Background: Maillard reaction (MR) modifies food allergens with various glycation structures during thermal processing of foods.
Results: We show that pyrraline, a glycation structure, enhances potential allergenicity of ovalbumin, a model allergen, by promoting scavenger receptor class A-mediated allergen uptake by dendritic cells.
Conclusion: Pyrraline could act as a pathogenesis-related component in food allergies.
Significance: We reveal how MR links to food allergies.
Keywords: Allergen, Carbohydrate, Dendritic Cells, Glycation, Scavenger Receptor
Abstract
The Maillard reaction (also referred to as “glycation”) takes place between reducing sugars and compounds with free amino groups during thermal processing of foods. In the final stage of the complex reaction cascade, the so-called advanced glycation end products (AGEs) are formed, including proteins with various glycation structures. It has been suggested that some AGEs could have immunostimulatory effects. Here, we aimed to identify specific glycation structure(s) that could influence the T-cell immunogenicity and potential allergenicity of food allergens, using ovalbumin (OVA, an egg white allergen) as a model allergen. OVA was specifically modified with representative glycation structures: Nϵ-carboxymethyl lysine (CM-OVA), Nϵ-carboxyethyl lysine (CE-OVA), pyrraline (Pyr-OVA), or methylglyoxal-derived arginine derivatives (MGO-OVA). As well as AGE-OVA, a crude glycation product in thermal incubation of OVA with glucose, only Pyr-OVA, and not other modified OVAs, was efficiently taken up by bone marrow-derived murine dendritic cells (BMDCs). The uptake of Pyr-OVA was reduced in scavenger receptor class A (SR-A)-deficient BMDCs, but not in cells treated with inhibitors of scavenger receptor class B, galectin-3, or blocking antibodies against CD36, suggesting that pyrraline binds to SR-A. Compared with other modified OVAs, Pyr-OVA induced higher activation of OVA-specific CD4+ T-cells in co-culture with BMDCs. Furthermore, compared with native OVA, AGE-OVA and Pyr-OVA induced higher IgE production in mice. Pyrraline could induce better allergen uptake by DCs via association with SR-A and subsequently enhance CD4+ T-cell activation and IgE production. Our findings help us to understand how Maillard reaction enhances the potential allergenicity of food allergens.
Introduction
The Maillard reaction (MR, also referred to as “glycation” or nonenzymatic glycosylation)2 is a complex chemical reaction between reducing sugars and compounds with free amino groups, such as proteins and amino acids, and takes place during the thermal processing and storage of foods. Via formation of Amadori products such as fructoselysine (“early stage”), the MR modifies lysine and arginine residues with various types of glycation structures including Nϵ-carboxymethyl lysine (CML), Nϵ-carboxyethyl lysine (CEL), pentosidine, pyrraline, and the methylglyoxal-derived hydroimidazolone MG-H1 in advanced stages of the reaction cascade (1, 2). The relatively stable products formed at late stages of the Maillard reaction are collectively called advanced glycation end products (AGEs). Because many allergenic foods such as eggs are often subjected to thermal processing before consumption, the possible involvement of AGEs in the pathology of food allergies is of great concern.
The MR reportedly alters IgE binding and mediator release capacities of some food allergens by glycations (3–7). Evidence has also accumulated to show that some glycation structures of AGEs could function as immune epitopes for dendritic cells (DCs) (8–11). DCs express several receptors known to bind AGEs, e.g., the receptor for AGEs (RAGE) (12, 13), galectin-3 (14), macrophage scavenger receptor class A type I and II (SR-A) (15), scavenger receptor class B type I (SR-B) (16), and CD36 (17). Importantly, receptors expressed on the cell surface mediate antigen uptake and maturation in DCs. It has been suggested that AGEs influence DC maturation via association with RAGE (3–5). Moreover, we previously demonstrated that AGEs of ovalbumin (OVA, a major egg allergen) produced by thermal incubation with glucose are taken up by DCs via association with SR-A and possess higher CD4+ T-cell immunogenicity than the native form of the allergen (11).
Glycation structures produced by the MR are heterogeneous. It is still not known which glycation structure(s) bind to the receptor(s) expressed on the cell surface of DCs and how this influences cellular events. In this study, we aimed to identify glycation structure(s) that enhance the CD4+ T-cell immunogenicity of food allergens. To this end, we used OVA as a model allergen and modified it with representative glycation structures, i.e., CML, Nϵ-carboxyethyl lysine (CEL), pyrraline (Pyr), or arginine derivatives such as MG-H1, to assess the T-cell immunogenicity of these modifications. We found that modification with Pyr significantly enhanced the CD4+ T-cell immunogenicity and potential allergenicity of OVA.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J (B6) mice and BALB/c mice (female, 8–10 weeks) were purchased from Charles River Laboratories International. OT-II mice expressing a transgenic T-cell receptor for I-Ab restricted OVA323–339 (18), OT-I mice expressing a transgenic CD8+ T-cell receptor for MHC class I-restricted OVA257–264, and SR-A-deficient mice on a B6 background (15) were purchased from Jackson Laboratories and bred at the animal facility of the Paul-Ehrlich-Institut. The mice were housed under specified pathogen-free conditions, and animal experiments were performed in compliance with German legislation.
Preparation of CM-, CE-, MGO-, or Pyr-OVA
Carboxymethylation, carboxyethylation, and arginine derivatization was accomplished with endotoxin-free OVA (Seikagaku Corporation, Tokyo, Japan) as described by Glorieux et al. (19). Briefly, OVA was dissolved in 0.067 mm phosphate buffer (pH 7.4). Glyoxylic acid was added to the OVA solution at a 5:1 molar ratio of glyoxylic acid and lysine residues in OVA for carboxymethylation (CM-OVA), whereas pyruvic acid was added to the protein solution at a 15:1 molar ratio of pyruvic acid to lysine residues for carboxyethylation (CE-OVA). After adjusting the solution to pH 7.4 with 0.5 n NaOH, NaBH3CN (8.8 mmol/g OVA for CM and 41 mmol/g OVA for CE) was added, and the solution was heated at 40 °C for 20 h, followed by dialysis against distilled water and lyophilization. For arginine derivatization (MGO-OVA), methylglyoxal was added to the OVA solution at a 2:1 molar ratio of methylglyoxal to lysine residues, and the solution was incubated for 25 h at 40 °C. The solution was then dialyzed against water and lyophilized. Levels of CML and CEL in the modified OVA were quantified by GC/MS after acid hydrolysis (20), whereas levels of MG-H1 were quantified by amino acid analysis (21). The presence of CML and CEL was also verified by ELISA using mAbs against glycation structures (11).
Modification of OVA with pyrraline (Pyr-OVA) was performed as described by Henle and Bachmann (22). Briefly, 3-deoxyglucosone (3-DG) and OVA were dissolved in 0.1 n sodium acetate buffer at a 4:1 ratio to lysine residues. The resulting mixture was freeze-dried and heated for 1, 2, or 4 h at 70 °C. After adjusting to room temperature, the powder was mixed with water and then lyophilized. Modification with Pyr was quantified using a reverse phase HPLC photodiode array detector after enzymatic hydrolysis (23). For quantification of the total lysine and arginine modification, the contents of the respective unmodified amino acids were determined in all OVA samples by amino acid analysis (24). For analysis of protein aggregation, modified OVAs were applied to SDS-PAGE with 4 to 20% acrylamide gradient in nonreducing conditions. Separated proteins were quantified by densitometry.
Preparation of AGE-OVA
AGE-OVA was prepared as described previously (11). Briefly, 1 mm endotoxin free OVA was incubated with 1 m glucose in 100 mm sodium phosphate buffer (pH 7.4) at 50 °C for 6 weeks. Native OVA and thermally incubated OVA without glucose under the same conditions were used as controls. The endotoxin concentration in AGE-OVA was less than 0.25 endotoxin units/pg of protein.
Analysis of the Secondary Structure of OVAs
The secondary structure of OVA samples was analyzed by CD spectroscopy (a J-810S spectropolarimeter; Jasco, Germany).
Generation of Bone Marrow-derived Murine Dendritic cells (BMDCs)
Bone marrow cells were cultured in RPMI 1640 supplemented with 10% FCS, 1 mm sodium pyruvate, 10 mm HEPES, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mm 2-mercaptoethanol, and 100 ng/ml rGM-CSF (R&D Systems) for 8 days. In the cultures, more than 80% of the cells were CD11b+ and CD11c+ cells.
Assessment of T-cell Activation and Cytokine Production
Splenic CD4+ and CD8+ T-cells were isolated from OT-II mice and OT-I mice, respectively, via an isolation kit (Miltenyi Biotec). To evaluate T-cell activation, CD4+ T-cells (8.0 × 105 cells/ml) or CD8+ T-cells (1.6 × 106 cells/ml) were co-cultured with BMDCs (1.6 × 105 cells/ml) in the presence of different forms of OVA for 24–72 h. Culture supernatants were harvested at 24 h to determine the concentration of IL-2 and at 72 h to determine the concentrations of IFN-γ and IL-17A by ELISA (eBioscience). To evaluate T-cell proliferation, CD4+ T-cells were stained with carboxyfluorescein diacetate succinimidyl ester (Invitrogen), and co-cultured with BMDCs in the presence of either form of OVA. After 96 h, carboxyfluorescein diacetate succinimidyl ester intensity of CD4+ T-cells was measured by flow cytometry, LSR II (BD Bioscience).
Assessment of the Uptake of Glycated OVA by BMDCs
OVA samples were conjugated with FITC using a FluoroTag FITC conjugation kit (Sigma-Aldrich), according to the manufacturer's instructions. BMDCs (1.0 × 106 cells/ml) were incubated for 15 min with FITC conjugates of samples. To evaluate the uptake levels, only samples with a comparable FITC/protein molar ratio were used. Following the incubation with FITC conjugates, BMDCs were stained with both phycoerythrin-conjugated anti-mouse CD11b and allophycocyanin-conjugated anti-mouse CD11c mAbs (eBioscience). The FITC intensity of CD11b+ CD11c+ cells was then analyzed by flow cytometry.
To inhibit possible uptake mediated by receptor, the following inhibitors were added to the BMDCs 30 min before the addition of FITC-conjugated samples: 3 mg/ml mannan (Sigma-Aldrich) for the MR, 150 mm lactose (Sigma-Aldrich) for galectin-3, and 10 μg/ml BLT-1 (Merck) for SR-B. To block possible uptake mediated by CD36, 100 μg/ml anti-CD36 antibodies (Abcam) or isotype control antibodies were added to the BMDCs as described above.
To verify endocytosis of OVA samples in BMDCs, cells were fixed with 4% paraformaldehyde (Thermo Scientific) after incubation with FITC conjugates for 15 min and then stained with DAPI (Invitrogen). The cells were then stained with anti-early endosome antigen 1 (EEA-1) antibodies (Merck Millipore). FITC conjugates were localized in the cells using a laser scanning microscope (LSM 510; Carl Zeiss).
Assessment of BMDC Maturation
BMDCs (1 × 106 cells/ml) were stimulated with 10 μg/ml of LPS or 50 μg/ml of either form of OVA for 18 h. BMDCs were then treated with anti-mouse CD16/CD32 mAb to block IgG receptors and stained with FITC-conjugated anti-mouse CD40, CD80, CD86, or MHC class II molecule mAb. Additionally, the cells were stained with phycoerythrin-conjugated anti-mouse CD11b and allophycocyanin-conjugated anti-mouse CD11c mAbs to gate the DC population. FITC intensity of CD11b+CD11c+ cells was measured by flow cytometry. Antibodies used for this experiment were purchased from eBioscience.
Assessment of the Potential Allergenicity of OVA Samples
BALB/c mice (female, 8–10 weeks) were intraperitoneally sensitized twice with 10 μg of native OVA, AGE-OVA, or Pyr-OVA plus 1 mg of Alum Adjuvant (Thermo Scientific) at 2-week intervals. One week after each immunization, blood was harvested to measure serum levels of OVA-specific IgE and IgG1 antibodies by ELISA (25). Additionally, 2 weeks after the final immunization, mice were challenged by feeding OVA. The body core temperature of mice on 4 days of OVA challenges was measured.
Statistical Analysis
Significant differences between mean values were assessed by analysis of variance followed by a Student's t test. A p value of < 0.05 was considered significant.
RESULTS
Modifying OVA with Glycation Structures Did Not Alter the Secondary Structure of the Allergen
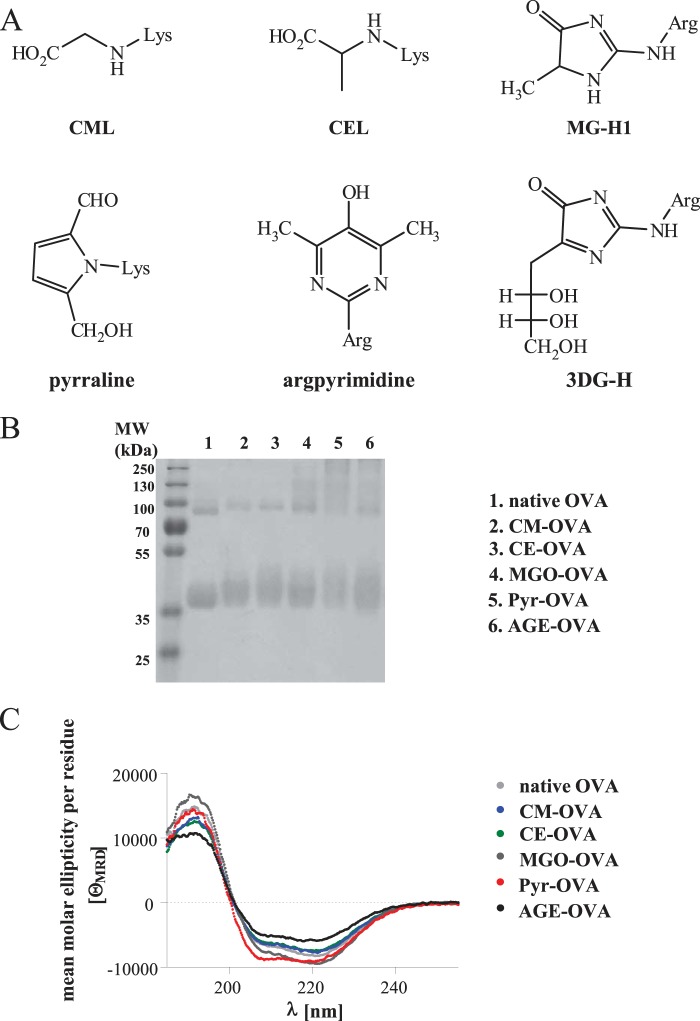
To identify glycation structures enhancing T-cell immunogenicity of a food allergen, we modified OVA to contain CML, CEL, pyrraline, or MGO-derivatives of arginine, such as MG-H1 (Fig. 1A). The modification levels of glycation structures were determined by GC-MS or reverse phase HPLC photodiode array detector after hydrolysis (Table 1). Carboxymethylation and carboxyethylation were highly selective reactions, yielding 81% lysine modification by CML and 77% by CEL, respectively. The formation of pyrraline by incubation of OVA with 3-DG was less selective, because that reactive dicarbonyl leads to various side reactions. Thus 51% of lysine in Pyr-OVA had reacted to pyrraline, in addition to 21% of lysine residues, which were modified with unknown products. Also, with this lysine modification, 25% of arginine in Pyr-OVA were modified with 3-DG-derived hydroimidazolinone (3DG-H), and 14% were modified with unknown products, respectively, although modification of lysine is preferred in the incubation with 3-DG. Derivatization of OVA with MGO, under the present conditions, exclusively modified arginine (not lysine), but was only a little selective regarding the products. Although 18% of arginine in MGO-OVA was derived by MG-H1 and 13% was derived by argpyrimidine, 40% of the arginine products remained unknown, because of diverse side reactions with the reactive dicarbonyl MGO.
FIGURE 1.
Glycation structures. A, scheme of glycation structures. B, SDS-PAGE profiles. MW, molecular weight. C, CD spectra of native OVA and CM-, CE-, MGO-, Pyr-, or AGE-OVA.
TABLE 1.
Levels of modification by glycation structures in OVA samples
| Sample | CML | CEL | MG-H1/argpyrimidine | Pyr/3DG-H | Specific modificationa |
Total modificationa |
||
|---|---|---|---|---|---|---|---|---|
| Lys | Arg | Lys | Arg | |||||
| μmol/g protein | μmol/g protein | μmol/g protein | μmol/g protein | |||||
| CM-OVA | 334.6 | 73.8 (CM) | 81.2 | NDb | ||||
| CE-OVA | 363.3 | 80.1 (CE) | 77.2 | ND | ||||
| MGO-OVA | 53.6/38.0 | 17.6 (MG-H1) | ND | 71.1 | ||||
| 12.5 (argpyrimidine) | ||||||||
| Pyr-OVA | 236.4/116.2 | 51.1 (Pyr) | 25.1 (3DG-H) | 72.4 | 39.7 | |||
a Values are percentages in all Lys or Arg of protein.
b ND, not detectable.
AGE-OVA, a crude glycation product from late stages of the MR, was prepared by incubating OVA with glucose at 50 °C for 6 weeks (11). Diffuse bands of glycated proteins detected by SDS-PAGE analysis also confirmed the modification of OVA by glycation structure (Fig. 1B). CML (28 μmol/g protein) and Pyr (0.9 μmol/g protein) were detected in AGE-OVA. The CD spectra of native OVA, modified OVAs, and AGE-OVA were similar, indicating that the modifications did not alter the secondary structure of the allergen (Fig. 1C).
Pyr-OVA Showed Higher CD4+ T-cell Immunogenicity than Native Allergen
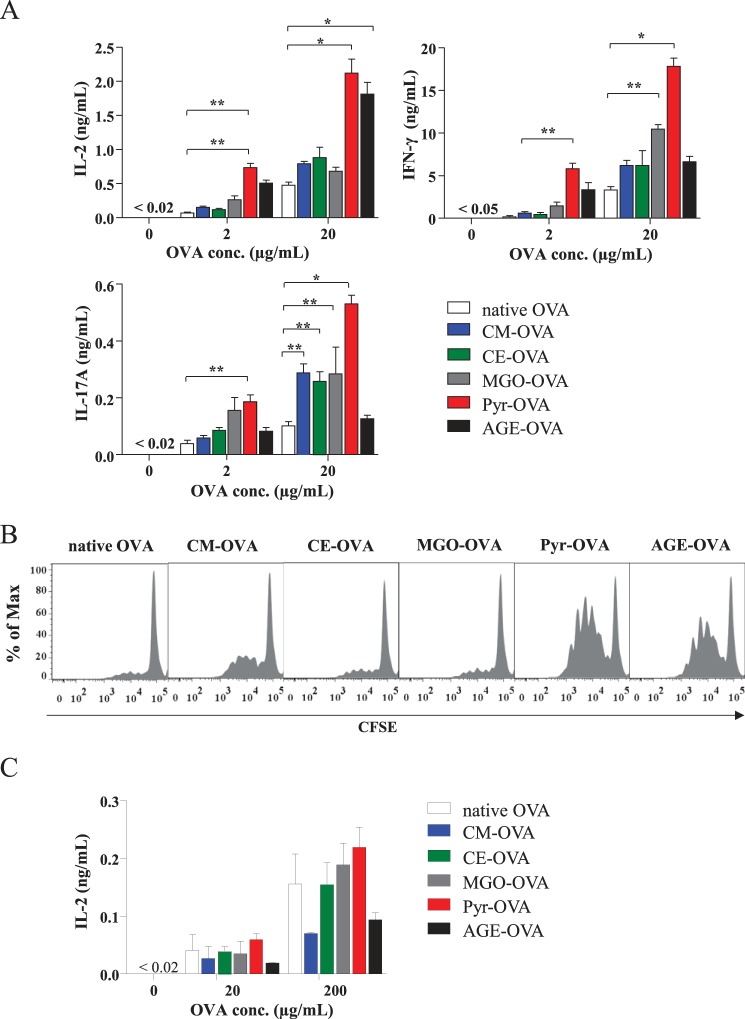
To assess the T-cell immunogenicity of glycated OVAs, OVA-specific CD4+ T-cells were isolated from OT-II mice and co-cultured with BMDCs in the presence of the OVA samples. After 24 h of co-culture, OT-II CD4+ T-cells showed increased IL-2 production in response to Pyr-OVA and AGE-OVA in comparison to native OVA and other modified OVA at 2.0 and 20 μg/ml (Fig. 2A). After 72 h of co-culture, 20 μg/ml of all modified OVAs, but especially Pyr-OVA, induced significantly higher production of IFN-γ, a Th1 cytokine, and IL-17A, a Th17 cytokine, in OT-II CD4+ T-cells, when compared with native OVA (Fig. 2A). In contrast, the same concentration of AGE-OVA did not enhance IFN-γ and IL-17A production. Viability of T-cells might be weakened at late stage of culture because of overstimulation of T-cell by AGE-OVA or toxicity of this crude glycation product. Indeed, OT-II CD4+ T-cells showed increased proliferation after 96 h of co-culture with BMDCs in the presence of Pyr-OVA and AGE-OVA at 2.0 μg/ml, a lower concentration (Fig. 2B).
FIGURE 2.
Enhanced activation of OVA-specific CD4+ T-cells by Pyr-OVA. A, CD4+ T-cells were isolated from OT-II mice, and co-cultured with BMDCs in the presence of 2.0 or 20 μg/ml of native OVA or CM-, CE-, MGO-, Pyr-, or AGE-OVA. Concentrations (conc.) of IL-2 in culture supernatants after 24 h and concentrations of IFN-γ and IL-17A in culture supernatants after 72 h were measured by ELISA. *, p < 0.001; **, p < 0.01. The data are representative of two independent experiments. B, carboxyfluorescein succinimidyl ester (CFSE)-stained CD4+ T-cells were co-cultured with BMDCs and stimulated with 2.0 μg/ml of either form of OVA. After 96 h, the carboxyfluorescein diacetate succinimidyl ester intensity of CD4+ T-cells was measured by flow cytometry. C, CD8+ T-cells were isolated from OT-I mice and co-cultured with BMDCs in the presence of 20 or 200 μg/ml of OVA samples. Concentrations of IL-2 in culture supernatants after 24 h were measured by ELISA. The data are representative of three independent experiments.
We also assessed the CD8+ T-cell immunogenicity of glycated OVA using CD8+ T-cells isolated from OT-I mice. All modified OVAs and native OVA induced similar or even not significantly different levels of IL-2 production by OT-I cells (Fig. 2C). These results suggest that Pyr-OVA has higher CD4+ T-cell immunogenicity, but similar CD8+ T-cell immunogenicity when compared with native and other glycated OVAs.
As mentioned above, Pyr-OVA contained not only pyrraline but also 3DG-H and other unknown products. Protein aggregation was also induced during formation of Pyr-OVA by incubation of OVA with 3-DG. To examine whether enhanced CD4+ T-cell activation by Pyr-OVA was due to pyrraline, OVA was incubated with 3-DG at 70 °C for shorter duration to reduce levels of unspecific modification. Pyr-OVA1 and Pyr-OVA2 prepared by 1 or 2 h of incubation reduced the levels of unspecific products (Table 2) and protein aggregation (Fig. 3A), compared with Pyr-OVA3 prepared by 4 h of incubation, which was also used in experiments for Fig. 2. However, Pyr-OVA1 and Pyr-OVA2 still induced higher CD4+ T-cell activation than native OVA (Fig. 3B). Enhanced CD4+ T-cell activation by Pyr-OVAs depended on Pyr modification levels. OVA incubated at 70 °C for 4 h without 3-DG, a control for Pyr-OVA3, induced only a basal level of CD4+ T-cell activation, as did native OVA (Fig. 3B) The results indicate that (i) pyrraline is a glycation structure to enhance CD4+ T-cell immunogenicity of OVA, and that (ii) protein aggregation would not contribute to the enhanced CD4+ T-cell activation by Pyr-OVA.
TABLE 2.
Levels of modification by glycation structures in Pyr-OVA samples
OVA and 3-DG were dissolved in 0.1 n sodium acetate buffer, freeze-dried, and heated at 70 °C. Pyr-OVA1, 2, and 3 were products of heat treatment for 1, 2, and 4 h, respectively. Levels of lysine and arginine modification in the OVA samples were analyzed.
| Sample | Pyrraline | 3DG-H | Lys with pyrralinea | Arg with 3DG-Ha | Total Lys with modificationa | Total Arg with modificationa |
|---|---|---|---|---|---|---|
| μmol/g protein | μmol/g protein | |||||
| Pyr-OVA1 | 50.7 | 7.8 | 10.9 | 1.7 | 12.6 | 0.6 |
| Pyr-OVA2 | 143.5 | 33.8 | 31.0 | 7.3 | 36.4 | 11.8 |
| Pyr-OVA3 | 236.4 | 116.2 | 51.1 | 25.1 | 72.4 | 39.7 |
a Values are percentages in all Lys or Arg of OVA.
FIGURE 3.
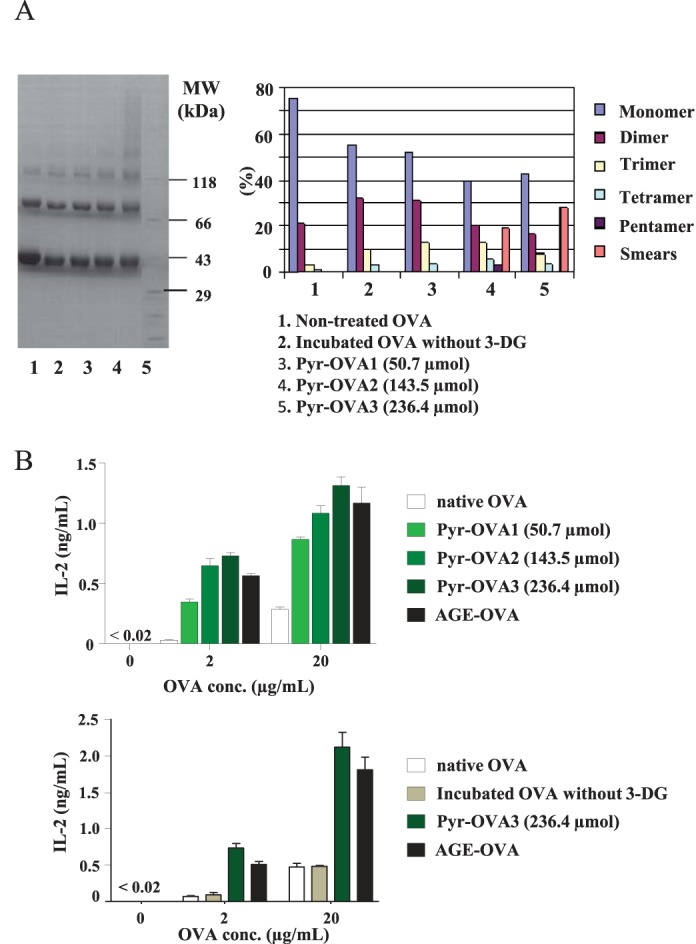
The concentration-dependent effect of Pyr on CD4+ T-cell activation. A, for analysis of protein aggregation, 5.0 μg of native OVA, OVA modified with pyrraline at different molecular ratio by incubation with 3-DG at 70 °C for 1, 2, or 4 h (see Table 2), or OVA incubated at 70 °C for 4 h without 3-DG, a control for Pyr-OVA, were applied to SDS-PAGE with 4 to 20% acrylamide gradient in nonreducing condition. Separated proteins were quantified by densitometry. MW, molecular weight. B, CD4+ T-cells isolated from OT-II mice were co-cultured with BMDCs in the presence of 2 or 20 μg/ml of OVA samples. Concentrations of IL-2 in culture supernatants after 24 h were measured by ELISA. The data are representative of two independent experiments.
Pyr-OVA Was Taken Up by DCs via Association with SR-A
DCs express several receptors that bind to AGEs such as SR-A, SR-B, CD36, and galectin-3, which mediate endocytotic uptake of ligands (26–28). To investigate whether the enhanced CD4+ T-cell immunogenicity of OVA by Pyr modification was derived from the DCs, we examined the uptake of OVA samples by BMDCs.
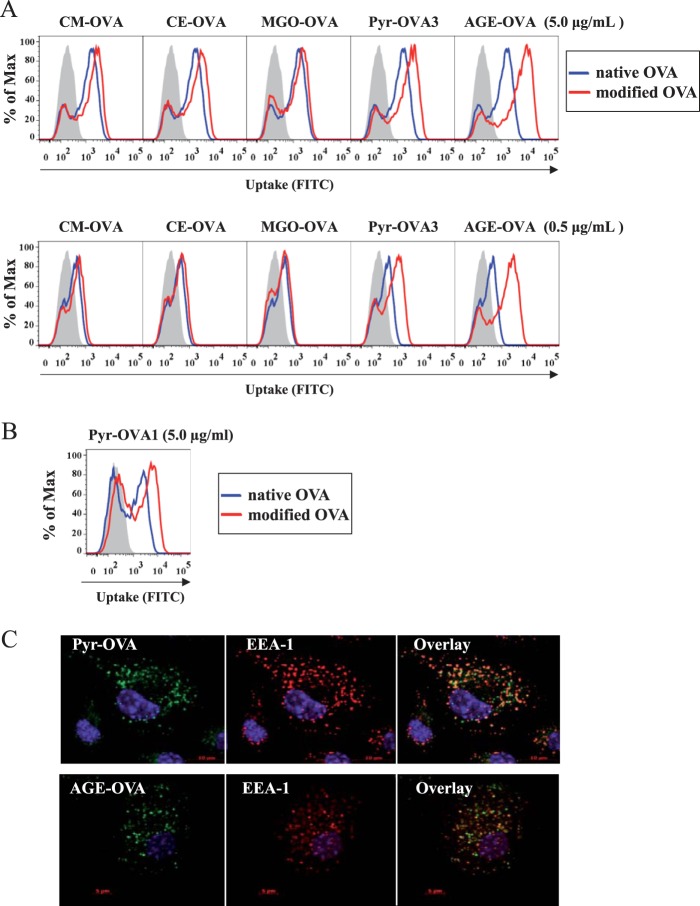
OVA samples were conjugated with FITC at similar FITC/protein concentration ratio, e.g., 0.46 in native OVA, 0.58 in CM-OVA, 0.43 in CE-OVA, 0.63 in MGO-OVA, 0.36 in Pyr-OVA3, and 0.42 in AGE-OVA. FITC intensity of BMDCs incubated with the samples, an indication of uptake by DCs, was measured by flow cytometry. Both AGE-OVA and Pyr-OVA3 were taken up more efficiently by BMDCs than native and other modified OVAs (Fig. 4A). The enhanced uptake of AGE-OVA and Pyr-OVA was more pronounced when BMDCs were incubated at a lower concentration, 0.5 μg/ml of OVA samples, compared with 5.0 μg/ml.
FIGURE 4.
Enhanced uptake of Pyr-OVA by BMDCs. A, wild-type BMDCs were incubated with 0.5 or 5.0 μg/ml of FITC-conjugated native OVA or AGE-, CM-, CE-, MGO-OVA, or Pyr-OVA3 (see Table 2) for 15 min. B, wild-type BMDCs were incubated with 5.0 μg/ml of FITC-conjugated native OVA or Pyr-OVA1 (see Table 2). DC uptake of the OVA samples was analyzed by flow cytometry. The gray areas represent cells cultured with medium only. C, after the incubation, the cells were treated with anti-EEA1 antibodies to stain early endosome compartments. DC uptake of the samples was analyzed by confocal microscopy.
Pyr-OVA1 was modified with a lower level of pyrraline and possessed more free lysine residues for FITC conjugation, compared with Pyr-OVA3. Therefore, Pyr-OVA1 could be conjugated with a higher concentration of FITC compared with Pyr-OVA3. As shown in Fig. 4B, Pyr-OVA1 with a FITC/protein concentration ratio of 0.79 was also highly taken up by DCs, compared with native OVA with a similar ratio of 0.82. The results further indicate the enhanced uptake of Pyr-OVA by BMDCs.
Confocal microscopy confirmed that the majority of AGE-OVA and Pyr-OVA was not attached on the cell surface of DCs but located within endosomal compartments expressing EEA-1, an early endosome-associated protein, inside the cells (Fig. 4C). In addition to antigen uptake, cell maturation is required for DCs to gain their full T-cell stimulatory capacity. To examine the influence of Pyr on DC maturation, the expression of co-stimulatory molecules CD40, CD80, CD86, and MHC class II molecules on the cell surface of BMDCs were analyzed after stimulation with OVA samples. LPS, a positive stimulus for DC maturation, enhanced the expression of co-stimulatory and MHC class II molecules on the cell surface, whereas none of the OVA samples changed the basal expression (Fig. 5), suggesting that Pyr modification does not induce BMDC maturation.
FIGURE 5.
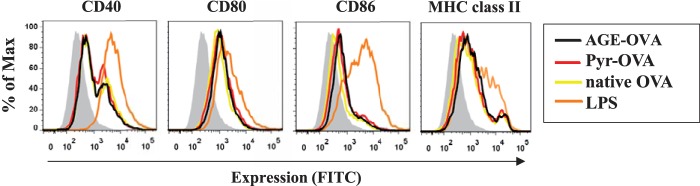
Lack of effect of Pyr-OVA and other glycated OVAs on maturation of BMDCs. BMDCs were stimulated with 50 μg/ml of native OVA, AGE-OVA, or Pyr-OVA or with 10 μg/ml of LPS for 18 h. Expression of CD40, CD80, CD86, and MHC class II molecules on the cell surface of stimulated BMDCs was analyzed by flow cytometry. The gray areas represent cells cultured with medium only. The data are representative of three independent experiments.
We also tried to identify receptor(s) involved in the uptake of Pyr-OVA using SR-A-deficient BMDCs, as well as antibodies blocking CD36 and inhibitors of SR-B or galectin-3. Moreover, we used mannan to inhibit the involvement of the mannose receptor in OVA uptake, because natural carbohydrate residues of OVA bind to the receptor (29, 30). Uptake of Pyr-OVA and AGE-OVA was considerably reduced in SR-A-deficient BMDCs (Fig. 6A). Additional treatment of SR-A-deficient BMDCs with BLT-1, an inhibitor of SR-B (31), or lactose, an inhibitor of galectin-3 (32), did not reduce uptake of the OVA samples (Fig. 6, B and C). Similarly, blocking CD36 using antibodies against the receptor did not reduce uptake of the OVA samples (Fig. 6B). In contrast, treatment of SR-A-deficient DCs with mannan substantially reduced the uptake of all OVA samples, although some uptake of Pyr-OVA and AGE-OVA was still observed (Fig. 6C). These results suggest that SR-A, in addition to the mannose receptor, plays a crucial role in the uptake of Pyr-OVA by BMDCs via association with the glycation structure, whereas the mannose receptor alone is accountable for native OVA uptake by DCs.
FIGURE 6.
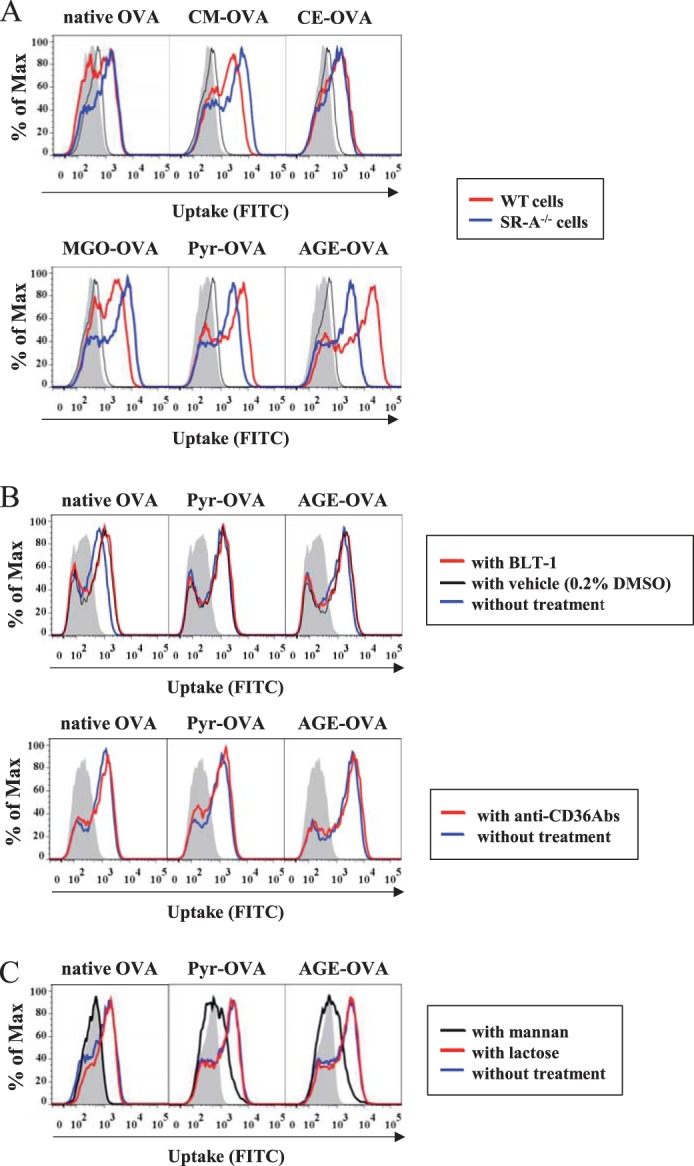
SR-A-mediated uptake of Pyr-OVA by BMDCs. A, wild-type or SR-A-deficient BMDCs were incubated with 5.0 μg/ml of FITC-conjugated native OVA or AGE-, CM-, CE-, MGO-, and Pyr-OVA for 15 min. B, before incubation with FITC-conjugated native OVA or AGE- or Pyr-OVA, SR-A-deficient BMDCs were treated with BLT-1 (top panels) or anti-CD36 blocking antibodies (bottom panels) for 30 min. C, before incubation with FITC-conjugated native OVA or AGE- or Pyr-OVA, SR-A-deficient BMDCs were treated with mannan or lactose for 30 min. DC uptake of the OVA samples was analyzed by flow cytometry. The gray areas represent cells cultured with medium only. The data are representative of three independent experiments.
Next, to examine whether the expression of SR-A in DCs is a prerequisite for Pyr-OVA enhanced CD4+ T-cell activation, we co-cultured OT-II CD4+ T-cells with SR-A-deficient or wild-type BMDCs in the presence of Pyr-OVA. OT-II CD4+ T-cells co-cultured with SR-A-deficient BMDCs produced less IL-2 in response to Pyr-OVA and AGE-OVA than those co-cultured with wild-type DCs (Fig. 7). The results suggest that SR-A expressed in DCs is involved in activating OVA-specific CD4+ T-cells by Pyr-OVA.
FIGURE 7.
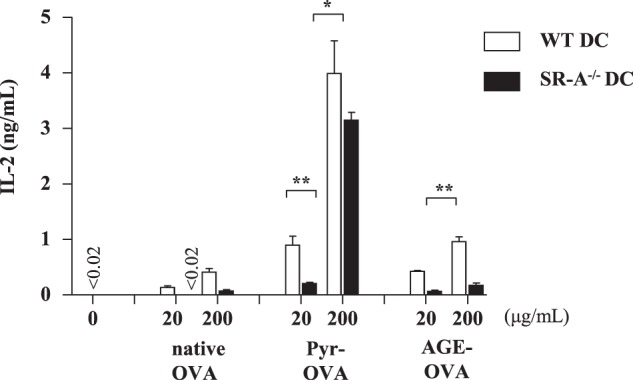
Engagement of SR-A in enhanced CD4+ T-cell activation by Pyr-OVA. CD4+ T-cells isolated from OT-II mice were co-cultured with wild-type or SR-A-deficient BMDCs in the presence of 20, or 200 μg/ml of native OVA, AGE-OVA, or Pyr-OVA for 24 h. Concentrations of IL-2 in culture supernatants were measured by ELISA. *, p < 0.001. The data are representative of two independent experiments.
Pyr-OVA and AGE-OVA Induced Higher IgE Production Than Native Allergen
Finally, we investigated the potential allergenicity of Pyr-OVA and AGE-OVA. BALB/c mice were sensitized twice with OVA samples in combination with ALUM adjuvant. After the first sensitization, OVA-specific IgG1 antibodies, a Th2 type IgG subclass, were detected by ELISA in the sera. Pyr-OVA or AGE-OVA induced higher levels of OVA-specific IgG1 antibodies than did native OVA (Fig. 8A). After the second sensitization, Pyr-OVA or AGE-OVA developed significantly higher levels of OVA-specific IgE antibodies than native OVA (Fig. 8B). OVA-specific IgG2a antibodies, a Th1 type IgG subclass, were only at marginal levels in all groups (data not shown). Moreover, upon oral challenges by feeding with a diet containing OVA, a significant reduction of body core temperature was observed in Pyr-OVA- or AGE-OVA-sensitized mice but not in mice sensitized to unmodified OVA in comparison to the PBS control (Fig. 8C). Therefore, Pyr-OVA and AGE-OVA possibly possess higher allergenicity, compared with unglycated OVA.
FIGURE 8.
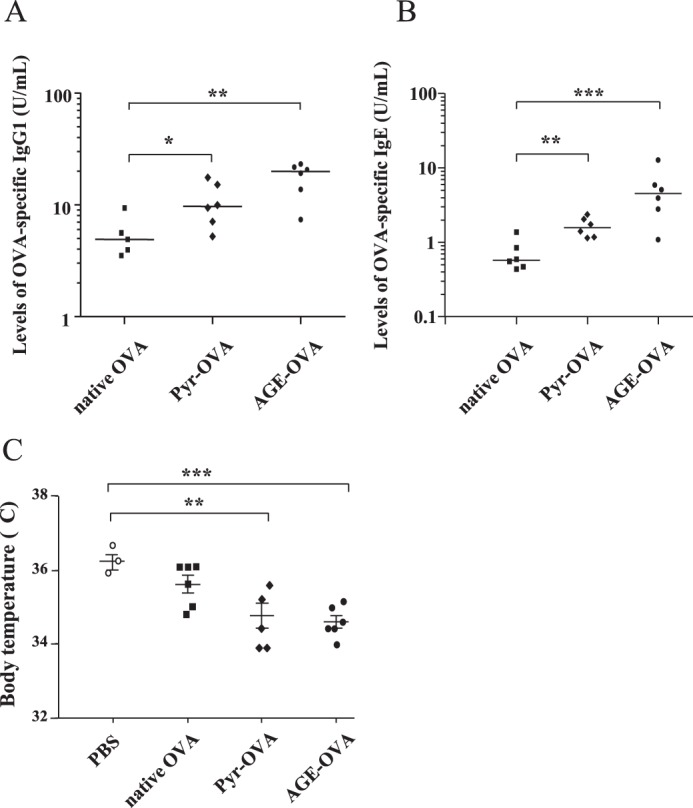
Enhanced IgE production by Pyr-OVA and AGE-OVA. BALB/c mice were sensitized with 10 μg of native OVA, Pyr-OVA, or AGE-OVA plus ALUM at a 2-week interval in total twice. One week after each sensitization, blood was harvested. A and B, serum levels of IgG1 antibodies (A) and IgE antibodies specific for OVA (B) were determined by ELISA after the first and second sensitization, respectively. C, 2 weeks after the final sensitization, mice were challenged by oral feeding with a diet containing OVA. The body temperature of mice on 4 days of OVA feeding was measured. Each symbol presents data of individual mice. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The data are representative of two independent experiments.
DISCUSSION
Our results indicate that modifying OVA containing the lysine derivative Pyr, a glycation structure produced by the advanced MR, promotes uptake of the allergen by DCs via binding to SR-A; this in turn significantly enhances OVA-specific CD4+ T-cell activation. SR-A has been suggested to transfer its ligand to the MHC class II loading pathway for efficient CD4+ T-cell activation (30, 33, 34). Consistent with this SR-A function, Pyr modification enhanced the CD4+ T-cell immunogenicity, but not the CD8+ T-cell immunogenicity of OVA. Because Th2 cells, a subset of CD4+ T-cells, play a critical role in inducing IgE production by B-cells, Pyr could potentially enhance the allergenicity of food allergens. Indeed, we found that Pyr-OVA and AGE-OVA induced significantly stronger IgE production in mice than native OVA did. The present study is the first to identify a specific glycation structure that enhances the CD4+ T-cell immunogenicity and potential allergenicity of a food allergen.
Pyr-OVA enhanced activation of OT-II cells on a C57BL/6 background, which genetically tend to develop Th1 and Th17 cells. We also observed that Pyr-OVA enhanced Th2 cytokine production by DO11.10 cells on a BALB/c background, which genetically tend to develop Th2 cells (data not shown). Neither Pyr-OVA nor AGE-OVA induced a bias in T-cell polarization. Thus enhanced IgE production by Pyr-OVA and AGE-OVA is apparently not due to enhanced Th2 cell polarization by the modification but instead to SR-A-mediated uptake by DCs and subsequent increased stimulation of the CD4+ T-cell population. In contrast, Buttari et al. (10) recently showed that AGEs of plasma β2 glycoprotein I (β2 GPI) triggered maturation of monocyte-derived human DCs and polarized allogenic naive CD4+ T-cells into Th2 cells in a co-culture with matured DCs. The different observations could be explained by variations in the glycation structures in AGEs and in the expression profiles of receptors in these DCs. AGEs of β2GPI appear to bind RAGE in human DCs, which might confer a T-cell stimulatory capacity that promotes Th2 polarization. In our experiments, Pyr-OVA and AGE-OVA did not induce cell maturation but were taken up by SR-A. This is consistent with other studies showing evidence that SR-A acts as an endocytic receptor for antigen uptake (35), whereas RAGE acts as a receptor triggering maturation of DCs (36).
SR-A is a trimer comprised of a transmembrane domain, a spacer region, a helical coiled-coil domain, a collagenous domain, and a C-terminal cysteine-rich domain (37–39). A variety of ligands such as oxidized or acetylated LDL and chemically modified proteins including AGEs bind to the collagen-like domain of SR-A (35, 39). However, information about the binding of specific glycation structure(s) to SR-A has been limited. We showed that SR-A mediates uptake by DCs of Pyr-OVA and AGE-OVA, but not CM-, CE-, and MGO-OVAs. The result indicates that Pyr, but not CML, CEL, and MGO, binds to SR-A. It is consistent with a previous study by Nagai et al. (40) showing that MGO-modified bovine serum albumin (MG-BSA in their study) was not taken up by RAW264.7, a macrophage-derived cell line expressing SR-A. Moreover, glyoxal-modified BSA, which contained high amounts of CML, was also not taken up by the cell line, supporting the hypothesis that SR-A does not bind to CML.
Together with Pyr, however, CML might contribute to the enhanced CD4+ T-cell immunogenicity of AGE-OVA. The concentration of CML (28 μmol/g protein) was higher than that of Pyr (0.9 μmol/g protein) in AGE-OVA. CM-OVA tended to induce higher DC uptake and CD4+ T-cell activation than native OVA, although the enhancement effect of CML modification was significantly lower than that of Pyr modification. Moreover, treatment of SR-A-deficient DCs with mannan, an inhibitor for binding of natural mannose residues in OVA to mannose receptor, almost completely inhibited the uptake of native OVA, whereas the uptake of AGE-OVA appeared not to be completely inhibited. These results suggest that an unknown receptor expressed on the surface of DCs might partially mediate the uptake of AGE-OVA by binding to CML. Galectin-3, CD36, and SR-B were not the receptors for CML, because treatment of DCs with the specific inhibitors lactose and BLT-1 or with blocking antibodies against CD36 did not reduce uptake of CM-OVA (data not shown). Further studies are required to identify a receptor that binds to CML.
In summary, we demonstrate that Pyr is capable of inducing SR-A-mediated allergen uptake by DCs and enhancing CD4+ T-cell immunogenicity of OVA. High amounts of Pyr have been detected in roasted peanuts, an allergenic food (41). However, information about the profile of glycation structures in thermally processed or stored allergenic foods is still limited. Identifying glycation structures in such foods should provide further insights into the potential allergenicity of food allergens.
Acknowledgments
We thank Prof. Harald Kolmar (Technische Universität Darmstadt), Dr. Max Bastian and Dr. Stefan Schülke (Paul-Ehrlich-Institut) for helpful discussion, and Dorothea Kreuz, Manja Burggraf, and Susanne Siebeneicher (Paul-Ehrlich-Institut) for technical assistance.
This work was supported by funds from the Christian Doppler Research Association and Biomay (to G. G. and P. B.).
- MR
- Maillard reaction
- AGE
- advanced glycation end product
- BMDC
- bone marrow-derived dendritic cell
- CE
- Nϵ-carboxyethyl
- CEL
- Nϵ-carboxyethyl lysine
- CM
- Nϵ-carboxymethyl
- CML
- Nϵ-carboxymethyl lysine
- DC
- dendritic cell
- 3DG-H
- 3-deoxyglucosone-derived hydroimidazolinone
- MG-H1
- methylglyoxal-derived hydroimidazolone 1
- MGO
- methylglyoxal
- OVA
- ovalbumin
- Pyr
- pyrraline
- SR-A
- scavenger receptor class A
- SR-B
- scavenger receptor class B
- RAGE
- receptor for AGE.
REFERENCES
- 1. Henle T. (2005) Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 29, 313–322 [DOI] [PubMed] [Google Scholar]
- 2. Ames J. M. (2007) Evidence against dietary advanced glycation endproducts being a risk to human health. Mol. Nutr. Food Res. 51, 1085–1090 [DOI] [PubMed] [Google Scholar]
- 3. Gruber P., Vieths S., Wangorsch A., Nerkamp J., Hofmann T. (2004) Maillard reaction and enzymatic browning affect the allergenicity of Pru av 1, the major allergen from cherry (Prunus avium). J. Agric. Food Chem. 52, 4002–4007 [DOI] [PubMed] [Google Scholar]
- 4. Vissers Y. M., Iwan M., Adel-Patient K., Stahl Skov P., Rigby N. M., Johnson P. E., Mandrup Müller P., Przybylski-Nicaise L., Schaap M., Ruinemans-Koerts J., Jansen A. P., Mills E. N., Savelkoul H. F., Wichers H. J. (2011) Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6. The necessity of degranulation assays. Clin. Exp. Allergy 41, 1631–1642 [DOI] [PubMed] [Google Scholar]
- 5. Jiménez-Saiz R., Belloque J., Molina E., López-Fandiño R. (2011) Human immunoglobulin E (IgE) binding to heated and glycated ovalbumin and ovomucoid before and after in vitro digestion. J. Agric. Food Chem. 59, 10044–10051 [DOI] [PubMed] [Google Scholar]
- 6. Taheri-Kafrani A., Gaudin J. C., Rabesona H., Nioi C., Agarwal D., Drouet M., Chobert J. M., Bordbar A. K., Haertle T. (2009) Effects of heating and glycation of β-lactoglobulin on its recognition by IgE of sera from cow milk allergy patients. J. Agric. Food Chem. 57, 4974–4982 [DOI] [PubMed] [Google Scholar]
- 7. Iwan M., Vissers Y. M., Fiedorowicz E., Kostyra H., Kostyra E., Savelkoul H. F., Wichers H. J. (2011) Impact of Maillard reaction on immunoreactivity and allergenicity of the hazelnut allergen Cor a 11. J. Agric. Food Chem. 59, 7163–7171 [DOI] [PubMed] [Google Scholar]
- 8. Ge J., Jia Q., Liang C., Luo Y., Huang D., Sun A., Wang K., Zou Y., Chen H. (2005) Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells. Arterioscler. Thromb. Vasc. Biol. 25, 2157–2163 [DOI] [PubMed] [Google Scholar]
- 9. Price C. L., Sharp P. S., North M. E., Rainbow S. J., Knight S. C. (2004) Advanced glycation end products modulate the maturation and function of peripheral blood dendritic cells. Diabetes 53, 1452–1458 [DOI] [PubMed] [Google Scholar]
- 10. Buttari B., Profumo E., Capozzi A., Facchiano F., Saso L., Sorice M., Riganò R. (2011) Advanced glycation end products of human β2 glycoprotein I modulate the maturation and function of DCs. Blood 117, 6152–6161 [DOI] [PubMed] [Google Scholar]
- 11. Ilchmann A., Burgdorf S., Scheurer S., Waibler Z., Nagai R., Wellner A., Yamamoto Y., Yamamoto H., Henle T., Kurts C., Kalinke U., Vieths S., Toda M. (2010) Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity. Role of macrophage scavenger receptor class A type I and II. J. Allergy Clin. Immunol. 125, 175–183 [DOI] [PubMed] [Google Scholar]
- 12. Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. (1992) Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 267, 14987–14997 [PubMed] [Google Scholar]
- 13. Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. (1992) Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 267, 14998–15004 [PubMed] [Google Scholar]
- 14. Vlassara H., Li Y. M., Imani F., Wojciechowicz D., Yang Z., Liu F. T., Cerami A. (1995) Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE). A new member of the AGE-receptor complex. Mol. Med. 1, 634–646 [PMC free article] [PubMed] [Google Scholar]
- 15. Suzuki H., Kurihara Y., Takeya M., Kamada N., Kataoka M., Jishage K., Ueda O., Sakaguchi H., Higashi T., Suzuki T., Takashima Y., Kawabe Y., Cynshi O., Wada Y., Honda M., Kurihara H., Aburatani H., Doi T., Matsumoto A., Azuma S., Noda T., Toyoda Y., Itakura H., Yazaki Y., Kodama T. (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 [DOI] [PubMed] [Google Scholar]
- 16. Ohgami N., Nagai R., Miyazaki A., Ikemoto M., Arai H., Horiuchi S., Nakayama H. (2001) Scavenger receptor class B type I-mediated reverse cholesterol transport is inhibited by advanced glycation end products. J. Biol. Chem. 276, 13348–13355 [DOI] [PubMed] [Google Scholar]
- 17. Ohgami N., Nagai R., Ikemoto M., Arai H., Kuniyasu A., Horiuchi S., Nakayama H. (2001) Cd36, a member of the class b scavenger receptor family, as a receptor for advanced glycation end products. J. Biol. Chem. 276, 3195–3202 [DOI] [PubMed] [Google Scholar]
- 18. Turner S. J., Jameson S. C., Carbone F. R. (1997) Functional mapping of the orientation for TCR recognition of an H2-Kb-restricted ovalbumin peptide suggests that the beta-chain subunit can dominate the determination of peptide side chain specificity. J. Immunol. 159, 2312–2317 [PubMed] [Google Scholar]
- 19. Glorieux G., Helling R., Henle T., Brunet P., Deppisch R., Lameire N., Vanholder R. (2004) In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int. 66, 1873–1880 [DOI] [PubMed] [Google Scholar]
- 20. Wellner A., Huettl C., Henle T. (2011) Formation of Maillard reaction products during heat treatment of carrots. J. Agric. Food Chem. 59, 7992–7998 [DOI] [PubMed] [Google Scholar]
- 21. Henle T., Walter A., Haessner R., Klostermeyer H. (1994) Detection and identification of a protein-bound imidazolone resulting from the reaction of arginine residues and methylglyoxal. Z. Lebensm. Unters. Forsch. 199, 55–58 [Google Scholar]
- 22. Henle T., Bachmann A. (1996) Synthesis of pyrraline reference material. Z. Lebensm. Unters. Forsch. 202, 72–74 [DOI] [PubMed] [Google Scholar]
- 23. Förster A., Kühne Y., Henle T. (2005) Studies on absorption and elimination of dietary maillard reaction products. Ann. N.Y. Acad. Sci. 1043, 474–481 [DOI] [PubMed] [Google Scholar]
- 24. Henle T., Walter H., Krause I., Klostermeyer H. (1991) Efficient determination of individual maillard compounds in heat-treated milk products by amino acid analysis. Int. Dairy J. 1, 125–135 [Google Scholar]
- 25. Burggraf M., Nakajima-Adachi H., Hachimura S., Ilchmann A., Pemberton A. D., Kiyono H., Vieths S., Toda M. (2011) Oral tolerance induction does not resolve gastrointestinal inflammation in a mouse model of food allergy. Mol. Nutr. Food Res. 55, 1475–1483 [DOI] [PubMed] [Google Scholar]
- 26. Plüddemann A., Neyen C., Gordon S. (2007) Macrophage scavenger receptors and host-derived ligands. Methods 43, 207–217 [DOI] [PubMed] [Google Scholar]
- 27. Araki N., Higashi T., Mori T., Shibayama R., Kawabe Y., Kodama T., Takahashi K., Shichiri M., Horiuchi S. (1995) Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur. J. Biochem. 230, 408–415 [DOI] [PubMed] [Google Scholar]
- 28. Lepur A., Carlsson M. C., Novak R., Dumić J., Nilsson U. J., Leffler H. (2012) Galectin-3 endocytosis by carbohydrate independent and dependent pathways in different macrophage like cell types. Biochim. Biophys. Acta 1820, 804–818 [DOI] [PubMed] [Google Scholar]
- 29. Huntington J. A., Stein P. E. (2001) Structure and properties of ovalbumin. J. Chromatogr. B Biomed. Sci. Appl. 756, 189–198 [DOI] [PubMed] [Google Scholar]
- 30. Burgdorf S., Kautz A., Böhnert V., Knolle P. A., Kurts C. (2007) Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 [DOI] [PubMed] [Google Scholar]
- 31. Nieland T. J., Penman M., Dori L., Krieger M., Kirchhausen T. (2002) Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. U.S.A. 99, 15422–15427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherayil B. J., Weiner S. J., Pillai S. (1989) The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 170, 1959–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abraham R., Singh N., Mukhopadhyay A., Basu S. K., Bal V., Rath S. (1995) Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J. Immunol. 154, 1–8 [PubMed] [Google Scholar]
- 34. Nicoletti A., Caligiuri G., Törnberg I., Kodama T., Stemme S., Hansson G. K. (1999) The macrophage scavenger receptor type A directs modified proteins to antigen presentation. Eur. J. Immunol. 29, 512–521 [DOI] [PubMed] [Google Scholar]
- 35. Gordon S. (2002) Pattern recognition receptors. Doubling up for the innate immune response. Cell 111, 927–930 [DOI] [PubMed] [Google Scholar]
- 36. Nogueira-Machado J. A., Volpe C. M., Veloso C. A., Chaves M. M. (2011) HMGB1, TLR and RAGE. A functional tripod that leads to diabetic inflammation. Expert Opin. Ther. Targets 15, 1023–1035 [DOI] [PubMed] [Google Scholar]
- 37. Doi T., Higashino K., Kurihara Y., Wada Y., Miyazaki T., Nakamura H., Uesugi S., Imanishi T., Kawabe Y., Itakura H. (1993) Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J. Biol. Chem. 268, 2126–2133 [PubMed] [Google Scholar]
- 38. Andersson L., Freeman M. W. (1998) Functional changes in scavenger receptor binding conformation are induced by charge mutants spanning the entire collagen domain. J. Biol. Chem. 273, 19592–19601 [DOI] [PubMed] [Google Scholar]
- 39. Acton S., Resnick D., Freeman M., Ekkel Y., Ashkenas J., Krieger M. (1993) The collagenous domains of macrophage scavenger receptors and complement component C1q mediate their similar, but not identical, binding specificities for polyanionic ligands. J. Biol. Chem. 268, 3530–3537 [PubMed] [Google Scholar]
- 40. Nagai R., Matsumoto K., Ling X., Suzuki H., Araki T., Horiuchi S. (2000) Glycolaldehyde, a reactive intermediate for advanced glycation end products, plays an important role in the generation of an active ligand for the macrophage scavenger receptor. Diabetes 49, 1714–1723 [DOI] [PubMed] [Google Scholar]
- 41. Wellner A., Nusspickel L., Henle T. (2012) Glycation compounds in peanuts. Eur. Food Res. Technol. 234, 423–429 [Google Scholar]