Abstract
The marine natural product didemnin B, currently in clinical trials as an antitumor agent, has several potent biological activities apparently mediated by distinct mechanisms. Our initial investigation of didemnin B resulted in the discovery of its GTP-dependent binding of the translation elongation factor EF1 alpha. This finding is consistent with the protein synthesis inhibitory activity of didemnin B observed at intermediate concentrations. To begin to dissect the mechanisms involved in the cytostatic and immunosuppressive activities of didemnin B, observed at low concentrations, additional didemnin-binding proteins were sought. Here we report the purification of a 36-kDa glycosylated didemnin-binding protein from bovine brain lysate. Cloning of the human cDNA encoding this protein revealed a strong sequence similarity with palmitoyl protein thioesterase (PPT), an enzyme that removes palmitate from H-Ras and the G alpha s subunits of heterotrimeric GTP-binding proteins in vitro. Mutations in PPT have recently been shown to be responsible for infantile neuronal ceroid lipofuscinosis, which is a severe brain disorder characterized by progressive loss of brain function and early death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto J. A., Newman R. A., Bignami G. S., Raybould T. J., Raber M. N., Esparza L., Walters R. S. Phase II clinical and pharmacological study of didemnin B in patients with metastatic breast cancer. Invest New Drugs. 1992 Jul;10(2):113–117. doi: 10.1007/BF00873128. [DOI] [PubMed] [Google Scholar]
- Bott R., Subramanian E., Davies D. R. Three-dimensional structure of the complex of the Rhizopus chinensis carboxyl proteinase and pepstatin at 2.5-A resolution. Biochemistry. 1982 Dec 21;21(26):6956–6962. doi: 10.1021/bi00269a052. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Camp L. A., Hofmann S. L. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993 Oct 25;268(30):22566–22574. [PubMed] [Google Scholar]
- Camp L. A., Verkruyse L. A., Afendis S. J., Slaughter C. A., Hofmann S. L. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994 Sep 16;269(37):23212–23219. [PubMed] [Google Scholar]
- Cho H., Cronan J. E., Jr "Protease I" of Escherichia coli functions as a thioesterase in vivo. J Bacteriol. 1994 Mar;176(6):1793–1795. doi: 10.1128/jb.176.6.1793-1795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H. G., Davies B., Hoth D., Suffness M., Plowman J., Flora K., Grieshaber C., Leyland-Jones B. Didemnin B. The first marine compound entering clinical trials as an antineoplastic agent. Invest New Drugs. 1986;4(3):279–284. doi: 10.1007/BF00179597. [DOI] [PubMed] [Google Scholar]
- Chung J., Grammer T. C., Lemon K. P., Kazlauskas A., Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994 Jul 7;370(6484):71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- Crampton S. L., Adams E. G., Kuentzel S. L., Li L. H., Badiner G., Bhuyan B. K. Biochemical and cellular effects of didemnins A and B. Cancer Res. 1984 May;44(5):1796–1801. [PubMed] [Google Scholar]
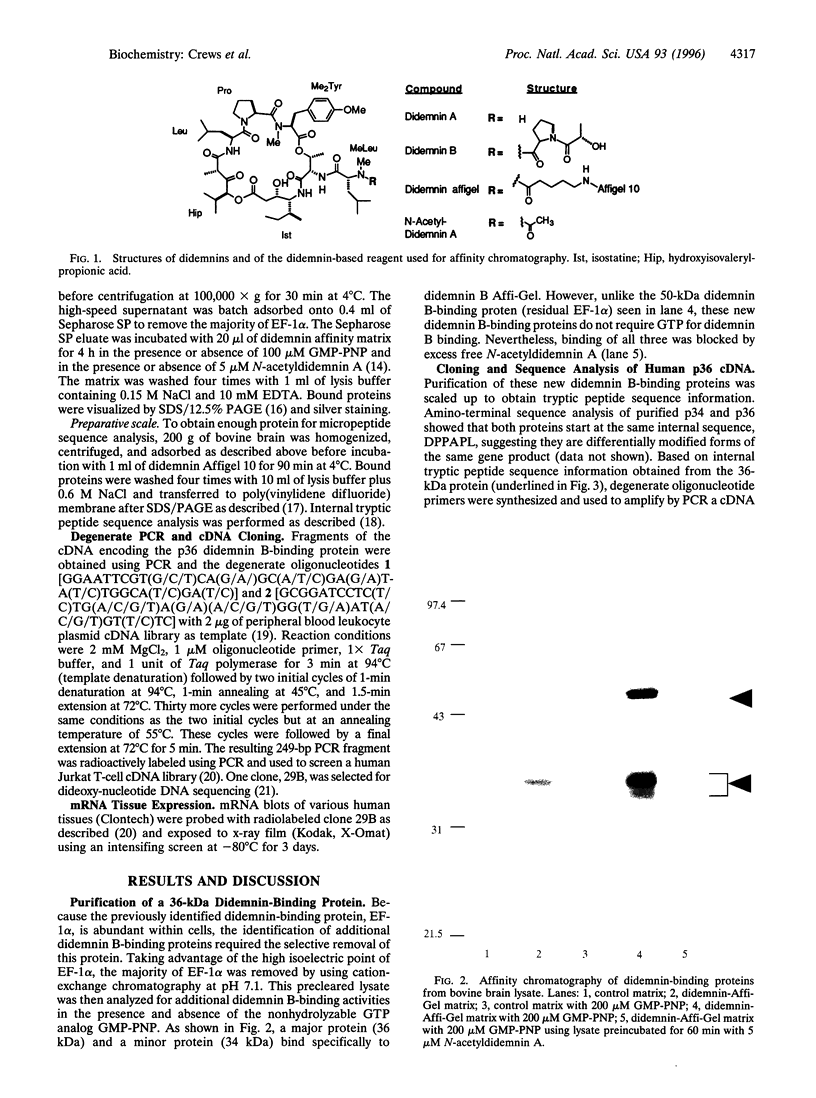
- Crews C. M., Collins J. L., Lane W. S., Snapper M. L., Schreiber S. L. GTP-dependent binding of the antiproliferative agent didemnin to elongation factor 1 alpha. J Biol Chem. 1994 Jun 3;269(22):15411–15414. [PubMed] [Google Scholar]
- Derewenda Z. S., Sharp A. M. News from the interface: the molecular structures of triacylglyceride lipases. Trends Biochem Sci. 1993 Jan;18(1):20–25. doi: 10.1016/0968-0004(93)90082-x. [DOI] [PubMed] [Google Scholar]
- Hall N. A., Thomas-Oates J. E., Dell A., Haltia M., Lake B. D., Patrick A. D. Stored dolichyl pyrophosphoryl oligosaccharides in Batten disease. Am J Med Genet. 1992 Feb 15;42(4):580–585. doi: 10.1002/ajmg.1320420431. [DOI] [PubMed] [Google Scholar]
- Heaney-Kieras J., Kieras F. J., Wisniewski K. E. Glycoprotein metabolism in neuronal ceroid lipofuscinosis fibroblasts. Biochem Med Metab Biol. 1992 Oct;48(2):137–142. doi: 10.1016/0885-4505(92)90058-7. [DOI] [PubMed] [Google Scholar]
- Ivy G. O., Kanai S., Ohta M., Sato Y., Otsubo K., Kitani K. Leupeptin causes an accumulation of lipofuscin-like substances in liver cells of young rats. Mech Ageing Dev. 1991 Mar;57(3):213–231. doi: 10.1016/0047-6374(91)90048-5. [DOI] [PubMed] [Google Scholar]
- Ivy G. O. Protease inhibitors as a model for NCL disease, with special emphasis on the infantile and adult forms. Am J Med Genet. 1992 Feb 15;42(4):555–560. doi: 10.1002/ajmg.1320420427. [DOI] [PubMed] [Google Scholar]
- Ivy G. O., Schottler F., Wenzel J., Baudry M., Lynch G. Inhibitors of lysosomal enzymes: accumulation of lipofuscin-like dense bodies in the brain. Science. 1984 Nov 23;226(4677):985–987. doi: 10.1126/science.6505679. [DOI] [PubMed] [Google Scholar]
- Jacobs A. J., Blessing J. A., Munoz A. A phase II trial of didemnin B (NSC No. 325319) in advanced and recurrent cervical carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1992 Mar;44(3):268–270. doi: 10.1016/0090-8258(92)90055-n. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A., Salituro F., Rich D. H., Hofmann T. Conformational flexibility in the active sites of aspartyl proteinases revealed by a pepstatin fragment binding to penicillopepsin. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6137–6141. doi: 10.1073/pnas.79.20.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani K., Ohta M., Kanai S., Nokubo M., Sato Y., Otsubo K., Ivy G. O. Morphological, physiological and biochemical alterations in livers of rodents induced by protease inhibitors: a comparison with old livers. Adv Exp Med Biol. 1989;266:75–92. doi: 10.1007/978-1-4899-5339-1_6. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane W. S., Galat A., Harding M. W., Schreiber S. L. Complete amino acid sequence of the FK506 and rapamycin binding protein, FKBP, isolated from calf thymus. J Protein Chem. 1991 Apr;10(2):151–160. doi: 10.1007/BF01024778. [DOI] [PubMed] [Google Scholar]
- Li L. H., Timmins L. G., Wallace T. L., Krueger W. C., Prairie M. D., Im W. B. Mechanism of action of didemnin B, a depsipeptide from the sea. Cancer Lett. 1984 Jul;23(3):279–288. doi: 10.1016/0304-3835(84)90095-8. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Malfetano J. H., Blessing J. A., Jacobs A. J. A phase II trial of Didemnin B (NSC #335319) in patients with previously treated epithelial ovarian cancer. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1993 Feb;16(1):47–49. doi: 10.1097/00000421-199302000-00012. [DOI] [PubMed] [Google Scholar]
- Marciniszyn J., Jr, Hartsuck J. A., Tang J. Mode of inhibition of acid proteases by pepstatin. J Biol Chem. 1976 Nov 25;251(22):7088–7094. [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F. Didemnin B: a new immunosuppressive cyclic peptide with potent activity in vitro and in vivo. Transplantation. 1985 Jul;40(1):49–56. [PubMed] [Google Scholar]
- Ng Ying Kin N. M., Palo J., Haltia M., Wolfe L. S. High levels of brain dolichols in neuronal ceroid-lipofuscinosis and senescence. J Neurochem. 1983 May;40(5):1465–1473. doi: 10.1111/j.1471-4159.1983.tb13592.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D. M., Holoye P. Y., Forman A., Winn R., Perez-Soler R., Dakhil S., Rosenthal J., Raber M. N., Hong W. K. Phase II clinical trial of didemnin B in previously treated small cell lung cancer. Invest New Drugs. 1994;12(3):243–249. doi: 10.1007/BF00873966. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P., Jungbjer B., Månsson J. E., Rynmark B. M., Boström K., Hagberg B., Norén L., Santavuori P. Large alterations in ganglioside and neutral glycosphingolipid patterns in brains from cases with infantile neuronal ceroid lipofuscinosis/polyunsaturated fatty acid lipidosis. J Neurochem. 1987 Dec;49(6):1772–1783. doi: 10.1111/j.1471-4159.1987.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Vesa J., Hellsten E., Verkruyse L. A., Camp L. A., Rapola J., Santavuori P., Hofmann S. L., Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995 Aug 17;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Peterson B. L., Allen S. L., Browning S. M., Duggan D. B., Schiffer C. A. A phase II trial of didemnin B in myeloma. A Cancer and Leukemia Group B (CALGB) study. Invest New Drugs. 1994;12(1):41–43. doi: 10.1007/BF00873234. [DOI] [PubMed] [Google Scholar]
- Whittaker R. G., Manthey M. K., Le Brocque D. S., Hayes P. J. A microtiter plate assay for the characterization of serine proteases by their esterase activity. Anal Biochem. 1994 Aug 1;220(2):238–243. doi: 10.1006/abio.1994.1333. [DOI] [PubMed] [Google Scholar]
- Wisniewski K. E., Kida E. Proteinase inhibitor alpha 1-antichymotrypsin has different expression in various forms of neuronal ceroid lipofuscinosis. Exp Neurol. 1990 Oct;110(1):121–126. doi: 10.1016/0014-4886(90)90056-x. [DOI] [PubMed] [Google Scholar]
- Wolfe L. S., Gauthier S., Haltia M., Palo J. Dolichol and dolichyl phosphate in the neuronal ceroid-lipofuscinoses and other diseases. Am J Med Genet Suppl. 1988;5:233–242. doi: 10.1002/ajmg.1320310626. [DOI] [PubMed] [Google Scholar]
- el-Shirbiny A. M. Prostatic specific antigen. Adv Clin Chem. 1994;31:99–133. doi: 10.1016/s0065-2423(08)60334-0. [DOI] [PubMed] [Google Scholar]