ∼20% of that of uncleaved human IL-6 by 30 min of incubation, and activity remained at that level after 60 and 120 min (Fig. 5). Human meprin B also decreased the activity of human IL-6 over time but with a different pattern compared with human meprin A. Human meprin B decreased the activity of human IL-6 to ∼82% in 30 min, 42% in 60 min, and 35% after 2 h of incubation (Fig. 5). The activities for the human meprin A- and B-treated human IL-6 correlate more with the loss of the 22-kDa IL-6 band on the Western blot analysis than with the 21-kDa IL-6 band. However, the loss of immunologically recognizable forms of human IL-6 did not directly correlate with loss of biological activity.
FIGURE 5.
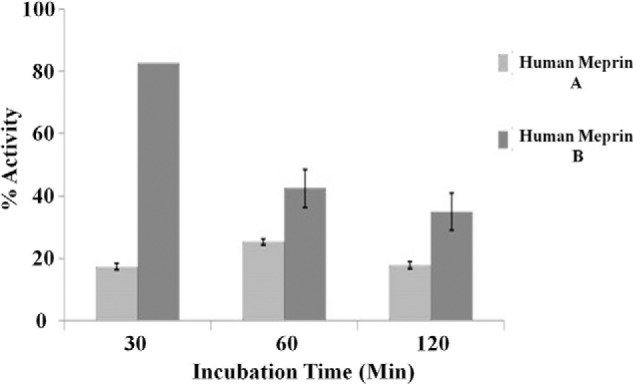
Human meprin A and meprin B decrease the bioactivity of human IL-6. Recombinant human homomeric meprin A and B (0.1 μm) and human IL-6 (1 μm) were incubated for several lengths of time, as indicated on the x axis. The percentage of activity of the meprin-cleaved IL-6 was determined by comparing the proliferation of B9 cells incubated with meprin-cleaved IL-6 with the proliferation of B9 cells incubated with uncleaved IL-6 (which was considered 100% activity) (n = 3).
FIGURE 6.
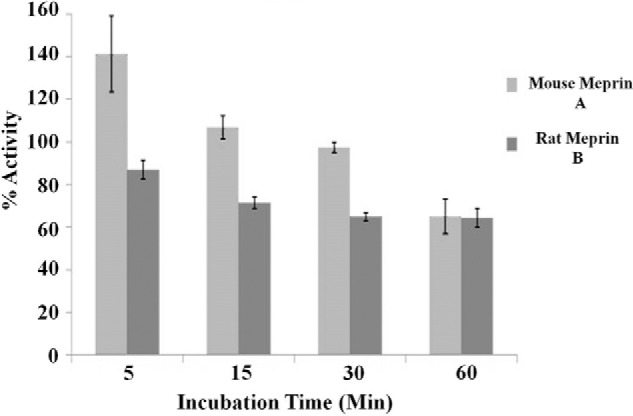
Rodent meprin A and meprin B decrease the bioactivity of murine IL-6. Recombinant mouse and rat meprin A and B (0.1 μm each) were incubated with murine IL-6 (1 μm) for 5–120 min, as indicated on the x axis. The percentage of activity of the meprin-cleaved IL-6 was determined by comparing the proliferation of B9 cells incubated with meprin-cleaved IL-6 with the proliferation of B9 cells incubated with uncleaved IL-6 (which was considered 100% activity) (n = 3).
For the rodent isoforms, both meprin A and B decreased the activity of murine IL-6 by 40% after 1 h. However, with short-term incubations (5 min), meprin A increased the biological activity of IL-6. This increase was not observed for meprin B-treated IL-6. Meprin B decreased IL-6 biological activity ∼18% in 5 min, 30% in 15 min, and 40% in 30 min. As observed with the human IL-6 activity values, there was no direct correlation between loss of immunologically recognizable forms of IL-6 and biological activity.
Cell-bound Meprins Cleave Exogenous IL-6
Meprin metalloproteases are often found in vivo as membrane-bound meprin B and heteromeric meprin αβ isoforms, such as in certain portions of the intestine and kidney proximal tubule cells (17, 18). Because MCDK cells do not express meprin genes in culture, the cells were transfected with full-length meprin β constructs to produce meprin B (β/β) at the cell surface or with both meprin α and β to produce membrane-bound heteromeric meprin A (α2β2 or α3β1) at the cell surface. All of these isoforms have been found to exist in vivo (34). The cell-anchored meprins are expressed in the latent form (with the prosequence) in culture, and proteolysis with a trypsin-like enzyme is necessary for activation. The meprins were activated by limited trypsin digestion, and the trypsin was then inhibited and removed from the culture medium. The meprin-expressing MDCK cells were then incubated with exogenous rhIL-6 to determine whether activated meprins were able to cleave exogenous rhIL-6. Fig. 7 shows a Western blot analysis of IL-6 mobility after exposure to activated or inactive meprins. Lanes 1 and 5 in Fig. 7A are controls that show the mobility of IL-6 after incubation with MDCK cells not transfected with meprins. The upper band at ∼26 kDa is the diglycosylated form of rhIL-6. The majority of the rhIL-6 is monoglycosylated; the band of ∼22 kDa. Fig. 7A (lanes 2–4 and lanes 6–8) shows that the membrane-bound activated forms of meprin B and heteromeric A cleaved IL-6 to a faster-moving form. As seen in Fig. 7B, latent forms (non-trypsinized) of meprin B and heteromeric meprin A did not produce the same mobility change as active meprins (compare lane 1 to lane 2, containing active and inactive meprin B, respectively, and lane 3 to lane 4, containing active and inactive forms of heteromeric meprin A). These data provide evidence that cells expressing active membrane-bound forms of meprins are able to cleave exogenous IL-6.
FIGURE 7.
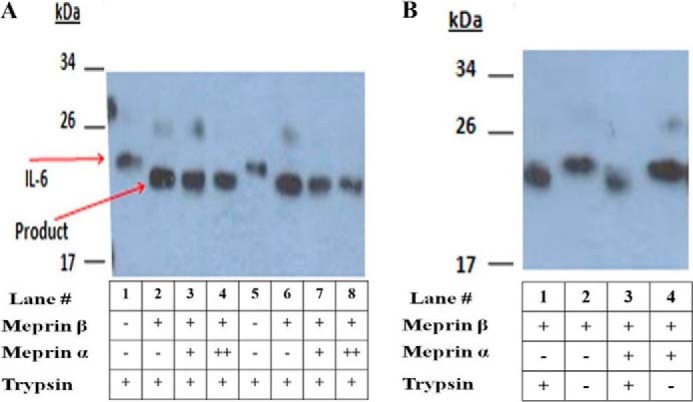
Interaction of human IL-6 with rat meprin B and rat meprin A expressed in MDCK cells. Full-length DNA constructs encoding rat meprin β and rat meprin α were transfected into MDCK cells. The resulting membrane-bound meprin B and heteromeric meprin A isoforms were activated by limited trypsin digestion as described under “Materials and Methods.” After trypsin was removed from the culture, exogenous rhIL-6 was added to the cells, and 24 h of incubation followed. Medium containing rhIL-6 was then isolated, and proteins were subjected to SDS-PAGE and probed with a polyclonal antibody against IL-6. Lanes with columns indicating + for both meprin β and meprin α represent MDCK cells that were transiently transfected with meprin β and meprin α constructs in a 1:1 ratio. Lanes with columns indicating + for the meprin β and ++ for the meprin α constructs represent MDCK cells that were transfected with a 2:1 molar ratio of the meprin α and meprin β constructs, respectively. A, lanes 1 and 5 contain medium from untransfected cells treated with trypsin. Lanes 2-4 and 6–8 contain medium transfected with meprin constructs and treated with trypsin to activate meprins. B, lanes 1 and 2 contain medium from cells transfected with meprin β with and without trypsin treatment, respectively. Lanes 3 and 4 contain medium from cells transfected with meprin α and β with and without trypsin, respectively.
DISCUSSION
This work identifies and characterizes a new substrate for meprin metalloproteases. Both meprin A and meprin B cleave IL-6 with micromolar affinity, and both human and murine IL-6 are substrates of meprins for their respective species isoforms of meprin A and meprin B. Furthermore, meprin cleavage of IL-6 decreases its biological activity. That is, it decreases the ability of IL-6 to stimulate growth of B9 cells. Lastly, it has been shown that membrane-bound active meprins expressed by MDCK cells are able to cleave extracellular IL-6. Elevated levels of IL-6 are correlated with increased inflammation found in the meprin null mice in an experimental model of IBD, reinforcing the hypothesis that meprin is a modulator of inflammation by acting upon IL-6 and decreasing its activity (9, 35).
To date, meprins are the only proteases whose interaction with IL-6 has been characterized kinetically. Furthermore, it has been found that IL-6 is a good substrate for meprins. Villin and actin are the best substrates of meprins identified so far, with Km values of ∼1 μm (20). Meprins cleave IL-6 with Km values in the range of those of proIL-18 (4.7 versus 5.5 μm and 12.0 versus 1.5 μm for meprin A and meprin B, respectively) (16).
The C terminus of IL-6 is an ideal location for proteolytic attack because it is solvent-exposed. The MS data in this study show that three to five amino acids are cleaved from the C terminus of IL-6. The Ala182-Leu183-Arg184 peptide was absent from the meprin-digested, tryptic peptides of IL-6. On the basis of the known specificity of meprin A, we propose that the putative bond cleaved in IL-6 is the Ala182-Leu183 bond in the C terminus (17, 22). Although a recent analysis of meprin cleavage specificity using whole protein substrates indicates that meprin α cleavage is quite nonspecific at the P1 and P1′ positions of peptide bonds, alanine and leucine are preferred amino acids for cleavage at the P1 and P1′ positions (17, 22, 24). Gingipains, trypsin-like cysteine proteases secreted by bacteria that cause gingivitis, have also been found to cleave IL-6 at the C terminus. The Arg-gingipain cleaves rhIL-6 at the Arg181-Ala182 bond, and the Lys-gingipain cleaves IL-6 at the Lys173-Glu174 bond (36). This study also found that the C terminus is important for the biologic activity of IL-6 because the loss of four C-terminal amino acids is enough to significantly decrease the biologic activity of IL-6 (37, 38).
Although meprin deactivates IL-6 with time of incubation, there is no direct correlation between the loss of the 22- or 21-kDa forms of IL-6 seen on the Western blot analyses and the loss of IL-6 activity. For the human IL-6 studies, meprin A treatments decreased biological activity by ∼80% by 30 min, and activity remained at this level at 1 and 2 h. The immunologically recognized 22-kDa band totally shifts to the product 21-kDa band in the first 30 min and is decreased in intensity by 50% by 30 min, 85% by 1 h, and 90% by 2 h. Thus, the residual 20% biological activity for up to 2 h correlates more with the loss of the 22-kDa band than with the 21-kDa band that varies over this time period. For human meprin B studies, the loss of the 22-kDa form (40% at 30 min and 65% at 1 h) roughly correlates with the loss of biological activity for 30 and 1 h (20% at 30 min and 60% at 1 h). Biological activity remains at 35% at 2 h, whereas there is very little of the 22-kDa band at this time point. The 21-kDa band decreases from 70 to 60 to 50% of original intensity at 30 min, 1 h, and 2 h, respectively. Although the pattern of loss of the 21-kDa band does not correlate with the loss of biological activity, the fact that this band and biological activity persists after 2 h of incubation suggests that this isoform retains some activity.
For the rodent form of IL-6, there is the complication of what appears to be an activation of IL-6 biological activity after a short incubation (5 min) with meprin A. As a consequence, after 5 min of incubation, 140% activity is observed, and after 1 h of incubation, when there is little to no recognized band at 22 kDa or 21 kDa, 40% biological activity remains. One interpretation of these results is that mouse meprin A creates an immunologically unrecognized form of IL-6 that is biologically active. That is, it enhances B9 cell proliferation either through the IL-6 receptor or in another manner. Truncated forms may bind receptors with higher affinity than IL-6. Another possibility is that meprin A activates a contaminant protein in the mouse IL-6 preparation that enhances B9 cell proliferation. For rodent meprin B, IL-6 biological activity was decreased ∼40% in 30 min and 1h, the 22-kDa band was decreased 35% at 30 min and 70% at 1 h, and the 21-kDa band decreased 30% between 30 min and 1 h. Thus, again, the loss of immunologically recognizable forms of IL-6 did not directly correlate with the loss of biological activity.
Part of the difficulty in the quantitative assessment of biological activity is that it is measured as a function of B9 cell proliferation. B9 cells are incubated with IL-6 that has been treated with meprin A or B for various lengths of time, and cell growth is measured over a 72-hour time period. The complexity of the cell-based assay does not result in exact correlations with specific bands, and even when there are no or vanishingly small amounts of immunologically recognized bands on Western blot analyses, some biological activity remains (e.g. 20–40% for the human and rodent forms). Whatever the reason for “activation” with very short incubations of rodent IL-6 with meprin A, and the observation of biological activity when there is little evidence of immunologically recognizable forms of IL-6, our studies are consistent with the proposition that the removal of three to five amino acids and further degradation of the cytokine results in inactivation of the cytokine with time.
In vivo, secreted and membrane-bound meprins are ideally localized for keeping IL-6 activity under control, allowing for a robust but appropriate immune response. The PMN-derived serine proteases (neutrophil elastase, proteinase 3, and cathepsin C) also cleave IL-6, decreasing the activity of this cytokine. These proteases cleave IL-6 at locations other than the C terminus. Neutrophil elastase cleaves IL-6 at the N terminus, cathepsin C cleaves IL-6 between the first and second IL-6 helices, and proteinase 3 cleaves IL-6 at a small helical structure outside of the main four α-helical bundle but not at the C terminus (39). It is likely that the PMN-derived proteases work in concert with the meprins to keep IL-6 activity under control. Upon release of IL-6 from epithelial cells after injury, epithelial cell-based meprins are readily available to act upon IL-6. Later on during the immune response, after monocyte/macrophage infiltration, meprins expressed by these immune cells are positioned to act upon the IL-6 expressed by both monocytes and macrophages.
Our data indicate that the meprins, meprin B in particular, may be better suited to cleave IL-6 in vivo than the PMN-derived proteases. In vitro studies of PMN-derived proteases use higher concentrations of enzyme (333 μm) and substrate (7.7 μm) compared with those in this study. This implies that these proteases have lower affinities for IL-6 than the meprins. Furthermore, meprin B has an acidic pH optimum, whereas meprin A and the polymorphonuclear-derived proteases have neutral pH optimums (32, 40). There is a considerable decrease of pH values at sites of inflammation, therefore making meprin B more capable of cleaving IL-6 in vivo.
This work provides insight into how meprins are involved in the pathogenesis of inflammatory diseases where IL-6 is an aggravating factor. In addition, this work suggests that meprins may be considered for replacement therapy for IBD patients who display low meprin expression levels.
Acknowledgments
We thank Dr. S. Gaylen Bradley (Pennsylvania State College of Medicine) for critical reading of the manuscript; Dr. Anne Stanley, Dr. Bruce Stanley, and the Pennsylvania State College of Medicine Mass Spectrometry Core Facility for help with mass spectrometric analysis; and the National Cancer Institute, National Institutes of Health Biological Resources Branch for the recombinant human IL-6.
This work was supported, in whole or in part, by National Institutes of Health grant R01 DK019691 (to J. S. B.). This work was also supported by Pennsylvania Department of Health tobacco settlement funds (to J. S. B.).
- IBD
- inflammatory bowel disease
- MDCK
- Madin-Darby canine kidney
- MEM
- minimal essential medium
- mIL-6
- murine IL-6
- hIL-6
- human IL-6
- hrIL-6
- recombinant human IL-6.
REFERENCES
- 1. Rose-John S. (2012) IL-6 trans-signaling via the soluble IL-6 receptor. Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 8, 1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doan T. (2007) in Lippincott's Illustrated Reviews. Immunology (Harvey R. A., ed) p. 384, Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 3. Jones L. L., Vignali D. A. (2011) Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol. Res. 51, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grivennikov S., Karin E., Terzic J., Mucida D., Yu G. Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., Karin M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanaka T., Kishimoto T. (2012) Targeting interleukin-6. All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 8, 1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisman E. Z., Tenenbaum A. (2010) The ubiquitous interleukin-6. A time for reappraisal. Cardiovasc. Diabetol. 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H., Wang H. Y., Bassel-Duby R., Maass D. L., Johnston W. E., Horton J. W., Tao W. (2007) Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am. J. Physiol. Heart Circ. Physiol. 292, H2408–H2416 [DOI] [PubMed] [Google Scholar]
- 8. Hänel K. H., Cornelissen C., Lüscher B., Baron J. M. (2013) Cytokines and the Skin Barrier. Int. J. Mol. Sci. 14, 6720–6745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atreya R., Neurath M. F. (2005) Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allergy Immunol. 28, 187–196 [DOI] [PubMed] [Google Scholar]
- 10. Yura R. E., Bradley S. G., Ramesh G., Reeves W. B., Bond J. S. (2009) Meprin A metalloproteases enhance renal damage and bladder inflammation after LPS challenge. Am. J. Physiol. Renal Physiol. 296, F135–F144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banerjee S., Jin G., Bradley S. G., Matters G. L., Gailey R. D., Crisman J. M., Bond J. S. (2011) Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G273–G282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaushal G. P., Haun R. S., Herzog C., Shah S. V. (2013) Meprin A metalloproteinase and its role in acute kidney injury. Am. J. Physiol. Renal Physiol. 304, F1150–F1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banerjee S., Oneda B., Yap L. M., Jewell D. P., Matters G. L., Fitzpatrick L. R., Seibold F., Sterchi E. E., Ahmad T., Lottaz D., Bond J. S. (2009) MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol. 2, 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herzog C., Haun R. S., Kaushal V., Mayeux P. R., Shah S. V., Kaushal G. P. (2009) Meprin A and meprin alpha generate biologically functional IL-1β from pro-IL-1β. Biochem. Biophys. Res. Commun. 379, 904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herzog C., Kaushal G. P., Haun R. S. (2005) Generation of biologically active interleukin-1β by meprin B. Cytokine 31, 394–403 [DOI] [PubMed] [Google Scholar]
- 16. Banerjee S., Bond J. S. (2008) Prointerleukin-18 is activated by meprin β in vitro and in vivo in intestinal inflammation. J. Biol. Chem., 283, 31371–31377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterchi E. E., Stöcker W., Bond J. S. (2008) Meprins, membrane-bound and secreted astacin metalloproteinases. Mol. Aspects Med. 29, 309–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broder C., Becker-Pauly C. (2013) The metalloproteases meprin α and meprin β. Unique enzymes in inflammation, neurodegeneration, cancer and fibrosis. Biochem. J., 450, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kruse M. N., Becker C., Lottaz D., Köhler D., Yiallouros I., Krell H. W., Sterchi E. E., Stöcker W. (2004) Human meprin α and β homo-oligomers. Cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem. J. 378, 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ongeri E. M., Anyanwu O., Reeves W. B., Bond J. S. (2011) Villin and actin in the mouse kidney brush border membrane bind to and are degraded by meprins. This interaction contributes to injury in ischemia reperfusion. Am. J. Physiol. Renal Physiol. 301, F871–F882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao J., Yura R. E., Matters G. L., Bradley S. G., Shi P., Tian F., Bond J. S. (2013) Meprin A impairs epithelial barrier function, enhances monocyte migration and cleaves the tight-junction protein occludin. Am. J. Physiol. Renal Physiol. 305, F714–F726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertenshaw G. P., Turk B. E., Hubbard S. J., Matters G. L., Bylander J. E., Crisman J. M., Cantley L. C., Bond J. S. (2001) Marked differences between metalloproteases meprin A and B in substrate and peptide bond specificity. J. Biol. Chem. 276, 13248–13255 [DOI] [PubMed] [Google Scholar]
- 23. Norman L. P., Matters G. L., Crisman J. M., Bond J. S. (2003) Expression of meprins in health and disease. Curr. Top. Dev. Biol. 54, 145–166 [DOI] [PubMed] [Google Scholar]
- 24. Becker-Pauly C., Barré O., Schilling O., Auf dem Keller U., Ohler A., Broder C., Schütte A., Kappelhoff R., Stöcker W., Overall C. M. (2011) Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol. Cell. Proteomics 10, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hengst J. A., Bond J. S. (2004) Transport of meprin subunits through the secretory pathway. Role of the transmembrane and cytoplasmic domains and oligomerization. J. Biol. Chem. 279, 34856–34864 [DOI] [PubMed] [Google Scholar]
- 26. Tsukuba T., Kadowaki T., Hengst J. A., Bond J. S. (2002) Chaperone interactions of the metalloproteinase meprin A in the secretory or proteasomal-degradative pathway. Arch. Biochem. Biophys. 397, 191–198 [DOI] [PubMed] [Google Scholar]
- 27. Simpson R. J., Hammacher A., Smith D. K., Matthews J. M., Ward L. D. (1997) Interleukin-6. Structure-function relationships. Protein Sci. 6, 929–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coulie P. G., Stevens M., Van Snick J. (1989) High- and low-affinity receptors for murine interleukin 6. Distinct distribution on B and T cells. Eur. J. Immunol. 19, 2107–2114 [DOI] [PubMed] [Google Scholar]
- 29. Bylander J. E., Bertenshaw G. P., Matters G. L., Hubbard S. J., Bond J. S. (2007) Human and mouse homo-oligomeric meprin A metalloendopeptidase. Substrate and inhibitor specificities. Biol. Chem. 388, 1163–1172 [DOI] [PubMed] [Google Scholar]
- 30. Ishmael S. S., Ishmael F. T., Jones A. D., Bond J. S. (2006) Protease domain glycans affect oligomerization, disulfide bond formation, and stability of the meprin A metalloprotease homo-oligomer. J. Biol. Chem. 281, 37404–37415 [DOI] [PubMed] [Google Scholar]
- 31. Bertenshaw G. P., Norcum M. T., Bond J. S. (2003) Structure of homo- and hetero-oligomeric meprin metalloproteases. Dimers, tetramers, and high molecular mass multimers. J. Biol. Chem. 278, 2522–2532 [DOI] [PubMed] [Google Scholar]
- 32. Bertenshaw G. P., Villa J. P., Hengst J. A., Bond J. S. (2002) Probing the active sites and mechanisms of rat metalloproteases meprin A and B. Biol. Chem. 383, 1175–1183 [DOI] [PubMed] [Google Scholar]
- 33. Scott T. R., Lillehoj H. S. (2006) Monoclonal antibodies against chicken interleukin-6. Vet. Immunol. Immunopathol. 114, 173–177 [DOI] [PubMed] [Google Scholar]
- 34. Bond J. S., Matters G. L., Banerjee S., Dusheck R. E. (2005) Meprin metalloprotease expression and regulation in kidney, intestine, urinary tract infections and cancer. FEBS Lett. 579, 3317–3322 [DOI] [PubMed] [Google Scholar]
- 35. Banerjee S., Jin G., Bradley S. G., Matters G. L., Gailey R. D., Crisman J. M., Bond J. S. (2011) Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G273–G282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banbula A., Bugno M., Kuster A., Heinrich P. C., Travis J., Potempa J. (1999) Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem. Biophys. Res. Commun. 261, 598–602 [DOI] [PubMed] [Google Scholar]
- 37. Ward L. D., Hammacher A., Zhang J. G., Weinstock J., Yasukawa K., Morton C. J., Norton R. S., Simpson R. J. (1993) Role of the C-terminus in the activity, conformation, and stability of interleukin-6. Protein Sci. 2, 1472–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krüttgen A., Rose-John S., Möller C., Wroblowski B., Wollmer A., Müllberg J., Hirano T., Kishimoto T., Heinrich P. C. (1990) Structure-function analysis of human interleukin-6. Evidence for the involvement of the carboxy-terminus in function. FEBS Lett. 262, 323–326 [DOI] [PubMed] [Google Scholar]
- 39. Bank U., Küpper B., Reinhold D., Hoffmann T., Ansorge S. (1999) Evidence for a crucial role of neutrophil-derived serine proteases in the inactivation of interleukin-6 at sites of inflammation. FEBS Lett. 461, 235–240 [DOI] [PubMed] [Google Scholar]
- 40. Korkmaz B., Moreau T., Gauthier F. (2008) Neutrophil elastase, proteinase 3 and cathepsin G. Physicochemical properties, activity and physiopathological functions. Biochimie 90, 227–242 [DOI] [PubMed] [Google Scholar]