Background: Methylation of OmpB has been implicated in rickettsial virulence.
Results: Native OmpBs purified from Rickettsia contain mono- and trimethyllysine at specific locations that coincide with those catalyzed by methyltransferases in vitro.
Conclusion: The number of trimethyllysine clusters in OmpBs correlates with degree of virulence.
Significance: This study provides new insight into methylation of OmpB and its correlation with virulence.
Keywords: Bacteria, Cell Surface Protein, Enzyme Catalysis, Mass Spectrometry (MS), Protein Methylation
Abstract
Methylation of rickettsial OmpB (outer membrane protein B) has been implicated in bacterial virulence. Rickettsial methyltransferases RP789 and RP027-028 are the first biochemically characterized methyltransferases to catalyze methylation of outer membrane protein (OMP). Methylation in OMP remains poorly understood. Using semiquantitative integrated liquid chromatography-tandem mass spectroscopy, we characterize methylation of (i) recombinantly expressed fragments of Rickettsia typhi OmpB exposed in vitro to trimethyltransferases of Rickettsia prowazekii RP027-028 and of R. typhi RT0101 and to monomethyltransferases of R. prowazekii RP789 and of R. typhi RT0776, and (ii) native OmpBs purified from R. typhi and R. prowazekii strains Breinl, RP22, and Madrid E. We found that in vitro trimethylation occurs at relatively specific locations in OmpB with consensus motifs, KX(G/A/V/I)N and KT(I/L/F), whereas monomethylation is pervasive throughout OmpB. Native OmpB from virulent R. typhi contains mono- and trimethyllysines at locations well correlated with methylation in recombinant OmpB catalyzed by methyltransferases in vitro. Native OmpBs from highly virulent R. prowazekii strains Breinl and RP22 contain multiple clusters of trimethyllysine in contrast to a single cluster in OmpB from mildly virulent R. typhi. Furthermore, OmpB from the avirulent strain Madrid E contains mostly monomethyllysine and no trimethyllysine. The native OmpB from Madrid E was minimally trimethylated by RT0101 or RP027-028, consistent with a processive mechanism of trimethylation. This study provides the first in-depth characterization of methylation of an OMP at the molecular level and may lead to uncovering the link between OmpB methylation and rickettsial virulence.
Introduction
Rickettsiae are obligatory intracellular infectious Gram-negative bacteria that are responsible for major rickettsiosis, which includes epidemic and endemic typhus, spotted fever, and scrub typhus (1, 2). Like all Gram-negative bacteria, rickettsiae contain several outer membrane proteins (OMPs)2 that are found in the outer leaflet of the outer membrane. OMPs provide the first line of communication with the extracellular environment, with prominent roles in molecular transport and bacterial infection and pathogenesis (3, 4). Rickettsial OMPs have been shown to participate in host cell attachment, invasion, internalization, and intracellular movement (5–7) and induce strong host humoral and cellular immune responses (8–10).
OmpB (outer membrane protein B) is an OMP that is present in all species of Rickettsia and belongs to the family of OMPs called autotransporters. The precursor of OmpB consists of a signal peptide, a large N-terminal passenger domain and a C-terminal β-barrel domain (11, 12). The passenger domain of rickettsial OmpB has been shown to participate in adhesion to mammalian cells in vitro, suggesting this may be the role of OmpB in the virulence of Rickettsia (5, 6, 13).
It has been known for many years that the passenger domain of OmpB of Rickettsia is methylated, and OmpBs from virulent strains are more extensively methylated (14, 15). More recent studies using genetic and biochemical approaches are consistent with the suggestion that methylation of OmpB may contribute to both (i) the host immunogenic response to OmpB itself and (ii) rickettsial virulence (16–18). Methylation of OmpB appears to enhance its antigenicity. For example, rabbit antiserum against recombinant OmpB is less reactive than antiserum against OmpB purified directly from Rickettsia (19). In addition, the immunoreactivity of chemically methylated recombinant OmpB is enhanced against sera from infected patients (20). Thus, methylation of recombinant OmpB could increase the efficacy of diagnostic reagents and help advance vaccine development against Rickettsia (21).
Protein methylation is a well established post-translational modification known to regulate functions of various proteins (22, 23), frequently through modulating protein-protein interactions (24, 25). The regulatory potential of this modification can be attributed in part to the multiple states of methylation at the ϵ-amino group of lysine, which can be mono-, di-, and trimethylated (26). Unlike the methylation of histones, which has been well established, methylation of outer membrane proteins is not well characterized, particularly at the molecular level.
We have recently characterized two rickettsial lysine methyltransferases (MTs) (27). They are RP789 (PKMT1) and RP027-028 (PKMT2) from Rickettsia prowazekii. RP027-028, as well as its counterpart in Rickettsia typhi, RT0101, catalyzes trimethylation, and RP789 catalyzes monomethylation of rOmpB fragments, monitored by incorporation of radioactive [methyl-3H] from [methyl-3H]AdoMet and Western blot analysis, whereas none of these MTs can methylate either histone or Escherichia coli protein. These are the first biochemically characterized MTs to catalyze the methylation of OMPs and appear to belong to a novel family of protein lysine MTs. The gene sequences of homologous MTs are found to be conserved in more than 40 rickettsial species and strains. The fact that these MTs recognize OmpB at many diverse sites suggests an unusual substrate recognition mechanism. Characterization of methylation in rickettsial OmpB at the molecular level is essential for elucidating the roles of methylation in bacterial virulence and pathogenesis. Moreover, findings on the rickettsial OmpB methylation may advance our understanding of the structure and function of protein lysine methylation in general. In addition, enzymatic methylation of recombinant OmpB may also provide improved methods over chemical methylation in efforts to advance diagnostic reagents and vaccine candidates.
To better understand OmpB methylation, we used semiquantitative integrated LC-MS/MS techniques to characterize the location, state and level of methylation. Our study provides the first characterization of methylation in an OMP and lays the foundation for further understanding how methylation of OmpB may contribute to the virulence of Rickettsia.
MATERIALS AND METHODS
OmpB Proteins
Native OmpB proteins from R. typhi and R. prowazekii strains Breinl, RP22, and Madrid E were extracted using 10 mm Tris-HCl (pH 7.6) at 45 °C for 30 min and purified as described previously (28). Each of the native OmpB proteins showed a single band at Mr 114,000 on SDS-polyacrylamide gel. Recombinant R. typhi OmpB fragments OmpB(AN) and OmpB(K), which corresponded to residues Met33 to Phe744 and Arg745 to Gly1353, respectively, were expressed and purified as previously described (20). Briefly, the bacterial proteins that were expressed in inclusion bodies were dissolved in 8 m urea and 1% deoxycholate, purified by ion exchange chromatography on DEAE-cellulose in 6 m urea. Soluble proteins were obtained by slow dialysis at stepwise decreasing concentrations of urea. No methylated lysine in recombinant OmpB(AN) and (K) were detected by LC-MS/MS. Protein concentrations were determined using Bio-Rad protein assays with bovine serum albumin as the standard.
Rickettsial MTs
Purified recombinant R. prowazekii RP789 (NP221139) and RP027-028 (ADE29537) and R. typhi RT0776 (YP067714) and RT0101 (YP067069) were used in enzymatic methylation of OmpB fragments in the present study. The MTs were prepared by the same method as previously described (27). No methyllysines in recombinant MTs were found as determined by LC-MS/MS.
Deletions of N-terminal Sequences of RP789 and RT0776
The plasmids expressing RP789ΔN (deletion of N-terminal 28 residues) and RT0776ΔN (deletion of N-terminal 27 residues) were constructed in pET28a by subcloning PCR-amplified DNA using the plasmids encoding RP789 and RT0776, respectively, as templates. Primer sequences are available on request. The constructs were confirmed by sequencing. Expression and purification was performed as described previously (27).
Radioactivity Assay of MT Activity
The standard assay mixture contained, in 50 μl of final volume, 8.3 mm sodium phosphate (pH 8.0), 0.16 mm [methyl-3H]AdoMet (34 mCi/mmol diluted from 10 Ci/mmol [methyl-3H]AdoMet, which has a >97% purity obtained from PerkinElmer, with the highest purity AdoMet purchased from New England Biolabs) and 2 μm OmpB(AN) or 1 μm OmpB(K). The reactions were initiated by adding MT to a final concentration of 0.26 μm and incubated at 37 °C. Aliquots of the reaction mixture were spotted at the indicated time onto Whatman 3MM cellulose filter paper discs (Fisher Scientific) and soaked briefly with 5% TCA. The paper discs were washed three times with 5% ice-cold TCA, followed by washing with ethanol:ether (1:1 by v/v) mixture. The amounts of acid precipitable radioactivity were determined using a PerkinElmer Wallace 1410 liquid scintillation counter. The assay conditions were optimized by varying pH. DTT, KCl, and cations reduce the activity of MT, and none of them was included in the assay.
Kinetic Analysis of the MTs
Initial rates of MT-catalyzed reactions were determined from the linear portions of the time courses of methylation at varying concentrations of OmpB(AN) up to 2 μm using the radioactivity assay at 37 °C. These initial rates were used to determine the Michaelis-Menten and catalytic constants. The reactions were initiated by the addition of the specified MT to a final concentration of 0.26 μm. Michaelis-Menten constants and maximum velocities were obtained by direct fit using KaleidaGraph. It should be noted that the initial rates thus determined represent the sum of initial rates of numerous enzymatic methylation reactions at multiple lysine residues in OmpB(AN) to three different methylation states whose rates may vary with wide ranges. The Michaelis-Menten and catalytic constants based on the radioactivity assay can only be considered as apparent Michaelis-Menten and catalytic constants.
Preparation of Proteins for LC-MS/MS Analysis
OmpB(AN) (10 μg) and OmpB(K) (5 μg) were methylated separately using 10 μg of specified MT in 50 μl of reaction mixtures containing 3.2 mm AdoMet (New England Biolabs) and 8.3 mm sodium phosphate (pH 8.0). After overnight incubation at 37 °C, the reaction mixture was evaporated to 20 μl using SpeedVac and mixed with SDS sample buffer. The proteins were separated by SDS-PAGE, and OmpB(AN) and (K) protein bands were excised from the gel and subjected to in-gel digestion. Native OmpB proteins (2 μg each) were separated by SDS-PAGE, excised from the gel, and processed for in-gel digestion. Multiple samples of enzymatically methylated rOmpB and native OmpB proteins were independently prepared and analyzed using LC-MS/MS.
In-gel Digestion
In-gel digestion of proteins was carried out as described with modifications (29). Briefly, the excised protein bands from SDS gels were washed using 50% methanol and 5% acetic acid, followed by reduction using 10 mm DTT. The protein samples were alkylated with 100 mm iodoacetamide in the dark. The gel pieces were dehydrated using acetonitrile and rehydrated with 100 mm (NH4)HCO3 twice. The gel pieces were mixed with 1 μg of sequencing grade chymotrypsin (Roche Applied Science) in 50 mm (NH4)HCO3 and digested overnight at 25 °C. The digested peptides were extracted with 50% (v/v) acetonitrile and 5% (v/v) formic acid. The volume was reduced to less than 20 μl by evaporation, and the final volume was adjusted to 20 μl using 1% formic acid. The samples were purified using Zip-Tip with C18 resin (Millipore, Billerica, MA) according to the manufacturer's protocol.
LC-MS/MS
LC-MS/MS was performed using an Eksigent nanoLC-Ultra two-dimensional system (Dublin, CA) coupled to an LTQ Orbitrap Elite mass spectrometer (Thermo Scientific, San Jose, CA). Peptide sample was first loaded onto a Zorbax 300SB-C18 trap column (Agilent, Palo Alto, CA) at a flow rate of 6 μl/min for 9 min and then separated on a reversed phase BetaBasic C18 PicoFrit analytical column (New Objective, Woburn, MA) using a 40-min linear gradient of 5–35% acetonitrile in 0.1% formic acid at a flow rate of 250 nl/min. Eluted peptides were sprayed into the mass spectrometer equipped with a nano-spray ionization source. Survey MS spectra were acquired in the Orbitrap at a resolution of 60,000. Each MS scan was followed by six data-dependent MS/MS scans in the linear ion trap with dynamic exclusion. Other mass spectrometry settings were as follows: spray voltage, 1.5 kV; full MS mass range, m/z 300–2000; and normalized collision energy, 35%.
Data files generated from the mass spectrometer were analyzed using Proteome Discoverer v1.3 software (Thermo Scientific) and the Mascot search engine running on a six-processor cluster at the National Institutes of Health (version 2.3). The search criteria were set to: database, Swiss-Prot (Swiss Institute of Bioinformatics); taxonomy, Bacteria; enzyme, chymotrypsin; maximal miscleavages, 3; variable modifications, methylation (K), dimethylation (K), trimethylation (K), oxidation (M), and deamidation (N, Q); fixed modifications, carbamidomethylation (C); peptide precursor mass tolerance, 25 ppm; and MS/MS fragment mass tolerance, 0.8 Da. Peptide-spectrum matches (PSM) were filtered to achieve an estimated false discovery rate of 1%.
In this study, mass errors of 3 ppm or less were routinely achieved with the Orbitrap mass spectrometer used in the present study. This high mass accuracy allowed us to differentiate between trimethylation and acetylation or between dimethylation and formylation, which have a mass difference of 0.03639 Da, equivalent to 11–45 ppm given that most peptide precursors had an m/z value between 400 and 1100 with a charge state of either +2 or +3. Even though a peptide mass tolerance of 25 ppm was used in database search, the application of a mass deviation filter (e.g., <5 ppm) established confidence that the tri- or di-methylation sites identified were not due to acetylation or formylation.
Fig. 1A shows the overall scheme of this analysis. The spectral matches of a peptide by MS/MS revealed the amino acid sequence, location, and state of methylation. In addition, LC-MS/MS of the peptides provides the number of peptide spectrum matches of all peptides at unmethylated and mono-, di-, and trimethylated states throughout the OmpB sequence. For multiple measurements, independently prepared samples were used. The MS data are reproducible in types and locations of methylation in independently prepared samples of OmpB and its fragments. The number of PSM correlates with the abundance of individual peptide (30). The observed numbers of PSM vary with the behavior of various peptides in ionization, ion detection, and chromatography and with the amount of protein samples. The variations are progressively lower for greater values of PSM. For comparison of methylations at different locations in OmpB, normalized fraction of methylation (from 0 to 1) is used to compare the relative levels of various methylation states at a specific lysine residue. The peptide coverage in LC-MS/MS analyses is close to the entire sequence of OmpB or the fragments in all cases. It should be pointed out that in the chymotryptic digests, the fragments containing Lys149 and Lys157 were consistently not detected, likely because of retaining by the chromatographic resins.
FIGURE 1.
The overall schemes of LC-MS/MS analysis (A) and OmpB and recombinant OmpB(AN) and OmpB(K) (B). A, the state, location, and normalized fraction of methylation in native OmpB and in enzymatically methylated rOmpB fragments catalyzed by rickettsial MTs were determined by LC-MS/MS analysis according to the outlined scheme. See “Materials and Methods” for details. B, the full-length R. typhi OmpB precursor consists of a signal peptide (amino acids 1–32) in red, a passenger domain (amino acids 33–1353) in green, and an autotransporter domain (amino acids 1354–1645) in blue. The corresponding residue numbers in R. typhi OmpB for recombinant OmpB(AN) and OmpB(K) are shown.
Data Analysis
Custom software PTMsite was used to compute the numbers of PSM at unmethylated and mono-, di-, and trimethylated states for all lysine residues throughout OmpB based on the results of LC-MS/MS of each sample. Normalized fractions of mono-, di-, and trimethylations of a specific lysine residue were calculated from the observed PSM numbers of respective mono-, di-, and trimethylated peptides divided by the total number of observed PSM numbers including unmethylated and mono-, di-, and trimethylated peptides of the corresponding lysine residue. For multiple trials of a protein, the normalized fractions were calculated from the sums of the PSM numbers of each methylated state at the lysine residue from multiple trials divided by the sum of PSM numbers of all methylation states of all trials at the same lysine residue.
RESULTS AND DISCUSSION
Kinetics of Methylation by Rickettsial MTs
Expression of the passenger domain of OmpB from R. typhi in E. coli produces inclusion bodies. However, recombinant OmpB (rOmpB) fragments OmpB(AN) and OmpB(K), which correspond to the N- and C-terminal halves of OmpB passenger domain (Fig. 1B), can be successfully purified and refolded to yield soluble protein substrates for analysis. Using the OmpB(AN) as the substrate of MTs, we analyzed the steady state kinetics of two MTs from R. prowazekii (RP789 and RP027-028) and two MTs from R. typhi (RT0766 and RT0101). Table 1 summarizes the steady state kinetic parameters based on the initial rates of the methylation of OmpB(AN) catalyzed by 0.26 μm each of the four MTs by monitoring the incorporation of radioactive [methyl-3H] from [methyl-3H]AdoMet to OmpB(AN). Monomethyltransferases RT0776 and RP789 exhibit an appreciably higher kcat than those of trimethyltransferases RT0101 and RP027-028. In addition, the enzyme efficiency (kcat/Km) of RP789 is higher than that of RT0776, whereas RT0101 is more active than RP027-028.
TABLE 1.
Kinetic parameters of Rickettsial methyltransferases
The steady state kinetics was monitored by the incorporation of radioactive [methyl-3H] from [methyl-3H]AdoMet into OmpB(AN) as described under “Materials and Methods.” The final concentration of methyltransferase was 0.26 μm.
| Methyltransferase | KmAN | kcat | kcat/Km |
|---|---|---|---|
| μm | s−1 | m−1·s−1 | |
| RT0101 | 0.48 ± 0.057 | 0.83 × 10−3 | 1.74 × 103 |
| RP027-028a | 10.1 ± 1.63 | 0.16 × 10−3 | 0.015 × 103 |
| RP789 | 0.29 ± 0.093 | 5.98 × 10−3 | 20.6 × 103 |
| RP789ΔN | 1.13 ± 0.17 | 3.32 × 10−3 | 2.93 × 103 |
| RT0776 | 1.71 ± 0.182 | 2.20 × 10−3 | 1.28 × 103 |
| RT0776ΔN | 3.07 ± 1.55 | 4.45 × 10−3 | 1.45 × 103 |
a RP027-028-catalyzed methylation was analyzed at 2 μm RP027-028 and 2–20 μm OmpB(AN) because of the low enzyme efficiency.
R. typhi RT0101- and RT0776-catalyzed Methylation of rOmpB
Because catalytic activity of MTs is relatively modest, we used 2 μm of trimethyltransferase to prepare enzymatically methylated rOmpB for the LC-MS/MS analysis. The time courses for the methylation of OmpB(AN) (2 μm) and OmpB(K) (1 μm) catalyzed by 2 μm trimethyltransferase RT0101 are shown in Fig. 2A and remain linear for at least 4 h. Similar time courses were also obtained for the methylation of rOmpB fragments catalyzed by 2 μm monomethyltransferase RT0776 (Fig. 2B).
FIGURE 2.
Time courses and normalized fractions of methylation in OmpB(AN) and OmpB(K) catalyzed by four rickettsial methyltransferases. A and B, time courses of RT0101- (A) and RT0776-catalyzed (B) methylation of 2 μm OmpB(AN) (●) or 1 μm OmpB(K) (■) in the presence of 0.16 mm [methyl-3H]AdoMet (34 mCi/mmol in A and 68 mCi/mmol in B), and 8.3 mm sodium phosphate (pH 8.0) were monitored using the radioactivity assay as described under “Materials and Methods.” The reaction was initiated by the addition of RT0101 or RT0776 to a final concentration of 2 μm. The control that contained MT alone (♦) is also shown. C, the normalized fractions of trimethylation at indicated lysine residues in OmpB(AN) and (K) that are catalyzed by RT0101 (open bars) and RP027-028 (solid bars) at 2 μm each are shown. The enzymatically methylated OmpB(AN) and (K) were prepared, and the methylated OmpB(AN) and (K) were analyzed using LC-MS/MS as described under “Materials and Methods.” The PSM values were combined from three independent trials each for RT0101 and RP027-028 and shown in supplemental Table S1. The correlation coefficient of the normalized fractions of trimethylations catalyzed by RT0101 and RP027-028 is 0.65. D, the normalized fractions of monomethylation at lysine residues in OmpB(AN) and (K) catalyzed by RT0776 (open bars) and RP789 (closed bars) at 2 μm are shown. Normalized fractions were determined as described for Fig. 2C. The PSM values were combined from three independent trials each for RT0776 and RP789 and shown in supplemental Table S2. The correlation coefficient of normalized fractions of monomethylation catalyzed by RT0776 and RP789 is 0.45.
To determine methylation profiles in rOmpB, OmpB(AN) and OmpB(K) were enzymatically methylated using 2 μm RT0101 and 3.2 mm AdoMet. After overnight incubation at 37 °C, the methylated OmpB(AN) and OmpB(K) were separated using SDS-PAGE followed by in-gel digestion and LC-MS/MS analysis. The numbers of PSM at four methylation states of all lysine residues in OmpB(AN) and OmpB(K) are shown in supplemental Table S1. The LC-MS/MS analysis reveals a total of 133 trimethyllysine-containing PSM distributed among 16 locations in OmpB. In contrast, nine monomethyllysine-containing PSM and one dimethyllysine-containing PSM were observed (supplemental Table S1). The predominance of trimethyllysine-containing PSM clearly shows that RT0101 is functioned mainly as a trimethyltransferase. The observed mono- and dimethylations could conceivably represent intermediates of trimethylation. Normalized fractions of trimethylation at all lysine residues in rOmpB were calculated for RT0101-catalyzed methylation (Fig. 2C). The normalized fractions reveal that specific lysine residues are trimethylated at varying levels from less than 1% to 36%. No single consensus sequences can be discerned based on the amino acid sequences adjacent to the trimethylated lysines. Our attempt to uncover amino acid sequence motifs of RT0101-catalyzed trimethylation using Motif-X (31) analysis reveals the presence of two motifs: (i) KX(G/A/V/I)Ν (present at Lys205, Lys232, Lys279, Lys667, Lys711, and Lys723) and (ii) KT(I/L/F) (present at Lys226, Lys232, Lys624, Lys635, and Lys667). The sequence at Lys232 and Lys667, KTIN, conforms to both motifs. The remaining sequences do not occur more than three times among the 16 sequences that are trimethylated by RT0101. It should be noted that our preliminary results show that RT0101 is unable to catalyze methylation of two synthetic 20-mer peptides, which contain either one or both of these conserved trimethylation motifs (data not shown), indicating that the conformation of OmpB or other unknown factors may also contribute to substrate recognition.
Supplemental Table S1 shows that RT0101 catalyzed methylation yields a disproportionally low number of PSM, which contains mono- and dimethylated lysine at 9 and 1, respectively, relative to 133 PSM for trimethylated lysine. This observation indicates that the trimethylation reaction likely proceeds via a processive instead of a distributive mechanism. With the former mechanism, the monomethyllysine formed remains enzyme-bound, and the release of product occurs when the lysine is fully trimethylated. This notion is supported by the time courses, monitored during the first 2 h, of the methylation of 2 μm OmpB(AN) catalyzed by 2 μm RT0101. The time course showed the PSM of trimethylated peptides increased steadily to 13, 28, and 56 at 30, 60, and 120 min, respectively, whereas the numbers of PSM of monomethylated peptides were 1, 1, and 6 at 30, 60, and 120 min, respectively. Thus, these data indicate that RT0101-catalyzed trimethylation of OmpB may proceed via a processive mechanism.
LC-MS/MS analysis of RT0776-catalyzed methylation of OmpB(AN) and OmpB(K) was also performed. As shown in supplemental Table S2, the total number of PSM containing monomethyllysine is 379 distributed among 34 lysine residues of a total 45 lysines, although the number of PSM containing di- and trimethyllysines is 6 and 2, respectively. The observation of extensive and predominant monomethylation (Fig. 2D) clearly indicates that RT0776 is mainly functioned as a monomethyltransferase that possesses an active site that can accommodate a wide variety of recognition sequences.
R. prowazekii RP789- and RP027-028-catalyzed Methylation of rOmpB
Unlike R. typhi, R. prowazekii causes epidemic typhus, which is transmitted by lice. MTs from heterologous species may produce products different from those catalyzed by enzymes from homologous species. To characterize the methylation of rOmpB catalyzed by MTs from the heterologous species, we analyzed methylation of R. typhi rOmpB using R. prowazekii RP027-028 and RP789, which possess 94 and 93% identities to their corresponding R. typhi orthologs RT0101 and RT0776, respectively. The locations and the PSM numbers of methylated peptides in rOmpB catalyzed by RP027-028 are summarized in supplemental Table S1, which are very similar to those observed with the methylation of rOmpB catalyzed by RT0101 (Fig. 2C). Similarly, the locations and PSM numbers of methylated peptides in rOmpB catalyzed by RP789 are shown in supplemental Table S2, which reveals 743, 47, and 46 PSM with mono-, di-, and trimethyllysines, respectively. These results show that RP027-028 and RP789 function essentially as a trimethyltransferase and monomethyltransferase, respectively, agreeing with the results observed for their R. typhi orthologs. However, unlike RT0776, RP789 catalyzes mono-, di-, and trimethylations to significantly higher normalized fractions than those catalyzed by RT0776, likely because RP789 and rOmpB are from two different species, whereas RT0776 and rOmpB are from the same species (Fig. 2D and supplemental Table S2). The appreciable differences of the products catalyzed by RT0776 and RP789 for the same substrate are unusual for enzymes sharing high identity. Clearly, heterologous monomethyltransferase does not accurately methylate rOmpB. However, the fact that both RP789 and RT0776 can catalyze methylation at many diverse sites suggests that they both possess active sites that can accommodate a variety of recognition sequences.
N-terminal Sequences in RT0776 and RP789 Contribute to Catalysis and Substrate Recognition
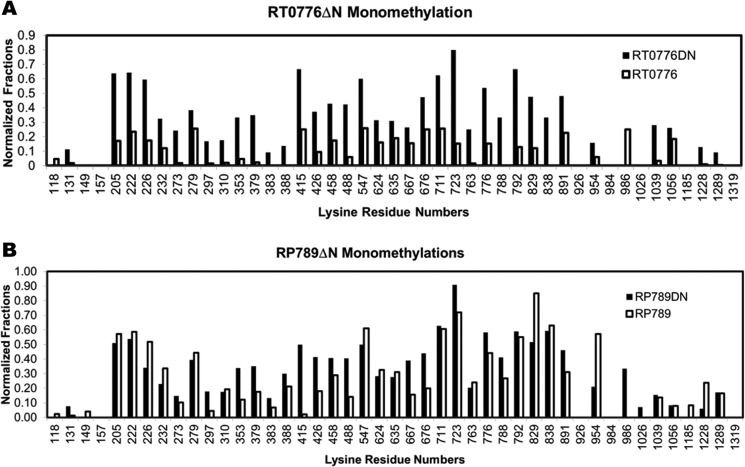
RT0776 shares 45% sequence identity with RT0101 but contains additional 27 N-terminal amino acid residues, which, as analyzed using SignalP 4.1 (32), are not conformed as a signal peptide that have been found in Gram-negative bacteria. Deleting the N-terminal 27 residues in RT0776, we generated RT0776ΔN, which was found to be enzymatically active as monitored by the incorporation of the radioactivity assay (Table 1). Unexpectedly, di- and trimethylation in rOmpB catalyzed by RT0776ΔN is elevated to 32 and 15 PSM, respectively, versus 6 and 2 by RT0776 (comparing supplemental Tables S3 and S2). In addition, the normalized fractions of monomethylation are significantly higher in the RT0776ΔN-catalyzed methylation than those in the RT0776-catalyzed methylation (Fig. 3A). Although the increase in observed mono-, di-, and trimethylation catalyzed by RT0776ΔN (versus the full-length construct) is surprising, the results suggest that the N-terminal sequence in RT0776 may participate in modulating both the catalytic activity and the states of methylation.
FIGURE 3.
The normalized fractions of RT0776ΔN- and RP789ΔN-catalyzed monomethylation at lysine residues of OmpB(AN) and (K). The normalized fractions of monomethylation at indicated Lys residues in OmpB(AN) and (K) that are catalyzed by RT0776ΔN (A, solid bars) and full-length RP0776 (A, open bars) and RP789ΔN (B, solid bars) and full-length RP789 (B, open bars) are shown. The enzymatically methylated OmpB(AN) and (K) were prepared and analyzed using LC-MS/MS as described under “Materials and Methods.” The PSM numbers were combined from three independent trials each for RP789ΔN and one trial for RT0776ΔN and shown in supplemental Table S3. The correlation coefficient of the normalized fractions of monomethylation catalyzed by RT0776 and RT0776ΔN is 0.68, and that by RP789 and RP789ΔN is 0.75.
RP789 contains additional N-terminal 28 residues relative to RP027-028. An N-terminal 28 residue-deleted RP789, RP789ΔN, was also constructed. Similar to RT0776ΔN, RP789ΔN is enzymatically active (Table 1) and also catalyzed additional mono-, di-, and trimethylation in rOmpB as shown in supplemental Table S3. Fig. 3B compares the location and the normalized fraction of monomethylation catalyzed by RP789 and RP789ΔN.
The normalized fractions of mono-, di-, and trimethylation in rOmpB catalyzed by RT0776, RT0776ΔN, RP789, and RP789ΔN are summarized in Table 2. Together, both RT0776ΔN and RP789ΔN produce higher normalized fractions of mono-, di-, and trimethylation than their full-length counterparts, indicating that the N-terminal domain in both RT0776 and RP789 may alter the products of monomethyltransferases by inhibiting their capacity to catalyze di- and trimethylation. Exactly how the N-terminal domain accomplishes this effect remains to be determined and will likely require structural studies of these enzymes. Interestingly, the plasticity exhibited by N-terminal truncated RT0776ΔN and RP789ΔN resembles the alteration of products noted earlier when rOmpB from R. typhi was used as a substrate of heterologous MT from R. prowazekii. Although the physiological function of the N-terminal domain remains to be established, the present results raise the possibility that the N-terminal sequence in rickettsial monomethyltransferases could be processed in vivo to alter its catalytic property and yield additional cellular function.
TABLE 2.
Normalized fractions of methylated peptides in OmpB(AN) and (K) catalyzed by RT0776, RT0776ΔN, RP789, and RP789ΔN
The normalized fractions of unmethylated, mono-, di-, and trimethyllysines were calculated from sums of the numbers of PSM for all lysine residues as described under “Materials and Methods.” Supplemental Tables S2 and S3 show the numbers of PSM of all lysyl residues as unmethylated, mono-, di-, and trimethyllysines in OmpB(AN) and (K) that were catalyzed by RT0776, RP789, RT0776ΔN, and RP789ΔN.
| Monomethylation | Dimethylation | Trimethylation | No methylation | |
|---|---|---|---|---|
| RT0776 | 0.093 | 0.001 | 0 | 0.883 |
| RT0776ΔN | 0.324 | 0.024 | 0.011 | 0.641 |
| RP789 | 0.237 | 0.015 | 0.015 | 0.734 |
| RP789ΔN | 0.313 | 0.034 | 0.036 | 0.617 |
Native OmpB from Virulent R. typhi Contains a Cluster of Highly Trimethylated Lysine Residues
OmpB from Rickettsia is methylated in vivo as revealed previously by amino acid composition analyses (14, 15). When the methylation profile in native OmpB purified directly from R. typhi was analyzed by LC-MS/MS, the numbers of PSM with mono-, di-, and trimethylation were found to be 300, 164, and 215, respectively (supplemental Table S4). The locations of trimethyllysines in native OmpB coincide with those observed for RT0101-catalyzed trimethylation of rOmpB (Fig. 4, A and B). A cluster of four residues at Lys667, Lys676, Lys711, and Lys723 show normalized fraction of trimethylation close to 100%. The same residues are also trimethylated by RT0101 and RP027-028 in vitro. However, the normalized fractions of trimethylation in native OmpB are appreciably higher than those observed in vitro with enzymatically methylated rOmpB using RT0101 (Figs. 2C and 4B).
FIGURE 4.
Locations of trimethylation in native OmpB purified from R. typhi Wilmington coincide with those in RT0101-catalyzed trimethylation in rOmpB. A, normalized fractions of mono- (red), di- (green), and trimethylations (purple) at indicated lysine residues in native OmpB from R. typhi are shown. Native OmpB protein (2 μg) from R. typhi Wilmington was prepared and analyzed by LC-MS/MS as described under “Materials and Methods.” The PSM values were combined from two independent trials and are shown in supplemental Table S4. B, the locations and normalized fractions of trimethylation in native OmpB purified from R. typhi (solid bars, panel B) are compared with those of the trimethylation in OmpB(AN) and (K) catalyzed by RT0101 (open bars; as in Fig. 2C). The correlation coefficient of the normalized fractions of trimethylation in native OmpB and those catalyzed by RT0101 is 0.76.
The high normalized fraction of trimethylation at specific lysine residues observed in native OmpB suggests the existence of a highly efficient cellular system in vivo through which those lysine residues in OmpB can be fully trimethylated. At present, little is known about the mechanism of in vivo trimethylation. Studying the trimethyltransferase catalyzed trimethylation of rOmpB in vitro would provide an approach to reconstitute the in vivo system. To this end, the evidence indicating the presence of a highly efficient in vivo trimethylation system appeared to be contrary to the observation that a high concentration of MTs is required to achieve an appreciable level of methylated rOmpB. This observation is consistent with the presence of product inhibition because of a slow release of the trimethylated lysine from the MT. However, the presence of cellular proteins or factors, which may interact with the methylated OmpB, would shift the equilibrium toward the methylated form of OmpB by facilitating the release of methylated lysine from its MT complex and/or reducing the population of methylated lysine-MT complex. Alternatively, the reduction of methylated MT population can also be accomplished via subcellular translocation (e.g., cytoplasm or periplasmic space), an effect of allosteric factors that could facilitate the post-translational modification. In addition, it is possible that in vivo methylation could occur before or during folding of the OmpB passenger domain where methylation sites could be more accessible than in the case of the fully folded OmpB.
Methylation in Native OmpBs from Virulent and Avirulent Strains of R. prowazekii
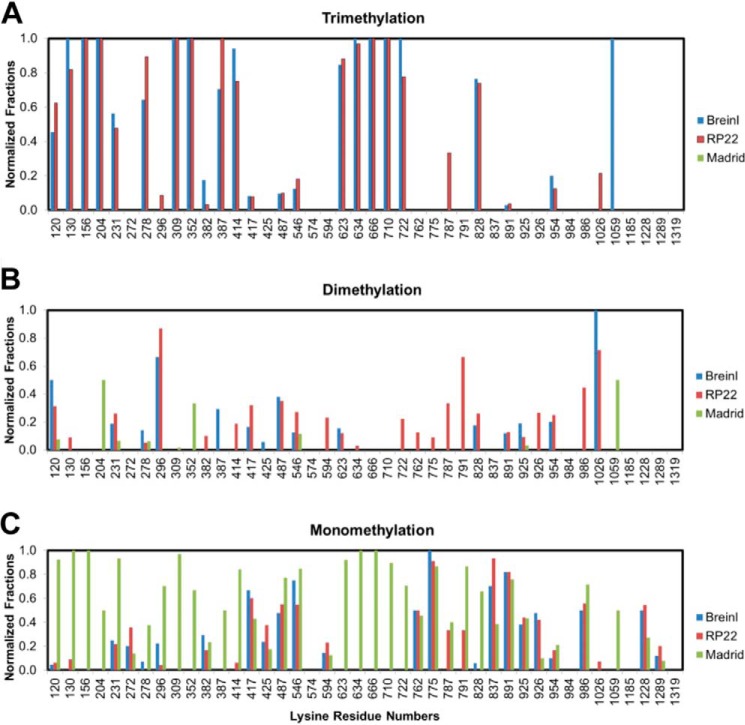
We next examined the methylation in native OmpBs from three strains of R. prowazekii (Madrid E, RP22, and Brein1). R. prowazekii Madrid E is known to be avirulent, whereas R. prowazekii RP22 and Breinl are both highly virulent. PSM numbers of peptides in the three native OmpBs as determined by LC-MS/MS are shown in supplemental Table S5. This table shows that the total numbers of PSM of trimethyllysine-containing peptides in native OmpB from Madrid E, RP22, and Breinl are 0, 271, and 139, respectively (supplemental Table S5). The absence of trimethyllysine in R. prowazekii Madrid E is consistent with the fact that the gene encoding RP027-028 is interrupted by a frameshift mutation, which generates the inactive RP027 and RP028 fragments (17, 27). Fig. 5 shows that the high normalized fraction of trimethylation occurs at specific locations in OmpBs from R. prowazekii strains RP22 and Breinl, and the high normalized fraction of monomethylation occurs in OmpB from R. prowazekii strain Madrid E, which is devoid of any trimethylation. The location, the state, and the normalized fraction of trimethylation and monomethylation in OmpBs from the two strains RP22 and Breinl are remarkably similar (Fig. 5, A and C). The correlation coefficient of the normalized fractions of trimethylations in native OmpBs from strains Breinl and RP22 is 0.90.
FIGURE 5.
Methylation in native OmpBs purified from R. prowazekii strains Breinl, RP22, and Madrid E. The locations and normalized fractions of tri- (A), di- (B), and monomethylation (C) in native OmpBs from R. prowazekii strains Breinl (blue), RP22 (red), and Madrid E (green) are shown. Native OmpBs purified from respective strains (2 μg each) were prepared and analyzed by LC-MS/MS as described under “Materials and Methods.” The PSM numbers of unmethylated and mono-, di-, and trimethyllysine-containing peptides in OmpBs from R. prowazekii strains Breinl, RP22, and Madrid E are shown in supplemental Table S5. The PSM numbers were obtained from two, two, and three independent trials of OmpBs from Breinl, RP22, and Madrid E, respectively.
The amino acid sequences at trimethylation sites abide closely to the recognition motifs found earlier based on the in vitro methylation of rOmpB catalyzed by RT0101 and RP027-028. For example, Lys130 (KILN), Lys204 (KIVN), and Lys231 (KTIN) in OmpB of R. prowazekii conform to the KX(G/A/V/I)N motif (Fig. 2C). No other motifs were found using Prosite in ExPASy (33). Similar to R. typhi OmpB, OmpBs from R. prowazekii strains RP22 and Breinl contain a cluster of highly trimethylated lysine residues at Lys623, Lys634, Lys666, Lys710, and Lys722. However, an additional cluster of highly trimethylated sites occurs at Lys120 to Lys231 and two doublets of highly trimethylated lysine residues in Lys309 to Lys414 with the trimethylation normalized fraction reaching nearly 100% at these sites. The amino acid sequences of OmpBs from R. prowazekii and R. typhi differ at these lysine residues and may account for the additional trimethyllysine in R. prowazekii. It is known that R. prowazekii strains RP22 and Breinl are highly virulent, whereas R. typhi is mildly virulent (34, 35).
Both RP789 and RP027-028 are active in RP22 and Breinl as shown by the extensive mono- and trimethylation observed in native OmpBs. The nearly 100% normalized fraction of trimethylation in OmpB from Breinl and RP22 supports the notion that in vivo OmpB trimethylation and monomethylation are catalyzed by trimethyltransferase, likely via a processive mechanism, and by monomethyltransferase, respectively. In the trimethyltransferase-catalyzed reaction, the reaction intermediates monomethyllysine and dimethyllysine remain enzyme-bound. Under this situation, the monomethyltransferase does not participate in the formation of trimethyllysine.
In comparison to the OmpB from R. prowazekii Madrid E, which is devoid of trimethyllysine, OmpB from R. typhi contains a single cluster of highly trimethylated lysines. The observation that trimethylation occurs in OmpBs from virulent strains but not in OmpB from avirulent strain is in agreement with earlier studies based on amino acid composition analysis (14). However, the present LC-MS/MS analyses show the location, state, and normalized fractions of the modified lysine residues. With this method we found multiple clusters of trimethyllysines in the highly virulent R. prowazekii strains RP22 and Breinl, a single cluster of trimethyllysines in the mildly virulent R. typhi, and none in the avirulent R. prowazekii Madrid E. The number of cluster of highly trimethylated lysines in OmpBs clearly correlates with the degree of virulence of the four strains of Rickettsia.
The correlation between the number of trimethyllysine clusters in OmpBs with the degree of virulence of the four different rickettsial strains suggests that most likely the trimethyllysine clusters in OmpB are associated with rickettsial virulence. It is known that the trimethylation of lysines may enhance cation-π electrostatic (36) and charge independent interactions (37). For example, trimethylation in calmodulin has been shown to modulate NAD kinase (38). Similarly, trimethylation was shown to promote interaction with polynucleotides (39) and participate in histone lysine methylation-mediated chromatin remodeling (40). A number of trimethyllysine-binding domains have been found in recent years (41). Association of methylated H3K9 with HP1 mediates the condensation of nucleosomes to heterochromatin in gene silencing (42). Thus, the presence of cluster of trimethyllysine in a given protein would elevate its valency and significantly enhance its affinity for protein-protein interaction. Additionally, multiple trimethyllysine clusters could further enhance protein-protein interactions. Therefore, it will be of interest to identify the putative rickettsial and human proteins that interact with the trimethyllysine clusters in OmpB that in turn may lead to additional molecular links on rickettsial virulence. It should be noted that the three-dimensional structure of OmpB, either from crystallographic study or from molecular modeling analysis, is not known at present. Thus, we cannot address the methylation profile in term of the structural features of OmpB.
OmpB Purified from the Avirulent Strain Madrid E Is Minimally Methylated by RT0101 and RP027-028
The observed differences in the normalized fraction of methylation between native OmpBs and in vitro methylated rOmpB prompted us to ask whether the native OmpBs can be further methylated by MTs in vitro. We chose native OmpB of R. prowazekii Madrid E as the substrate that contains predominantly monomethyllysine and is devoid of trimethyllysine. We observed that native OmpB from Madrid E is minimally trimethylated by either RT0101 or RP027-028 (Fig. 6). Both trimethyltransferases can generate only a few trimethyllysines in native OmpB from Madrid E. Our results show that only Lys352 in native OmpB was significantly trimethylated by RP027-028. This observation indicates that the preexisting methylation of lysine in the native OmpB prevents further methylation of these lysine residues to form trimethyllysine that is otherwise readily produced in rOmpB catalyzed by RT0101 or RP027-028. Together, these results are consistent with the earlier suggestion that the OmpB trimethylation catalyzed by RT0101 and by RP027-028 may proceed via a processive mechanism in which its reaction intermediates monomethyllysine and dimethyllysine are present as enzyme-bound complexes.
FIGURE 6.

Trimethylation in native OmpB of R. prowazekii strain Madrid E catalyzed by trimethyltransferases. Native OmpB of R. prowazekii Madrid E (2 μg) was methylated using 2 μm RT0101 or RP027-028. The methylated proteins were prepared and analyzed using LC-MS/MS as described under “Materials and Methods.” The locations and the normalized fractions of trimethylation catalyzed by RT0101 (solid bars) and RP027-028 (open bars) are shown.
Concluding Remarks
Methylation of OMPs has been implicated in contributing to bacterial virulence and pathogenesis. To investigate this further and to characterize methylation of rickettsial OmpB, we carried out an in-depth analysis comparing methylation profiles in rOmpB catalyzed by four different MTs and in native OmpB purified directly from virulent and avirulent bacteria, showing their profiles agree closely in location and state of methylation. Our results suggest that monomethylation of OmpB is carried out by monomethyltransferases (RT0776 and RP789), and trimethylation is carried out by trimethyltransferases (RT0101 and RP027-028). The near quantitative trimethylation of specific lysine residues found in native OmpB of virulent Rickettsia is consistent with the existence of an efficient cellular system of trimethylation of OmpB in vivo. Unlike typical lysine MTs, rickettsial MTs can recognize and methylate a diverse set of amino acid sequences. Our study reveals recognition motifs of rickettsial trimethyltransferases, which could be used to predict methylation sites in other OMPs. Additionally, heterologous MTs and N-terminal truncations of MTs significantly alter methylation profiles produced in OmpB. Interestingly, the number of cluster of trimethyllysines in OmpB correlates with the increasing virulence of four rickettsial strains. Furthermore, our study reveals that trimethylation may proceed via a processive mechanism such that monomethylation in OmpB could have an antagonistic effect on trimethylation.
In summary, our study provides the first characterization of methylation of OMPs from Gram-negative bacteria. The new findings on OmpB methylation may bring forward the development of new approaches of investigating the plausible link between OMP methylation and bacterial virulence and raise the possibility of targeting MT (43, 44) to advance new therapeutic strategy against Rickettsia.
Acknowledgments
We acknowledge Dr. Nicholas Noinaj (NIDDK, National Institutes of Health) for many valuable comments during the manuscript preparation and thank Drs. Zhiwen Zhang and Hua-Wei Chen (Naval Medical Research Center) for help in preparing OmpB(AN) and OmpB(K).
The work was supported by Naval Medical Logistic Command Award N62645 (to Georgetown University) and Work Unit 6000.RAD1.J.A0310 (to the Naval Medical Research Center).

This article contains supplemental Tables S1–S5.
- OMP
- outer membrane protein
- MT
- methyltransferase
- AdoMet
- S-adenosylmethionine
- PSM
- peptide spectrum match.
REFERENCES
- 1. Hackstadt T. (1996) The biology of rickettsiae. Infect. Agents Dis. 5, 127–143 [PubMed] [Google Scholar]
- 2. Walker D. H., Ismail N. (2008) Emerging and re-emerging rickettsioses. Endothelial cell infection and early disease events. Nat. Rev. Microbiol. 6, 375–386 [DOI] [PubMed] [Google Scholar]
- 3. Koebnik R., Locher K. P., Van Gelder P. (2000) Structure and function of bacterial outer membrane proteins. Barrels in a nutshell. Mol. Microbiol. 37, 239–253 [DOI] [PubMed] [Google Scholar]
- 4. Buchanan S. K. (1999) β-Barrel proteins from bacterial outer membranes. Structure, function and refolding. Curr. Opin. Struct. Biol. 9, 455–461 [DOI] [PubMed] [Google Scholar]
- 5. Chan Y. G., Cardwell M. M., Hermanas T. M., Uchiyama T., Martinez J. J. (2009) Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 11, 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uchiyama T., Kawano H., Kusuhara Y. (2006) The major outer membrane protein rOmpB of spotted fever group rickettsiae functions in the rickettsial adherence to and invasion of Vero cells. Microbes Infect. 8, 801–809 [DOI] [PubMed] [Google Scholar]
- 7. Li H., Walker D. H. (1998) rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24, 289–298 [DOI] [PubMed] [Google Scholar]
- 8. Dasch G. A., Samms J. R., Williams J. C. (1981) Partial purification and characterization of the major species-specific protein antigens of Rickettsia typhi and Rickettsia prowazekii identified by rocket immunoelectrophoresis. Infect. Immun. 31, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourgeois A. L., Dasch G. A. (1981) The species specific surface protein antigens of Rickettsia typhi. Immunogenicity and protective efficiency in guinea pig. In Rickettsiae and Rickettsial Diseases (Burgdorfer W., Anacker R. L., eds) pp. 71–80, Academic Press, New York [Google Scholar]
- 10. Feng H. M., Whitworth T., Olano J. P., Popov V. L., Walker D. H. (2004) Fc-dependent polyclonal antibodies and antibodies to outer membrane proteins A and B, but not to lipopolysaccharide, protect SCID mice against fatal Rickettsia conorii infection. Infect. Immun. 72, 2222–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barnard T. J., Dautin N., Lukacik P., Bernstein H. D., Buchanan S. K. (2007) Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat. Struct. Mol. Biol. 14, 1214–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulz G. E. (2002) The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 1565, 308–317 [DOI] [PubMed] [Google Scholar]
- 13. Renesto P., Samson L., Ogata H., Azza S., Fourquet P., Gorvel J.-P., Heinzen R. A., Raoult D. (2006) Identification of two putative rickettsial adhesins by proteomic analysis. Res. Microbiol. 157, 605–612 [DOI] [PubMed] [Google Scholar]
- 14. Ching W. M., Wang H., Davis J., Dasch G. A. (1993) Amino acid analysis and multiple methylation of lysine residues in the surface protein antigen of Rickettsia prowazekii. In Techniques in Protein Chemistry, pp. 307–314, Academic Press, Orlando, FL [Google Scholar]
- 15. Rodionov A. V. (1990) The nature of the post-translational modification of the common species-specific outer membrane protein of Rickettsia prowazekii. Bioorg. Khim. 16, 1687–1688 [PubMed] [Google Scholar]
- 16. Bechah Y., El Karkouri K., Mediannikov O., Leroy Q., Pelletier N., Robert C., Médigue C., Mege J.-L., Raoult D. (2010) Genomic, proteomic, and transcriptomic analysis of virulent and avirulent Rickettsia prowazekii reveals its adaptive mutation capabilities. Genome Res. 20, 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J.-Z., Hao J.-F., Walker D. H., Yu X.-J. (2006) A mutation inactivating the methyltransferase gene in avirulent Madrid E strain of Rickettsia prowazekii reverted to wild type in the virulent revertant strain Evir. Vaccine 24, 2317–2323 [DOI] [PubMed] [Google Scholar]
- 18. Chao C. C., Chelius D., Zhang T., Mutumanje E., Ching W. M. (2007) Insight into the virulence of Rickettsia prowazekii by proteomic analysis and comparison with an avirulent strain. Biochim. Biophys. Acta 1774, 373–381 [DOI] [PubMed] [Google Scholar]
- 19. Gilmore R. D., Jr., Cieplak W., Jr., Policastro P. F., Hackstadt T. (1991) The 120 kilodalton outer membrane protein (rOmp B) of Rickettsia rickettsii is encoded by an unusually long open reading frame. Evidence for protein processing from a large precursor. Mol. Microbiol. 5, 2361–2370 [DOI] [PubMed] [Google Scholar]
- 20. Chao C. C., Zhang Z., Wang H., Alkhalil A., Ching W. M. (2008) Serological reactivity and biochemical characterization of methylated and unmethylated forms of a recombinant protein fragment derived from outer membrane protein B of Rickettsia typhi. Clin. Vaccine Immunol. 15, 684–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan Y. G., Riley S. P., Chen E., Martinez J. J. (2011) Molecular basis of immunity to rickettsial infection conferred through outer membrane protein B. Infect. Immun. 79, 2303–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paik W. K., Paik D. C., Kim S. (2007) Historical review. The field of protein methylation. Trends Biochem. Sci. 32, 146–152 [DOI] [PubMed] [Google Scholar]
- 23. Paik W. K., Kim S. (1971) Protein methylation. Science 174, 114–119 [DOI] [PubMed] [Google Scholar]
- 24. Erce M. A., Pang C. N., Hart-Smith G., Wilkins M. R. (2012) The methylproteome and the intracellular methylation network. Proteomics 12, 564–586 [DOI] [PubMed] [Google Scholar]
- 25. Huang J., Berger S. L. (2008) The emerging field of dynamic lysine methylation of non-histone proteins. Curr. Opin. Genet. Dev. 18, 152–158 [DOI] [PubMed] [Google Scholar]
- 26. Botuyan M. V., Lee J., Ward I. M., Kim J.-E., Thompson J. R., Chen J., Mer G. (2006) Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abeykoon A. H., Chao C.-C., Wang G., Gucek M., Yang D. C., Ching W.-M. (2012) Two protein lysine methyltransferases methylate outer membrane protein B from rickettsia. J. Bacteriol. 194, 6410–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dasch G. A. (1981) Isolation of species-specific protein antigens of Rickettsia typhi and Rickettsia prowazekii for immunodiagnosis and immunoprophylaxis. J. Clin. Microbiol. 14, 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinter M., Sherman N. E. (2000) Protein Sequencing and Identification using Tandem Mass Spectroscopy, pp. 154–157, Wiley-Interscience [Google Scholar]
- 30. Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., van Wijk K. J. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS ONE 3, e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou M. F., Schwartz D. (2011) Biological sequence motif discovery using motif-x. Curr. Protoc. Bioinformatics 2011, 15–24 [DOI] [PubMed] [Google Scholar]
- 32. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004) Improved prediction of signal peptides. SignalP 3.0. J. Mol. Biol. 340, 783–795 [DOI] [PubMed] [Google Scholar]
- 33. Sigrist C. J., de Castro E., Cerutti L., Cuche B. A., Hulo N., Bridge A., Bougueleret L., Xenarios I. (2013) New and continuing developments at PROSITE. Nucleic Acids Res. 41, D344–D347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Botelho-Nevers E., Raoult D. (2011) Host, pathogen and treatment-related prognostic factors in rickettsioses. Eur. J. Clin. Microbiol. Infect. Dis. 30, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 35. Georgiades K., Raoult D. (2011) Genomes of the most dangerous epidemic bacteria have a virulence repertoire characterized by fewer genes but more toxin-antitoxin modules. PLoS One 6, e17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daze K. D., Hof F. (2013) The cation-π interaction at protein-protein interaction interfaces. Developing and learning from synthetic mimics of proteins that bind methylated lysines. Acc. Chem. Res. 46, 937–945 [DOI] [PubMed] [Google Scholar]
- 37. Lu Z., Lai J., Zhang Y. (2009) Importance of charge independent effects in readout of the trimethyllysine mark by HP1 chromodomain. J. Am. Chem. Soc. 131, 14928–14931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts D. M., Rowe P. M., Siegel F. L., Lukas T. J., Watterson D. M. (1986) Trimethyllysine and protein function. Effect of methylation and mutagenesis of lysine 115 of calmodulin on NAD kinase activation. J. Biol. Chem. 261, 1491–1494 [PubMed] [Google Scholar]
- 39. Granados E. N., Bello J. (1980) Interactions of poly(Nϵ,Nϵ,Nϵ-trimethyllysine) and poly(Nδ,Nδ,Nδ-trimethylornithine) with polynucleotides. Salt dissociation and thermal denaturation. Biochemistry 19, 3227–3233 [DOI] [PubMed] [Google Scholar]
- 40. Hughes R. M., Wiggins K. R., Khorasanizadeh S., Waters M. L. (2007) Recognition of trimethyllysine by a chromodomain is not driven by the hydrophobic effect. Proc. Natl. Acad. Sci. U.S.A. 104, 11184–11188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Musselman C. A., Lalonde M.-E., Côté J., Kutateladze T. G. (2012) Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 19, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zentner G. E., Henikoff S. (2013) Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 20, 259–266 [DOI] [PubMed] [Google Scholar]
- 43. Daze K. D., Pinter T., Beshara C. S., Ibraheem A., Minaker S. A., Ma M. C., Courtemanche R. J., Campbell R. E., Hof F. (2012) Supramolecular hosts that recognize methyllysines and disrupt the interaction between a modified histone tail and its epigenetic reader protein. Chem. Sci. 3, 2695–2699 [Google Scholar]
- 44. Wagner T., Jung M. (2012) New lysine methyltransferase drug targets in cancer. Nat. Biotechnol. 30, 622–623 [DOI] [PubMed] [Google Scholar]