Background: Tyrosine phosphorylation of WASP is required for macrophage functions.
Results: WASP phosphorylation is dependent on the Src tyrosine kinase Hck.
Conclusion: Hck is the predominant kinase that phosphorylates WASP in cells and is required for WASP-dependent functions.
Significance: Although many tyrosine kinases can phosphorylate WASP, Hck appears to be the predominant kinase to phosphorylate WASP in macrophages in response to physiological ligands.
Keywords: Chemotaxis, Extracellular Matrix, Invasion, Nonreceptor Tyrosine Kinase, Phagocytosis, Hck, Macrophage, WASP
Abstract
We have shown previously that tyrosine phosphorylation of Wiskott-Aldrich syndrome protein (WASP) is important for diverse macrophage functions including phagocytosis, chemotaxis, podosome dynamics, and matrix degradation. However, the specific tyrosine kinase mediating WASP phosphorylation is still unclear. Here, we provide evidence that Hck, which is predominantly expressed in leukocytes, can tyrosine phosphorylate WASP and regulates WASP-mediated macrophage functions. We demonstrate that tyrosine phosphorylation of WASP in response to stimulation with CX3CL1 or via Fcγ receptor ligation were severely reduced in Hck−/− bone marrow-derived macrophages (BMMs) or in RAW/LR5 macrophages in which Hck expression was silenced using RNA-mediated interference (Hck shRNA). Consistent with reduced WASP tyrosine phosphorylation, phagocytosis, chemotaxis, and matrix degradation are reduced in Hck−/− BMMs or Hck shRNA cells. In particular, WASP phosphorylation was primarily mediated by the p61 isoform of Hck. Our studies also show that Hck and WASP are required for passage through a dense three-dimensional matrix and transendothelial migration, suggesting that tyrosine phosphorylation of WASP by Hck may play a role in tissue infiltration of macrophages. Consistent with a role for this pathway in invasion, WASP−/− BMMs do not invade into tumor spheroids with the same efficiency as WT BMMs and cells expressing phospho-deficient WASP have reduced ability to promote carcinoma cell invasion. Altogether, our results indicate that tyrosine phosphorylation of WASP by Hck is required for proper macrophage functions.
Introduction
Monocyte/macrophage migration is a key step in host defense and during pathological processes such as atherosclerosis, chronic inflammation, and cancer (1). Monocyte migration from the circulation to the injured or infected site involves transmigration through the endothelial barrier and sub-endothelial basement membrane, a process termed transendothelial migration or diapedesis (2). This is followed by migration into the interstitial tissues, a three-dimensional environment. These processes are usually tightly regulated and are required for protective immunity but when inappropriately activated can result in chronic inflammatory diseases.
In vivo, macrophages have to migrate in diverse three-dimensional environments with heterogeneous compositions and rigidity (3). Leukocytes can use an amoeboid three-dimensional migration mode, which is independent of proteases for tissue infiltration (1, 4). Recent studies indicated that macrophages use an amoeboid mode to migrate into fibrillar collagen I but use mesenchymal migration in dense matrices such as Matrigel or gelled (dense) collagen I requiring matrix degradation (4, 5). During mesenchymal migration into Matrigel or gelled collagen I, macrophages formed three-dimensional podosomes at the tip of cell protrusions, with local proteolysis of the extracellular matrix (ECM)4 (4, 6). Podosomes have been linked with the ability of cells to perform chemotaxis and invade tissues, and both Src family kinases (SFKs) and WASP are among the proteins that regulate podosome formation (7–11).
WASP is a hematopoietic cell specific protein and an actin nucleation promoting factor regulating Arp2/3-dependent actin polymerization (12). WASP activity is required for several macrophage functions, in addition to podosome formation such as efficient fibronectin degradation, phagocytosis, and chemotaxis (9, 13–15). Remarkably, all of these WASP-mediated functions also require phosphorylation of WASP on tyrosine 291 (human numbering) (9, 14, 15). However, a number of tyrosine kinases have been demonstrated to phosphorylate WASP on this important residue (16). Therefore, identification of the tyrosine kinase required for WASP tyrosine phosphorylation is important for understanding the precise signaling cascade mediating these specific macrophage functions.
Among the SFKs, Hck, which is predominantly expressed in leukocytes, has been shown to phosphorylate WASP at tyrosine 291 in COS cells and this phosphorylation resulted in enhanced actin polymerization in vitro (17, 18), suggesting Hck may be a candidate for the phosphorylation of WASP in macrophages. Interestingly, Hck activation triggers the formation of podosome rosettes (11), suggesting that WASP is downstream of Hck in the signaling pathway leading to actin polymerization in podosomes (19). Additionally, mesenchymal three-dimensional migration of macrophages in Matrigel and organization of podosome rosettes are controlled by Hck (5). Diapedesis is also dependent on SFKs and WASP activity as reported in T cells, neutrophils, monocytes, dendritic cells, and NK cells (20–24). Thus, these observations suggest that Hck might play a role in WASP tyrosine phosphorylation and for WASP-mediated monocyte diapedesis and other macrophage functions. Here, we show that WASP is required for macrophage three-dimensional migration, it is tyrosine phosphorylated by Hck, mostly by the p61Hck isoform, and this phosphorylation is required for several macrophage functions, including efficient diapedesis.
EXPERIMENTAL PROCEDURES
Mice
All procedures involving mice were conducted in accordance with National Institutes of Health regulations concerning the use and care of experimental animals. All experiments were performed according to animal protocols approved by the animal care and use committee of the Albert Einstein College of Medicine or the Institut de Pharmacologie et de Biologie Structurale. Commercially available 129/svJ control and WASp−/− mice (25) were purchased from The Jackson Laboratory (Bar Harbor, ME). C57B16/J wild-type mice were purchased from Charles River, Inc. Hck−/− mice, backcrossed onto the C57B16/J background, were characterized previously (26).
Cells, Antibodies, and Reagents
RAW/LR5 cells, derived from the murine monocyte/macrophage RAW 264.7 cell line (27), were cultured in RPMI 1640 medium (Mediatech, Inc.) supplemented with 10% heat-inactivated newborn calf serum (Sigma) and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin). Control shRNA, shWASP, and shWASP-RAW/LR5 cells expressing human wild-type (WT) or mutant forms of WASP. All of the WASP rescue cell lines expressed equivalent levels of the exogenous WASP (Fig. 3 and Ref. 9). Murine bone marrow-derived macrophages (BMMs) were isolated and prepared according to Ref. 28 and were grown in α-minimal essential medium containing 15% fetal bovine serum, 360 ng/ml recombinant human CSF-1 (Chiron, Emeryville, CA) and antibiotics. Hck−/− bones were a generous gift from Dr. Clifford Lowell (University of California, San Francisco). 3B11 mouse endothelial cells were grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. All cells were maintained at 37 °C in a 5% CO2 atmosphere. Recombinant mouse CX3CL1 was purchased from R&D Systems. Rabbit anti-Hck (SC1428), mouse anti-WASP (B9), and protein A/G plus-agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin antibody was from Sigma (clone AC-15). HRP-conjugated mouse anti-phosphotyrosine (PY20) was from BD Transduction Laboratories. Rabbit anti-sheep erythrocyte IgG was from Diamedix (Miami, FL). Secondary antibodies conjugated to HRP were from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa Fluor dyes and conjugated phalloidin and secondary antibodies were from Molecular Probes.
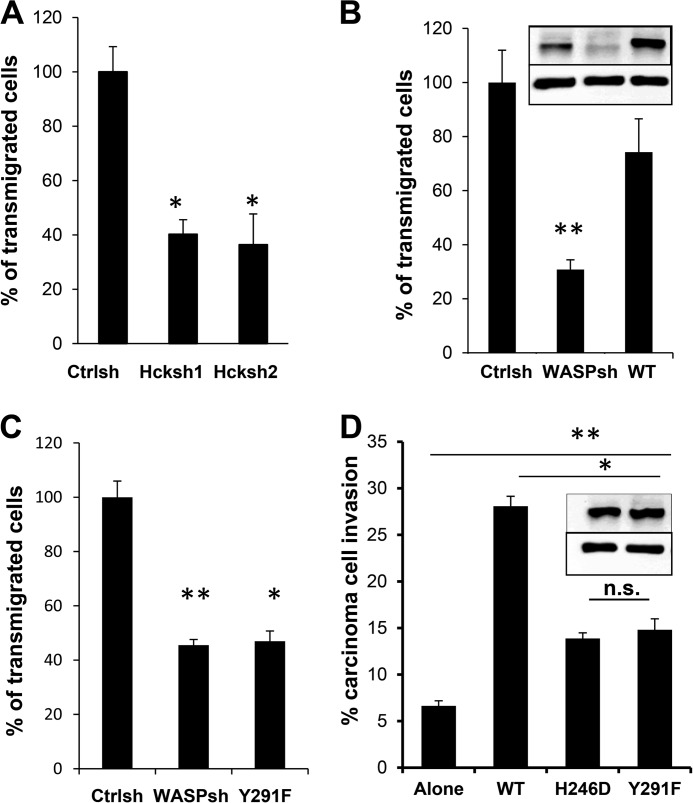
FIGURE 3.
Hck and tyrosine phosphorylation of WASP are required for diapedesis. A, RAW/LR5 cells were transfected with plasmids expressing two separate Hck shRNA targeting sequences, selected in puromycin for 48 h, and seeded on a TNFα-activated monolayer of 3B11 endothelial cells. Transmigration was allowed to proceed for 2 h, and the % of cells that completely crossed the endothelium (transmigrated) was scored. B, RAW/LR5 cells transduced with control shRNA plasmid (Ctrlsh), a plasmid targeting WASP (WASPsh), and WASP shRNA cells expressing either wild-type human WASP (WT) or WASP shRNA cells expressing a Y291F mutant form of WASP (C) were scored for their ability to transmigrate. D, quantification of the percent of MTLn3-GFP cells invading into a collagen I gel either in the absence or the presence WASP shRNA expressing wild-type (WT), inactive WASP (H246D), or phospho-deficient (Y291F) WASP (n = 3). Insets in B and D show representative Western blots of WASP expression level (above) and actin as a loading control (below) in the cells lines in the bars below. **, p < 0.001 compared with carcinoma cells alone and *, p < 0.05 compared with WASP shRNA cells expressing WT human WASP. n.s., not significant.
RNA-mediated Interference
Reduction of Hck expression was achieved through the retroviral infection of RAW/LR5 cells with short hairpin RNAs directed against Hck mRNA (sh1, 5′-GAAGAGCTCTACAATATCA-3′; sh2, 5′-AACTCGTGCTCCACTACAA-3′), using pSUPER.retro.puro plasmids according to the manufacturer's instructions (Oligoengine, Seattle, WA). Control cell lines were also generated using a nonspecific shRNA sequence (29). Plasmids were transiently transfected using FuGENE HD reagent (Roche Applied Science) according to the manufacturer's instructions and selected for 2 days in puromycin. The p61HckDN and p59HckDN constructs have been described in Carréno et al. (30).
Immunoprecipitation and Western Blotting
After the desired treatment, cells were lysed in ice-cold buffer A (25 mm Tris, 137 mm NaCl, 1% Nonidet P-40, 2 mm EDTA, 1 mm orthovanadate, 1 mm benzamidine, 10 μg/ml aprotinin, 10 μg/ml leupeptin, pH 7.4). Whole cell lysates were either used for immunoprecipitation with the indicated antibodies or mixed with 5× Laemmli sample buffer and boiled for 5 min at 100 °C. Lysates were precleared with control IgG prebound to protein A/G-agarose beads for 1 h at 4 °C followed by incubation with specific antibody prebound to beads. Specificity of the WASP antibody was confirmed as no WASP was detected in immunoprecipitates performed with isotype control IgG (data not shown). Total cell lysates and/or immunoprecipitates were resolved by SDS-PAGE, transferred onto PVDF membranes (Immobilon-P, Millipore), and after blocking, incubated with primary antibodies overnight at 4 °C followed by secondary antibodies conjugated to HRP. Signals were visualized using the SuperSignal West Pico Chemiluminescent Substrate (Pierce), and images were acquired and signal intensity was measured using a Kodak Image Station 440.
Chemotaxis was measured using 8-μm pore size inserts (Falcon; BD Biosciences) according to the manufacturer's instructions and described in Park and Cox (15). Briefly, the inserts were placed into 24-well plates containing RPMI 1640 for RAW/LR5 cells or α-MEM for BMMs in the presence or absence of CX3CL1 (50 ng/ml). Serum-starved RAW/LR5 cells (n = 500,000) or BMMs (n = 100,000) were then loaded onto the inserts and incubated at 37 °C for 4 h. Cell migration was quantified by counting the number of cells that migrated through the insert (at least 10 different randomly selected fields/well) and was compared with the number of migrated cells in the absence of any stimulant (fold increase). Data are represented as percent migration relative to the shRNA control cell or wild-type BMM population.
Fcγ receptor (FcγR)-mediated phagocytosis was performed as described (27). In brief, cells were incubated with a suspension of EIgG for 30 min at 37 °C in BWD (saturating numbers of EIgG/cell) (20 mm HEPES, 125 mm NaCl, 5 mm KCl, 5 mm dextrose, 10 mm NaHCO3, 1 mm KH2PO4, 1 mm CaCl2, and 1 mm MgCl2, pH 7.4). Non-internalized EIgG was then removed by washing, followed by hypotonic lysis. At least 50 cells were observed by phase contrast microscopy, and the number of EIgG in each cell was counted. Mean value of phagocytosis in WT BMMs or control shRNA cells was set arbitrarily to 100%.
Fibronectin matrix degradation assays were performed as described (9). Briefly, the indicated cell lines were plated, for 14 h under normal growth conditions, on glass coverslips sequentially coated with poly-l-lysine and Alexa Fluor 568-conjugated fibronectin (Sigma). Cells were then fixed in 3.7% formaldehyde in BWD, permeabilized with 0.2% Triton X-100 in BWD and stained with either Alexa Fluor 488 or Alexa Fluor 647 phalloidin as appropriate. Images acquired from a widefield microscope (×60/1.40 numerical aperture, phase 3 objective of an Olympus IX71 microscope coupled to a Sensicam cooled CCD camera), and were thresholded to delineate degraded areas. Areas of degraded matrix were then traced and measured in at least 10 different fields per experiment and expressed as degradation area per total number of cells per field. Mean value of degradation in WT BMMs or control shRNA cells was set arbitrarily to 100%.
Transmigration Assays
3B11 mouse endothelial cells were grown on glass coverslips and allowed to form a monolayer. Subsequently, they were stimulated with 50 ng/ml recombinant murine TNFα (Peprotech) for 14 h. To examine the effect of Hck in diapedesis, RAW/LR5 cells transfected with Hck-targeting sequences or a control non-targeting sequence were selected with 5 μg/ml puromycin for 48 h. Surviving cells were then used for transendothelial migration. The indicated RAW/LR5 cells were labeled with 1 mm CellTracker Green (CMFDA; Invitrogen) for 30 min followed by washing and seeding onto activated 3B11 monolayers. Cells were allowed to transmigrate for 1 or 2 h followed by fixation in 3.7% formaldehyde and staining with phalloidin and a rat antibody against ZO-1. RAW/LR5 cells were subsequently scored for their ability to transmigrate. Only those cells whose entire cell body was beneath the endothelial monolayer were considered to have transmigrated, whereas those partially through the monolayer were not included in the counting.
Carcinoma cell three-dimensional invasion assays were performed and quantified as described (31). Briefly, 80,000 MTLn3-GFP cells were plated on MatTek dishes in the presence or absence of 400,000 RAW/LR5. Cells were overlaid with 5.8 mg/ml type I collagen, incubated for 24 h prior to fixation. To quantify the MTLn3 cell invasion, confocal z-stacks were collected and GFP fluorescence in z-sections from 20 μm into the collagen and above was added up and divided by the sum of GFP fluorescence in all the z-sections. Data are reported as the percent of carcinoma cell invasion from an average of five independent fields per experiment.
Three-dimensional Migration Assay
Collagen I (Nutragen-Nutacon; 2 mg/ml final concentration) or Matrigel (BD Biosciences, batches from 8 to 12 mg/ml) were polymerized as a thick layer (1 to 1.5 mm) in the upper Transwell chambers as described (4). The lower chambers were filled with 800 μl of RPMI 1640 medium supplemented with 10% FCS and 50 ng/ml of murine recombinant CSF-1 (Immunotools). BMMs (5 × 104) were suspended in RPMI 1640 medium supplemented with 1% FCS and 10 ng/ml of mrM-CSF (Immunotools) and then seeded on the top of the matrices. The percentage of three-dimensional migration in fibrillar collagen I and in Matrigel was manually quantified after 48 h using ImageJ software.
Macrophage Infiltration Assay into Spheroids
This assay has been described previously in detail in Guiet et al. (32). Briefly, human breast tumor cells SUM159PT were cultured in three dimensions until cell spheroids reach a diameter of ∼0.5 μm. BMMs were stained with 0.5 mm CellTracker Red CMPTX (Molecular Probes, Invitrogen) and distributed (104 cells) into agar-coated wells containing a single spheroid and co-incubated for 3 days to allow BMM infiltration in spheroids. Formalin-fixed spheroids stained with DAPI were imaged using a Leica SP5 microscope (Leica Microsystems, Deerfield, IL) with a multiphoton source at 715 nm (coherent Chameleon) for z-stack acquisition of DAPI and CellTracker fluorescence (z-step, 1.2 μm). CellTracker-stained macrophages associated to spheroids were counted using the cell counter plugin of ImageJ software (National Institutes of Health, Bethesda, MD). Macrophages were classified “out of spheroids” when located in the first line of nuclei and “inside” when inside the first line of nuclei. At least three spheroids per condition were used.
Data Analysis
All results were calculated as the means ± S.E. Data were analyzed using Student's t test, and differences with a p value < 0.05 were regarded as significant. Error bars represent S.E.
RESULTS
Hck Mediates WASP Tyrosine Phosphorylation
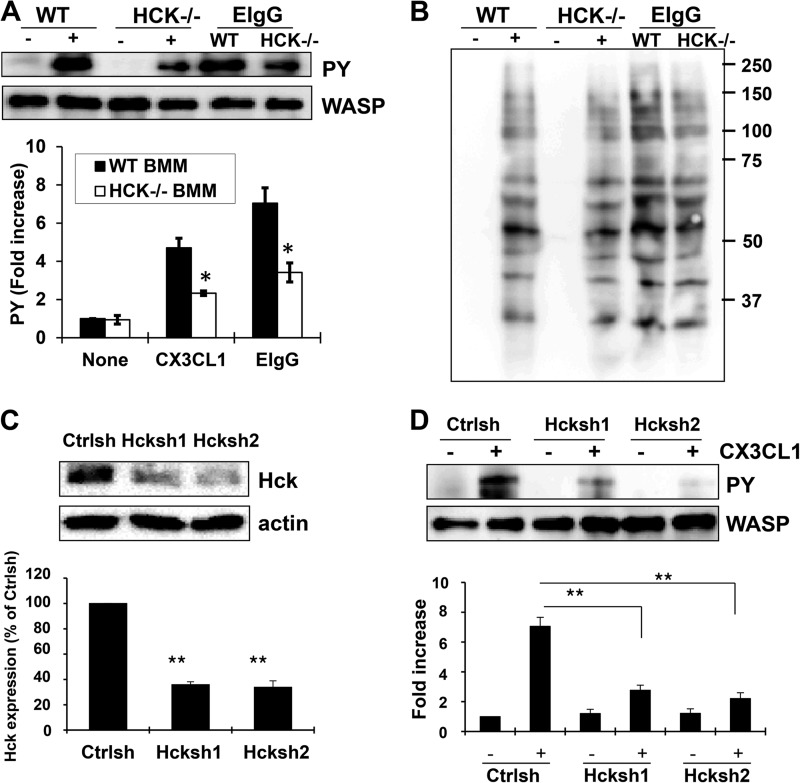
We previously showed that tyrosine phosphorylation of WASP was required for macrophage chemotaxis to CX3CL1 (15) and FcγR-mediated phagocytosis (14). Because SFKs are required for tyrosine phosphorylation of WASP (15), we speculated that Hck might be the major kinase mediating WASP tyrosine phosphorylation. For that reason, the effect of Hck deficiency on tyrosine phosphorylation of WASP was evaluated following stimulation of CX3CL1 or by ligation of the FcγR with opsonized red blood cells (EIgG). WASP was immunoprecipitated after CX3CL1 or EIgG treatment in wild-type (WT BMM) or Hck−/− bone marrow-derived macrophage (Hck−/− BMM) cells, and then the level of WASP phosphorylation was determined. WT BMM cells showed an increased in WASP phosphorylation after CX3CL1 or EIgG treatment, peaking at 1 or 5 min, respectively (Fig. 1A), consistent with results published previously (14, 15). WASP phosphorylation was significantly reduced in Hck−/− BMM cells under the same conditions (Fig. 1A). However, there was no reduction in the total level of tyrosine phosphorylation, indicating that Hck was not indirectly altering WASP phosphorylation by reducing tyrosine phosphorylation downstream of receptor signaling (Fig. 1B). Because it has been observed that there is some compensation by other SFK members following genetic deletion of one family member (26, 33), we employed a transient reduction approach using shRNA-mediated down-regulation of endogenous Hck. Retroviral delivery of Hck-specific shRNA (Hcksh1 and Hcksh2) in RAW/LR5 cells resulted in a ∼60% reduction of Hck protein expression compared with control shRNA-treated cells as determined by Western blot analysis (Fig. 1C). The ability of cells with reduced Hck expression to phosphorylate WASP in response to CX3CL1 was then evaluated and compared with control shRNA-treated cells. Despite the presence of low levels of Hck expression following shRNA treatment (∼40% Hck remaining), there was a dramatic reduction in CX3CL1-induced WASP tyrosine phosphorylation (Fig. 1D). A doublet is often observed in the phosphotyrosine immunoblot. This phenomenon is likely due to a mobility shift in WASP when it is phosphorylated, as observed in Cory et al. (17). These results suggest that although other kinases may phosphorylate WASP, Hck plays a major role in tyrosine phosphorylation of WASP in macrophages.
FIGURE 1.
Hck is required for tyrosine phosphorylation of WASP. A, wild-type and Hck−/− BMMs were untreated (− or none) or stimulated with either CX3CL1 for 1 min (+) or with EIgG for 5min. WASP was immunoprecipitated using WASP antibody, followed by Western blotting with either HRP-conjugated phospho-tyrosine (PY) and WASP antibodies. Blots were quantified by densitometry and normalized to the amount of immunoprecipitated WASP. Data are expressed as the fold increase as compared with WT prior to stimulation. ± S.E. (n = 3). *, p < 0.05. B, whole cell lysates of the same conditions were also probed with HRP conjugated phospho-tyrosine. C, RAW/LR5 cells were transfected with control shRNA (Ctrlsh) or two different Hck shRNA (Hcksh1 and Hcksh2) plasmids and selected for 2 days in puromycin. Hck and β-actin expression in these cells was analyzed by Western blotting with the respective antibodies and quantified as the percentage of Hck/β-actin signal intensity ratios relative to the control. D, control and Hck shRNA cells were stimulated with CX3CL1 for 1 min, and then WASP was immunoprecipitated using WASP antibody, followed by Western blotting with the HRP-conjugated phospho-tyrosine (PY) and WASP antibody. Data are expressed as the fold increase as compared with non-stimulated control shRNA cells. ± S.E. (n = 3), **, p < 0.01 compared with CX3CL1-induced tyrosine phosphorylation in control shRNA cells.
Hck Deficiency Results in a Defect of WASP-mediated Macrophage Functions
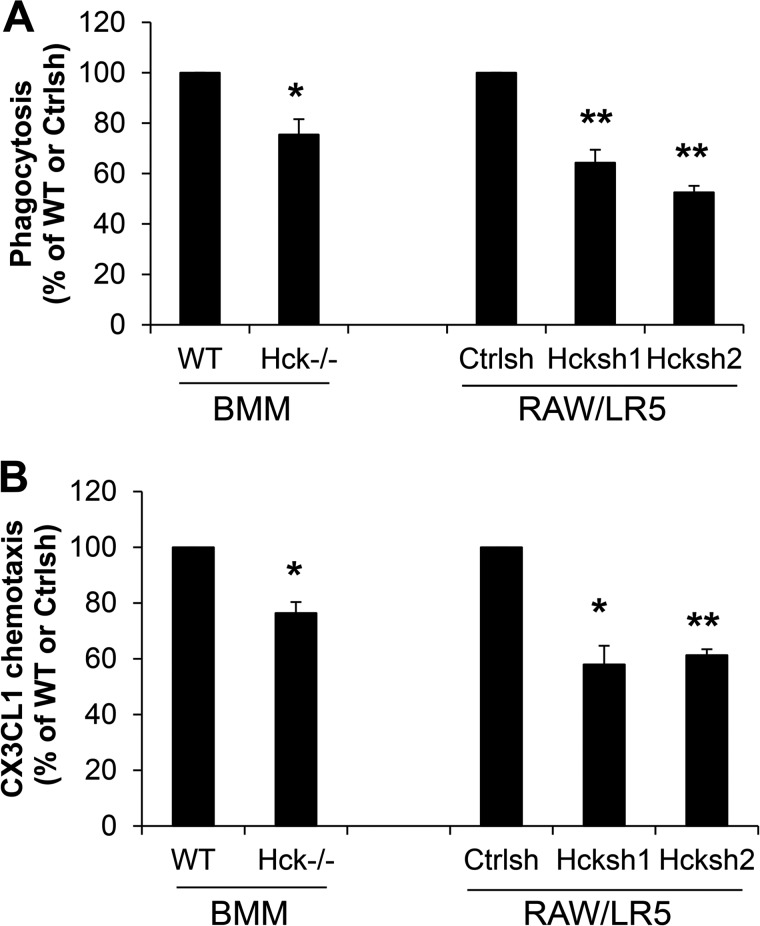
Our previous work established that macrophage functions such as phagocytosis, chemotaxis, and matrix degradation depend on tyrosine phosphorylation of WASP for optimal function (9, 14, 15). Therefore, we investigated the effect of Hck deficiency or reduced expression of Hck on macrophage functions. Initially, we determined the role of Hck in FcγR-mediated phagocytosis. Consistent with a role for Hck in WASP phosphorylation, altered Hck expression resulted in a significant inhibition of FcγR-mediated phagocytosis (Fig. 2A). It has also been shown that Hck plays a role in macrophage migration both in vivo and in vitro (5, 32, 34), although its role in macrophage chemotaxis has not been demonstrated formally. We then examined the requirement for Hck in chemotaxis and found a significant reduction in macrophage chemotaxis to CX3CL1 in both Hck−/− and Hck shRNA cells (Fig. 2B). Consistent with previous studies, which showed that although WASP was required for chemotaxis, it was not required for chemoattractant elicited F-actin-rich protrusions (15), we did not observe a decrease in F-actin-rich protrusions in Hck shRNA cells in response to CX3CL1 (p > 0.1). In the case of both assays, a greater inhibition in phagocytosis and chemotaxis was observed using transient shRNA in cell lines than in the primary BMMs. This result was consistent with the more dramatic reduction in WASP phosphorylation when the transient shRNA approach was used (Fig. 1, A and C).
FIGURE 2.
Hck regulates multiple macrophage functions. A, the ability of cells to undergo Fcγ-R mediated phagocytosis was determined as described under “Experimental Procedures.” The phagocytic index was calculated as the average number of ingested particles for 100 cells in three different experiments and expressed as a percentage of WT BMM or control shRNA (Ctrlsh) cells. *, p < 0.05 and **, p < 0.01 compared with WT BMMs or control shRNA cells. B, Hck−/− BMMs or Hck shRNA-treated cell migration in response to CX3CL1 was determined and expressed as a percentage of WT BMMs or control shRNA cells (n = 3). *, p < 0.05 and **, p < 0.01 compared with WT BMMs or control shRNA cells.
The migration of monocytes through the endothelium to sites of inflammation is a very important process during host defense against external pathogens. Given that WASP and SFKs are required for diapedesis (reviewed in Ref. 24), we hypothesized that Hck might play a role in the ability of cells to transmigrate across an endothelial monolayer. We therefore directly tested the effect of Hck deficiency in monocyte/macrophages following seeding on an activated monolayer of 3B11 endothelial cells. As shown in Fig. 3A, Hck shRNA cells showed significantly delayed transmigration at the early time points examined (1 and 2 h after seeding) compared with control cells. Because WASP can be phosphorylated by Hck (Fig. 1), we also tested the ability of WASP shRNA cells to transmigrate. Consistent with Hck shRNA cells, WASP shRNA cells showed considerably reduced diapedesis compared with Ctrlsh cells (Fig. 3B). Although rescue of transmigration occurred following expression of WT WASP (Fig. 3B), re-expression of a phospho-deficient (Y291F) WASP mutant did not increase the ability of cells to transmigrate (Fig. 3C). Because there was no statistical difference in the total number of cells counted in the assay between control and Hck shRNA or WASP shRNA and Y291F cells (p > 0.2), this indicated that reduced level of transmigration was not due to differences in adhesion.
Several studies have now shown that tumor associated macrophages promote carcinoma cell invasion both in vivo and in vitro (31, 35). We have recently demonstrated that WASP expression in macrophages was required for the ability of macrophages to stimulate carcinoma cell invasion (36). To examine whether WASP phosphorylation was required for this phenomenon shWASP cells expressing either WT or mutant forms of WASP were co-cultured with a breast tumor cell line (MTLn3-GFP) and the level of carcinoma cell invasion into a three-dimensional collagen gel was monitored. Cells expressing WT WASP were able to significantly stimulate carcinoma cell invasion (Fig. 3D). However, this invasion was significantly reduced (p = 0.01) if the carcinoma cells were co-cultured with macrophages containing an inactive form of WASP that lacked Cdc42 binding (H246D), which directly activates WASP and is a prerequisite for efficient WASP phosphorylation in macrophages (9, 37). Consistent with a major role for tyrosine phosphorylation in WASP function, co-culture with macrophages expressing phospho-deficient WASP (Y291F) also significantly reduced carcinoma cell invasion to the same extent as inactive WASP (Fig. 3D).
WASP Is Required for Migration of Macrophages in Third Dimension
The above results suggested that WASP was required for invasion in the third dimension because this assay requires the co-migration of both cell types into the three-dimensional gel (31). A previous study demonstrated that Hck−/− macrophages have a reduced ability to migrate inside high-density Matrigel (5), whereas they migrate normally when they use the amoeboid mode (32). As WASP appeared to be involved in Hck-mediated signaling, we tested the three-dimensional-migration capacity of WASP−/− BMMs in a fibrous collagen I or high density Matrigel as described in Van Goethem et al. (4). After 48 h, the number of Matrigel-infiltrated WASP−/− BMMs was significantly reduced by 50% compared with WT BMMs (Fig. 4A), whereas we observed no significant difference in three-dimensional migration in fibrous collagen I (Fig. 4B). This result indicated that WASP was a new effector of mesenchymal migration in dense matrices. Moreover, to further elaborate on the role of WASP in migration of macrophages in the third dimension, we next tested the effect of WASP deficiency on macrophage infiltration into spheroids. Tumor cell spheroids are cohesive three-dimensional organoids containing cells and several ECM proteins that mimic, to some extent, tumor tissue (32). Macrophage infiltration into such spheroids has been shown to combine mesenchymal and amoeboid migration modes (32). Here, we show that in the absence of WASP, cells have a significantly reduced ability to infiltrate into spheroids as compared with WT cells (Fig. 4, C and D).
FIGURE 4.
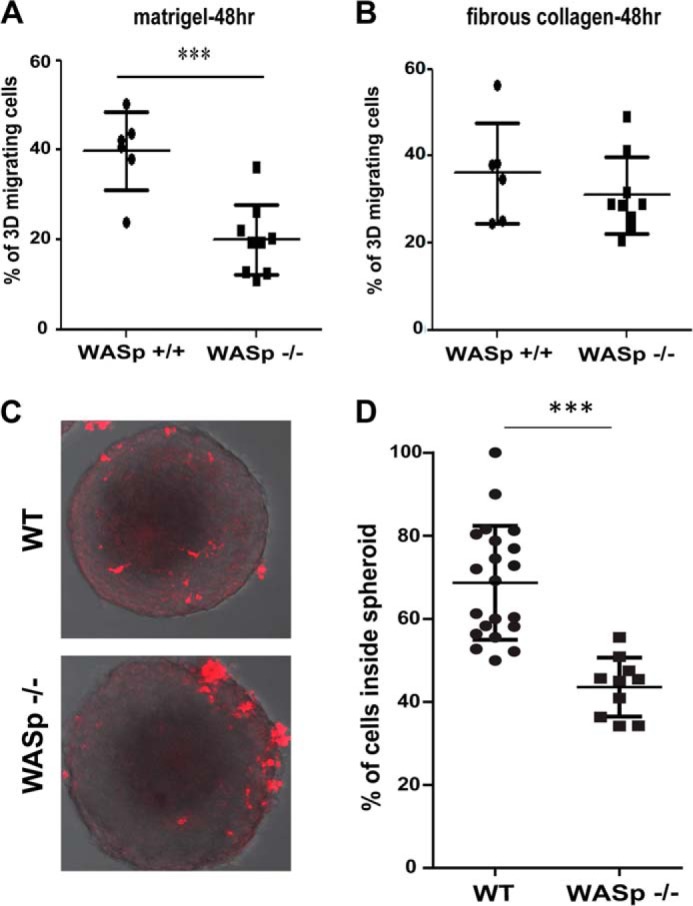
WASP is required for migration in dense ECMs and infiltration into tumor cell spheroids. BMMs from WT (WASp+/+) and WASP-deficient mice (WASp−/−) were seeded on the top of a thick layer of Matrigel (A; a poorly porous matrix) or fibrillar collagen I (B; a porous matrix), and the percentage of three-dimensional migrating cells was quantified after 48 h. Results are expressed as mean ± S.D. from n = 6 (WASp+/+) and n = 9 (WASp−/−) from three independent experiments. ***, p < 0.001. C, tumor cell spheroids were co-incubated for 3 days with CellTracker-stained BMMs, fixed, and examined with a multiphoton microscope. Spheroid cross-sections are shown. WT macrophages infiltrate the spheroid deeply, whereas WASP−/− macrophages are found outside the spheroid and infiltrated in the peripheral layer. D, quantitation of C, experiments were performed at least in triplicate and expressed as the percentage of macrophages inside spheroids (100% corresponds to macrophages inside plus macrophages adhering on the outer part of the spheroids). Results are expressed as mean ± S.D. (n = 5 WT and 3 WASP−/−; ***, p < 0.001). 3D, three-dimensional.
The p61 isoform of Hck controls WASP phosphorylation and is required for matrix degradation. The mesenchymal mode of migration requires ECM degradation mediated by podosomes (4). Matrigel is a complex mixture of ECM, including laminin and collagen IV with trace amounts of fibronectin. Previous studies have shown that Hck was required for degradation of gelatin, a form of hydrolyzed collagen (5). We examined the role of Hck in degradation of other matrix components and found that Hck−/− or Hck shRNA macrophages showed significantly reduced degradation of fibronectin (Fig. 5A), which was similar to our previous experiments showing that macrophages expressing a phospho-deficient form of WASP have impaired proteolytic activity toward fibronectin (9).
FIGURE 5.
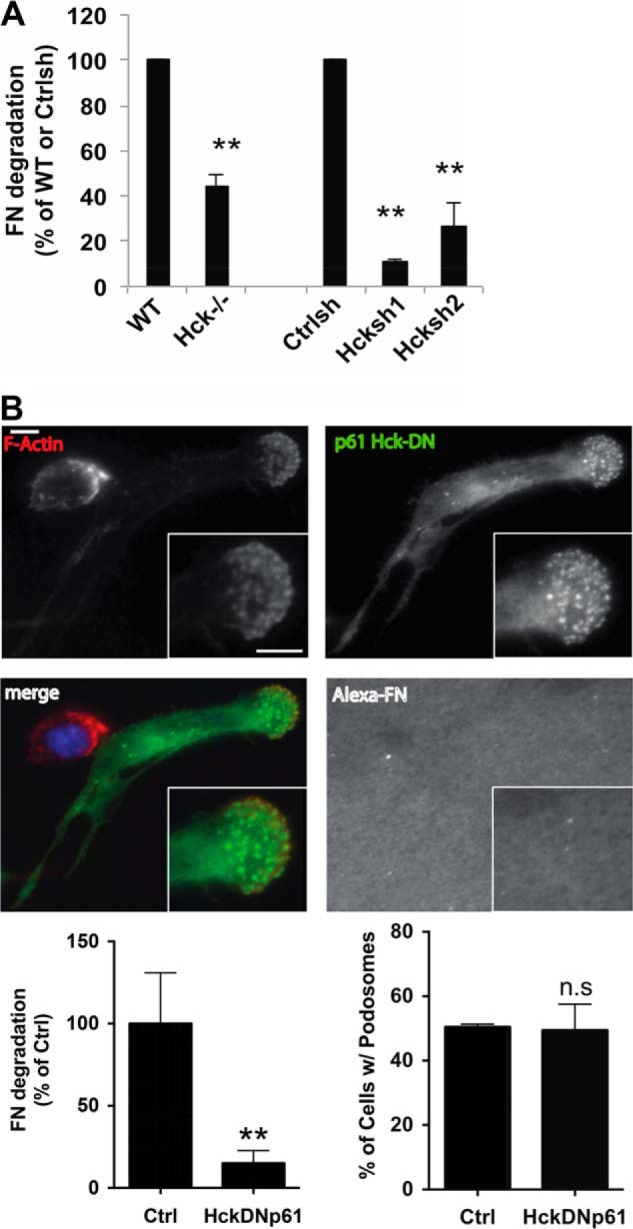
Hck, specifically the p61 isoform, regulates podosome-mediated matrix degradation. A, WT and Hck−/− BMMs or control shRNA (Ctrlsh), Hcksh1 and Hcksh2 cells were seeded on Alexa Fluor 568-conjugated fibronectin (Alexa-FN)-coated coverslips and incubated overnight. Degradation area per cell was determined and expressed as a percentage of WT BMM or control shRNA cells (n = 3). **, p < 0.01 compared with WT BMMs or control shRNA cells. B, representative image of RAW/LR5 cell expressing GFP-p61Hck DN stained with rhodamine phalloidin demonstrating the localization of p61HckDN to podosomes. The area of matrix degradation was measured and represented as percentage of the control cells shown in the lower left panel. The lower right panel shows the % of cells with podosomes. **, p < 0.01; n.s, non-significant; Ctrl, control.
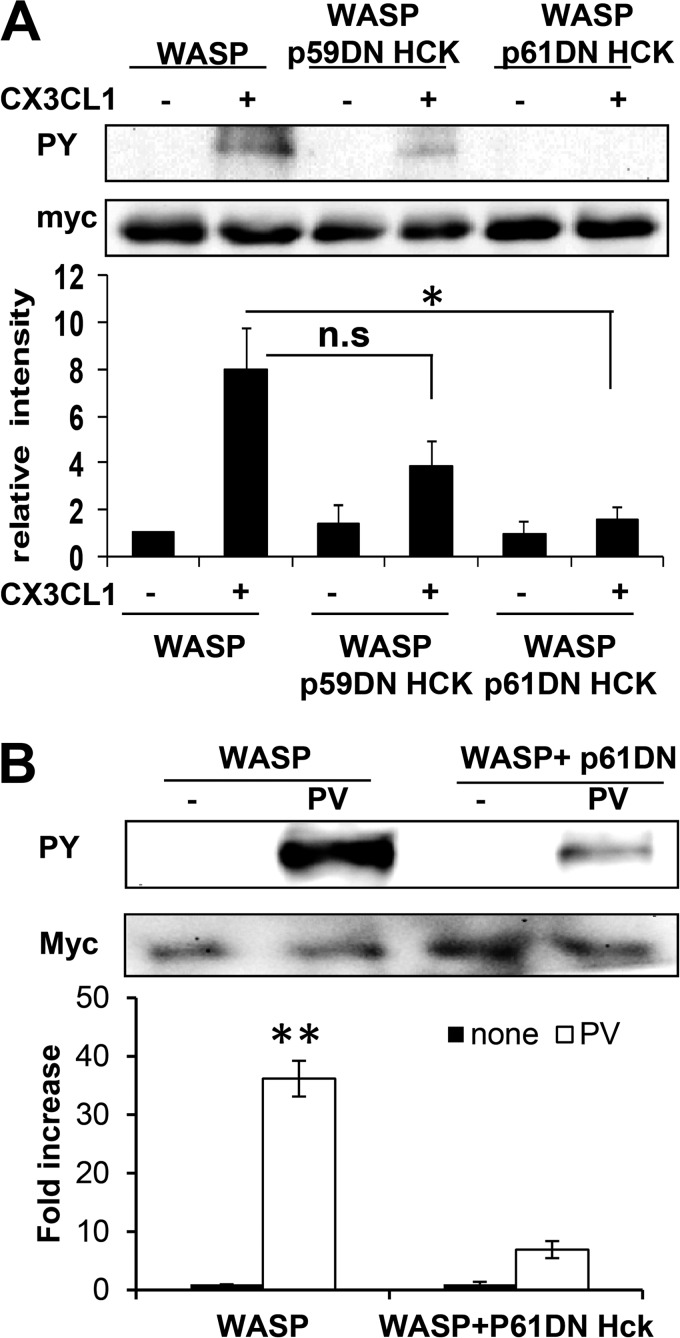
Hck is expressed as two different isoforms produced by alternative translation of the same mRNA: p59Hck and p61Hck (38). Earlier studies have demonstrated that activation of the p61 isoform of Hck triggers the formation of podosome rosettes with ECM proteolytic activity, whereas activation of p59Hck triggers the formation of cell protrusions (11, 30). To determine the role of p61Hck in WASP phosphorylation, a dominant-negative form of p61 Hck (p61DN Hck) was expressed in RAW/LR5 macrophages. As observed previously in human macrophages (11), p61DN Hck localized to podosomes in RAW/LR5 cells (Fig. 5B). Moreover, when plated overnight on Alexa Fluor-labeled fibronectin matrix, cells expressing p61DN Hck were still able to form podosomes similar to control cells. However, expression of p61DN Hck has significantly abrogated the ability of these cells to degrade the underlying matrix (Fig. 5B). Also, a dramatic reduction in WASP phosphorylation in response to CX3CL1 was observed in these cells compared with control cells (Fig. 6A). Expression of dominant-negative p59 Hck resulted in not significant inhibition of WASP phosphorylation (Fig. 6A), consistent with an important role for the p61Hck isoform. Maximal phosphorylation of WASP induced by pervanadate was also inhibited by expression of p61DN Hck (Fig. 6B). Taken together, these results suggest that the p61Hck isoform is mainly required for WASP phosphorylation upon CX3CL1 activation of macrophages and during podosome-mediated matrix degradation. Overall, our data indicates that p61Hck mediates WASP phosphorylation which is required for matrix degradation that may be regulated by functional podosome formation through WASP.
FIGURE 6.
The p61Hck isoform regulates WASP tyrosine phosphorylation. A, cells were transfected with either Myc-WASP alone or with dominant-negative form of p61Hck (p61DN) or p59Hck (p59DN) and then stimulated with CX3CL1 for 1 min, and then WASP was immunoprecipitated using a Myc antibody, followed by Western blotting with the HRP-conjugated phospho-tyrosine (PY) and Myc antibody as a control. Blots were quantified by densitometry and normalized to amount of WASP. A representative blot is shown, and data are expressed as the fold increase compared with WT prior stimulation. Shown are means ± S.E. (n = 3). B, cells were transfected with either Myc-WASP alone or with dominant-negative p61Hck (p61DN) and stimulated with pervanadate (PV) to induce maximal tyrosine phosphorylation. The graph shows the fold change in WASP phosphotyrosine (n = 3), mean ± S.E. **, p < 0.01 compared with untreated cells (none). n.s, not significant.
DISCUSSION
In leukocytes, tyrosine phosphorylation on Tyr-291 residue of WASP has been shown to play a significant role in various functions, including phagocytosis, chemotaxis, podosome dynamics, and matrix degradation. Several kinases have been proposed to mediate this tyrosine phosphorylation based on overexpression experiments of WASP with the kinase of interest (17, 39, 40), and WASP and Hck associate in chemoattractant stimulated neutrophils (18). However, the specific tyrosine kinase required to phosphorylate WASP in response to physiological ligands is still unclear. We previously showed that SFK activity was required to directly phosphorylate WASP and for chemotaxis to CX3CL1 in macrophages (15). Here, we clearly demonstrate that the SFK Hck phosphorylates WASP in macrophages in response to several stimuli and regulates WASP-mediated functions in macrophages.
Hck is predominantly expressed in leukocytes (41) and has been shown to play important roles in regulating actin-based processes such as chemotaxis and FcγR-mediated phagocytosis (42–44). Hck can phosphorylate WASP at Tyr-291 in vitro and this phosphorylation results in enhanced actin polymerization (17). Our data demonstrate that Hck phosphorylates WASP in vivo. Consistent with a role for Hck in WASP phosphorylation, reduced Hck expression resulted in a considerable inhibition of CX3CL1 chemotaxis, FcγR-mediated phagocytosis, and in the ability of cells to cross an endothelial barrier. All of these defects could be reproduced with the Y291F WASP point mutation. These defects may lead to profound alterations in the case of human diseases, including the ability of macrophages to promote carcinoma cell invasion (36) because WASP is also important for the ability of macrophages to invade in dense three-dimensional environments as well as invading into tumor spheroids (Fig. 4). Taken together, these results indicate that Hck plays a major role in tyrosine phosphorylation of WASP in macrophages and that WASP is a major regulator of several macrophage functions.
However, Hck−/− BMMs showed only minor effect in these macrophage functions consistent with only a partial reduction in the tyrosine phosphorylation of WASP. These results suggested that other kinases can phosphorylate WASP in macrophages in the absence of Hck because compensatory mechanisms have been already described in these particular knock-out mice (26, 33). WASP associates with both the Src kinase Fyn and with focal adhesion kinase Pyk-2 following the triggering of chemokine receptors on natural killer cells (23), and WASP phosphorylation following TCR ligation was reduced in Fyn-deficient Jurkat T cells (40). However, these two kinases are not likely to be the ones that phosphorylate WASP in vivo because Pyk-2 is not an SFK, and inhibitor studies have demonstrated that WASP phosphorylation is dependent on SFKs (15), and Fyn is not expressed in macrophages (45). One complication in the use of genetic knock-out mouse models is that they often do not show any obvious phenotypes due to gene compensation. Consistent with this idea, although the various hematopoietic Src kinases have been implicated in multiple functions using cell-based assays or other approaches, they do not seem to manifest as severe physiologic phenotypes in single knock-out models (26, 33, 46). Therefore, siRNA approaches may be more powerful in determining the true function of a particular SFK member.
Podosomes are adhesion structures prominent in cells of the myeloid lineage with proteolytic properties toward the ECM (47, 48). Interestingly, recent findings in diapedesis and cell migration in three-dimensional environments support a role for podosomes in cell invasion and motility (4, 20). Pharmacological inhibition of SFKs using the inhibitor PP2 resulted in loss of podosomes and inhibition of diapedesis in macrophages (11, 20). Also, Hck−/− BMMs contained undersized podosome rosettes and have a reduced migration in three-dimensional matrices compared with WT BMMs (5). Importantly, the data presented here demonstrated that Hck and WASP regulate the ability of macrophages to cross endothelial barriers and to migrate in dense three-dimensional matrices. Because WASP is also an important regulator of podosome formation, these findings strongly support a role for Hck and WASP in the regulation of matrix infiltration and motility of macrophages through podosome formation.
Hck is the only SFK which is expressed as two isoforms generated by alternative translation (41). The p59Hck isoform is associated with the plasma membrane, whereas p61Hck is associated with the membrane of lysosomes that contribute to the formation of podosome rosettes (11, 30, 49). Similar to this, we found that p61DN Hck localized to podosomes in RAW/LR5 macrophages. Interestingly, although cells expressing the p61DN Hck isoform still formed podosomes, they were unable to efficiently degrade fibronectin (Fig. 5B). This is consistent with our previous findings that WASP phosphorylation was not required for podosome formation but for podosome function (9). Moreover, expression of dominant-negative p61Hck resulted in severe inhibition of tyrosine phosphorylation of WASP in response to CX3CL1, whereas expression of dominant-negative p59Hck resulted in only slight inhibition of phosphorylation (Fig. 6A). The fact that a small fraction of p59Hck is associated with lysosomes (49, 50) could explain why the expression of the p59DN Hck mutant also has an effect on WASP phosphorylation in this context.
In conclusion, similarly to Hck, WASP plays a critical role in the three-dimensional mesenchymal migration mode of macrophages but has no effect on three-dimensional amoeboid migration. Specifically, the p61Hck isoform plays an important role in tyrosine phosphorylation of WASP induced by CX3CL1 stimulation. In our data, tyrosine phosphorylation of WASP is required for several macrophage functions, including diapedesis and extracellular matrix degradation. It also controls podosome assembly rates and actin dynamics (9). Therefore, p61Hck may affect podosome functionality via regulation of tyrosine phosphorylation of WASP.
Acknowledgments
We gratefully acknowledge Christel Verollet for providing migration and immunofluorescence experiments and critical reading of the manuscript. We thank members of the Condeelis and Segall laboratories for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant GM071828 (to D. C.) and ANR 2010-01301 MigreFlame, FRM DEQ 20110421312, and European Community's seventh framework programme (FP7–2007-2013) under Grant HEALTH-F4-2011-282095-TARKINAID.
- ECM
- extracellular matrix
- SFK
- Src family kinase
- WASP
- Wiskott-Aldrich syndrome protein
- BMM
- bone marrow-derived macrophages
- FcγR
- Fcγ receptor
- EIgG
- opsonized red blood cells.
REFERENCES
- 1. Friedl P., Weigelin B. (2008) Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969 [DOI] [PubMed] [Google Scholar]
- 2. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 3. Vérollet C., Charrière G. M., Labrousse A., Cougoule C., Le Cabec V., Maridonneau-Parini I. (2011) Extracellular proteolysis in macrophage migration: losing grip for a breakthrough. Eur. J. Immunol. 41, 2805–2813 [DOI] [PubMed] [Google Scholar]
- 4. Van Goethem E., Poincloux R., Gauffre F., Maridonneau-Parini I., Le Cabec V. (2010) Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J. Immunol. 184, 1049–1061 [DOI] [PubMed] [Google Scholar]
- 5. Cougoule C., Le Cabec V., Poincloux R., Al Saati T., Mège J. L., Tabouret G., Lowell C. A., Laviolette-Malirat N., Maridonneau-Parini I. (2010) Three-dimensional migration of macrophages requires Hck for podosome organization and extracellular matrix proteolysis. Blood 115, 1444–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Goethem E., Guiet R., Balor S., Charrière G. M., Poincloux R., Labrousse A., Maridonneau-Parini I., Le Cabec V. (2011) Macrophage podosomes go 3D. Eur J. Cell Biol. 90, 224–236 [DOI] [PubMed] [Google Scholar]
- 7. Destaing O., Sanjay A., Itzstein C., Horne W. C., Toomre D., De Camilli P., Baron R. (2008) The tyrosine kinase activity of c-Src regulates actin dynamics and organization of podosomes in osteoclasts. Mol. Biol. Cell 19, 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linder S., Nelson D., Weiss M., Aepfelbacher M. (1999) Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc. Natl. Acad. Sci. U.S.A. 96, 9648–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dovas A., Gevrey J. C., Grossi A., Park H., Abou-Kheir W., Cox D. (2009) Regulation of podosome dynamics by WASp phosphorylation: implication in matrix degradation and chemotaxis in macrophages. J. Cell Sci. 122, 3873–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cougoule C., Van Goethem E., Le Cabec V., Lafouresse F., Dupré L., Mehraj V., Mège J. L., Lastrucci C., Maridonneau-Parini I. (2012) Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur. J. Cell Biol. 91, 938–949 [DOI] [PubMed] [Google Scholar]
- 11. Cougoule C., Carréno S., Castandet J., Labrousse A., Astarie-Dequeker C., Poincloux R., Le Cabec V., Maridonneau-Parini I. (2005) Activation of the lysosome-associated p61Hck isoform triggers the biogenesis of podosomes. Traffic 6, 682–694 [DOI] [PubMed] [Google Scholar]
- 12. Takenawa T., Suetsugu S. (2007) The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 8, 37–48 [DOI] [PubMed] [Google Scholar]
- 13. Lorenzi R., Brickell P. M., Katz D. R., Kinnon C., Thrasher A. J. (2000) Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood 95, 2943–2946 [PubMed] [Google Scholar]
- 14. Park H., Cox D. (2009) Cdc42 regulates Fc gamma receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich syndrome protein (WASP) and neural-WASP. Mol. Biol. Cell 20, 4500–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park H., Cox D. (2011) Syk regulates multiple signaling pathways leading to CX3CL1 chemotaxis in macrophages. J. Biol. Chem. 286, 14762–14769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dovas A., Cox D. (2010) Regulation of WASp by phosphorylation: Activation or other functions? Commun. Integr. Biol. 3, 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cory G. O., Garg R., Cramer R., Ridley A. J. (2002) Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 277, 45115–45121 [DOI] [PubMed] [Google Scholar]
- 18. Shi Y., Dong B., Miliotis H., Liu J., Alberts A. S., Zhang J., Siminovitch K. A. (2009) Src kinase Hck association with the WASp and mDia1 cytoskeletal regulators promotes chemoattractant-induced Hck membrane targeting and activation in neutrophils. Biochem. Cell Biol. 87, 207–216 [DOI] [PubMed] [Google Scholar]
- 19. Vincent C., Maridonneau-Parini I., Le Clainche C., Gounon P., Labrousse A. (2007) Activation of p61Hck triggers WASp- and Arp2/3-dependent actin-comet tail biogenesis and accelerates lysosomes. J. Biol. Chem. 282, 19565–19574 [DOI] [PubMed] [Google Scholar]
- 20. Carman C. V., Sage P. T., Sciuto T. E., de la Fuente M. A., Geha R. S., Ochs H. D., Dvorak H. F., Dvorak A. M., Springer T. A. (2007) Transcellular diapedesis is initiated by invasive podosomes. Immunity 26, 784–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang H., Schaff U. Y., Green C. E., Chen H., Sarantos M. R., Hu Y., Wara D., Simon S. I., Lowell C. A. (2006) Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity 25, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuboi S. (2007) Requirement for a complex of Wiskott-Aldrich syndrome protein (WASP) with WASP interacting protein in podosome formation in macrophages. J. Immunol. 178, 2987–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stabile H., Carlino C., Mazza C., Giliani S., Morrone S., Notarangelo L. D., Santoni A., Gismondi A. (2010) Impaired NK-cell migration in WAS/XLT patients: role of Cdc42/WASp pathway in the control of chemokine-induced β2 integrin high-affinity state. Blood 115, 2818–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baruzzi A., Caveggion E., Berton G. (2008) Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol. Life Sci. 65, 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snapper S. B., Rosen F. S., Mizoguchi E., Cohen P., Khan W., Liu C. H., Hagemann T. L., Kwan S. P., Ferrini R., Davidson L., Bhan A. K., Alt F. W. (1998) Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9, 81–91 [DOI] [PubMed] [Google Scholar]
- 26. Lowell C. A., Soriano P., Varmus H. E. (1994) Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 8, 387–398 [DOI] [PubMed] [Google Scholar]
- 27. Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. (1997) Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186, 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanley E. R. (1997) Murine bone marrow-derived macrophages. Methods Mol. Biol. 75, 301–304 [DOI] [PubMed] [Google Scholar]
- 29. Gevrey J. C., Isaac B. M., Cox D. (2005) Syk is required for monocyte/macrophage chemotaxis to CX3CL1 (Fractalkine). J. Immunol. 175, 3737–3745 [DOI] [PubMed] [Google Scholar]
- 30. Carréno S., Caron E., Cougoule C., Emorine L. J., Maridonneau-Parini I. (2002) p59Hck isoform induces F-actin reorganization to form protrusions of the plasma membrane in a Cdc42- and Rac-dependent manner. J. Biol. Chem. 277, 21007–21016 [DOI] [PubMed] [Google Scholar]
- 31. Goswami S., Sahai E., Wyckoff J. B., Cammer M., Cox D., Pixley F. J., Stanley E. R., Segall J. E., Condeelis J. S. (2005) Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278–5283 [DOI] [PubMed] [Google Scholar]
- 32. Guiet R., Van Goethem E., Cougoule C., Balor S., Valette A., Al Saati T., Lowell C. A., Le Cabec V., Maridonneau-Parini I. (2011) The process of macrophage migration promotes matrix metalloproteinase-independent invasion by tumor cells. J. Immunol. 187, 3806–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Majeed M., Caveggion E., Lowell C. A., Berton G. (2001) Role of Src kinases and Syk in Fcγ receptor-mediated phagocytosis and phagosome-lysosome fusion. J. Leukoc. Biol. 70, 801–811 [PubMed] [Google Scholar]
- 34. Ernst M., Inglese M., Scholz G. M., Harder K. W., Clay F. J., Bozinovski S., Waring P., Darwiche R., Kay T., Sly P., Collins R., Turner D., Hibbs M. L., Anderson G. P., Dunn A. R. (2002) Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response. J. Exp. Med. 196, 589–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wyckoff J., Wang W., Lin E. Y., Wang Y., Pixley F., Stanley E. R., Graf T., Pollard J. W., Segall J., Condeelis J. (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029 [DOI] [PubMed] [Google Scholar]
- 36. Ishihara D., Dovas A., Hernandez L., Pozzuto M., Wyckoff J., Segall J. E., Condeelis J. S., Bresnick A. R., Cox D. (2013) Wiskott-Aldrich syndrome protein regulates leukocyte-dependent breast cancer metastasis. Cell Rep. 4, 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cammer M., Gevrey J. C., Lorenz M., Dovas A., Condeelis J., Cox D. (2009) The mechanism of CSF-1-induced Wiskott-Aldrich syndrome protein activation in vivo: a role for phosphatidylinositol 3-kinase and Cdc42. J. Biol. Chem. 284, 23302–23311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lock P., Ralph S., Stanley E., Boulet I., Ramsay R., Dunn A. R. (1991) Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization. Mol. Cell Biol. 11, 4363–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guinamard R., Aspenström P., Fougereau M., Chavrier P., Guillemot J. C. (1998) Tyrosine phosphorylation of the Wiskott-Aldrich syndrome protein by Lyn and Btk is regulated by CDC42. FEBS Lett. 434, 431–436 [DOI] [PubMed] [Google Scholar]
- 40. Badour K., Zhang J., Shi F., Leng Y., Collins M., Siminovitch K. A. (2004) Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guiet R., Poincloux R., Castandet J., Marois L., Labrousse A., Le Cabec V., Maridonneau-Parini I. (2008) Hematopoietic cell kinase (Hck) isoforms and phagocyte duties - from signaling and actin reorganization to migration and phagocytosis. Eur J. Cell Biol. 87, 527–542 [DOI] [PubMed] [Google Scholar]
- 42. Scholz G., Cartledge K., Dunn A. R. (2000) Hck enhances the adherence of lipopolysaccharide-stimulated macrophages via Cbl and phosphatidylinositol 3-kinase. J. Biol. Chem. 275, 14615–14623 [DOI] [PubMed] [Google Scholar]
- 43. Giagulli C., Ottoboni L., Caveggion E., Rossi B., Lowell C., Constantin G., Laudanna C., Berton G. (2006) The Src family kinases Hck and Fgr are dispensable for inside-out, chemoattractant-induced signaling regulating β 2 integrin affinity and valency in neutrophils, but are required for β2 integrin-mediated outside-in signaling involved in sustained adhesion. J. Immunol. 177, 604–611 [DOI] [PubMed] [Google Scholar]
- 44. Suzuki T., Kono H., Hirose N., Okada M., Yamamoto T., Yamamoto K., Honda Z. (2000) Differential involvement of Src family kinases in Fc gamma receptor-mediated phagocytosis. J. Immunol. 165, 473–482 [DOI] [PubMed] [Google Scholar]
- 45. Meng F., Lowell C. A. (1997) Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 185, 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vérollet C., Gallois A., Dacquin R., Lastrucci C., Pandruvada S. N., Ortega N., Poincloux R., Behar A., Cougoule C., Lowell C., Al Saati T., Jurdic P., Maridonneau-Parini I. (2013) Hck contributes to bone homeostasis by controlling the recruitment of osteoclast precursors. FASEB J. 27, 3608–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Linder S. (2007) The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 17, 107–117 [DOI] [PubMed] [Google Scholar]
- 48. Dovas A., Cox D. (2011) Signaling networks regulating leukocyte podosome dynamics and function. Cell Signal. 23, 1225–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carréno S., Gouze M. E., Schaak S., Emorine L. J., Maridonneau-Parini I. (2000) Lack of palmitoylation redirects p59Hck from the plasma membrane to p61Hck-positive lysosomes. J. Biol. Chem. 275, 36223–36229 [DOI] [PubMed] [Google Scholar]
- 50. Welch H., Maridonneau-Parini I. (1997) Hck is activated by opsonized zymosan and A23187 in distinct subcellular fractions of human granulocytes. J. Biol. Chem. 272, 102–109 [DOI] [PubMed] [Google Scholar]