Background: Mannose receptor family members are candidate mediators of intracellular collagen degradation.
Results: Despite common candidate collagen-binding domains and endocytic capacity throughout the family, only uPARAP/Endo180 and MR internalize collagens.
Conclusion: A multi-domain interplay in the active receptors governs collagen endocytosis.
Significance: Identification of the principal collagen receptors allows elucidation of the biological importance of intracellular collagen degradation.
Keywords: Collagen, Endocytosis, Extracellular Matrix, Protein Chimeras, Receptor Structure-Function, Intracellular Collagen Degradation, Mannose Receptor, Cancer Invasion, Mannose Receptor Family, uPARAP/Endo180
Abstract
Members of the well-conserved mannose receptor (MR) protein family have been functionally implicated in diverse biological and pathological processes. Importantly, a proposed common function is the internalization of collagen for intracellular degradation occurring during bone development, cancer invasion, and fibrosis protection. This functional relationship is suggested by a common endocytic capability and a candidate collagen-binding domain. Here we conducted a comparative investigation of each member's ability to facilitate intracellular collagen degradation. As expected, the family members uPARAP/Endo180 and MR bound collagens in a purified system and internalized collagens for degradation in cellular settings. In contrast, the remaining family members, PLA2R and DEC-205, showed no collagen binding activity and were unable to mediate collagen internalization. To pinpoint the structural elements discriminating collagen from non-collagen receptors, we constructed a series of receptor chimeras and loss- and gain-of-function mutants. Using this approach we identified a critical collagen binding loop in the suggested collagen binding region (an FN-II domain) in uPARAP/Endo180 and MR, which was different in PLA2R or DEC-205. However, we also found that an active FN-II domain was not a sufficient determinant to allow collagen internalization through these receptors. Nevertheless, this ability could be acquired by the transfer of a larger segment of uPARAP/Endo180 (the Cys-rich domain, the FN-II domain and two CTLDs) to DEC-205. These data underscore the importance of the FN-II domain in uPARAP/Endo180 and MR-mediated collagen internalization but at the same time uncover a critical interplay with flanking domains.
Introduction
The complex collagen structures of the extracellular matrix (ECM)2 undergo constant remodeling during development and homeostasis of adult tissues. In pathological conditions, the balance between collagen synthesis and turnover can be disrupted and this can result in excessive degradation or accumulation of collagen structures with severe consequences for the affected tissue(s). Osteoporosis, arthritis, fibrosis, and cancer represent such diseases (1, 2). Elucidating mechanisms of collagen remodeling is therefore of great importance to fully understand and ultimately stop the progression of these devastating diseases.
Two general pathways have been described for the turnover of collagen: an extracellular pathway, governed by certain secreted or membrane-bound proteases, including Cathepsin K and members of the matrix metalloproteinase (MMP) family, and an intracellular pathway, where partially fragmented collagen components are transported to lysosomal compartments for proteolytic degradation (3–6). Different cell surface receptors have been suggested to facilitate internalization of collagens, including certain β1 integrins with phagocytic functions (7) and, importantly, the family of endocytic receptors designated the mannose receptor (MR) family (for review see Ref. 8).
Studies on one member of the MR family, the urokinase plasminogen activator receptor-associated protein (uPARAP, Endo180, MRC2, CD280, denoted uPARAP from here on) (9–12), revealed the pivotal role of the intracellular collagen degradation pathway in biological and pathological processes. uPARAP has thus far been shown to be important in bone development (13–15), protection against fibrosis in the liver, lungs, and kidneys (16–18), and to promote growth and tissue destruction in cancer in mice (19, 20). uPARAP has also been shown to be up-regulated in a range of human cancers (20–24), and recently, a genome-wide association study suggested a link between the MRC2 gene encoding uPARAP and temporomandibular joint osteoarthritis (25).
The MR family, in addition to uPARAP, consists of MR (MRC1, CD206), the phospholipase A2 receptor (PLA2R, PLA2R1), and DEC-205 (Ly75, CD205). All are constitutively recycling endocytic type-1 transmembrane proteins (26–32) and they contain a highly conserved domain composition, including a cysteine-rich (Cys-rich) domain, a FN-II domain, and a number of C-type lectin-like domains (CTLD), of which most, however, possess no carbohydrate binding activity.
The four members are all candidate collagen receptors, as suggested due to a common putative collagen-binding region centered around the FN-II domain (8, 10, 30, 33–36). FN-II domains are well-characterized mediators of collagen binding in proteins such as fibronectin and MMP-2 and -9 (37–39) and several studies have demonstrated that this domain is essential for collagen binding and internalization in not only uPARAP but also MR (36, 40–43). A third receptor, PLA2R, has in addition been suggested to utilize the FN-II domain to mediate cellular adhesion to collagen (44).
Despite the expected functional redundancy in the MR family (8, 30, 33, 35, 36), no studies have experimentally investigated a function of PLA2R and DEC-205 in collagen degradation. Furthermore, the detailed mechanism of collagen interaction in the MR protein family remains elusive (41, 43, 45, 46). Therefore, to address the considerable gaps in the knowledge of MR family function and to dissect the mechanism of collagen interaction within the protein family, we initiated a comparative study of the four receptors with respect to collagen turnover and identified critical structural elements required for collagen binding and internalization.
EXPERIMENTAL PROCEDURES
Reagents
The following reagents were purchased from commercial sources: native, acid-extracted trypsin resistant collagen type I from rat tail (BD Biosciences, Franklin Lakes, NJ), native collagen type IV from human placenta, lysosomal cysteine protease inhibitor E64d, and protease inhibitor cocktail III (CalBioChem, Merck Biosciences, Darmstadt, Germany), Porcine Pancreatic PLA2 and mannose (Sigma), 125I (Perkin Elmer Life Sciences, Waltham, MO), Iodogen iodination reagent (Thermo Fisher Scientific, Waltham, MA), Mannose-BSA (Dextra Laboratories, Reading, UK), rabbit mAb against C-terminal DEC-205 (Epitomics, Burlingame, CA), Rat mAb against N-terminal DEC-205 (clone 205yekta, eBiosciences, San Diego, CA), Rabbit pAb against PLA2R (Novus Biologicals, Littleton, CO), Rat mAb against MR (Clone MR5D3, Abd Serotec, Oxford, UK), HRP-coupled rabbit-anti-rat pAb, HRP-coupled rabbit-anti-mouse pAb, and HRP-coupled Swine-anti-Rabbit pAb (Dako, Glostrup, Denmark), pcDNA5/FRT/TO and pcDNA5/FRT/TO/CAT expression vectors, Oregon-Green gelatin, Wheat germ agglutinin (WGA)-Alexa647, Hoechst 33342 NucBlue® Live ReadyProbesTM Reagent, prolong gold antifade mounting medium, and Lipofectamine2000 (Invitrogen, Grand Island, NY), USER (Uracil-Specific Excision Reagent) Enzyme, HindIII, SbfI, XhoI, and XmaI restriction enzymes (New England Biolabs, Ipswich, MA), skimmed milk powder (Isis, Aarhus, Denmark). PfuX7 polymerase was a kind gift from Dr. Anders Koefoed Holm at the Technical University of Denmark. The following cDNA clones were purchased from commercial sources (all cDNAs correspond to murine receptor DNA): MR (clone reference BC141338, Accession no. NM_008625), uPARAP (clone reference BC116642, Accession no. NM_008626), and DEC-205 (clone reference BC150734, Accession no. NM_013825) (Imagenes GmbH, Berlin, Germany), PLA2R synthetic cDNA (Accession no. NM_008867), synthetic uPARAP N-terminal sequence with FN-II domain (Gly179-Cys227) sequence replaced by equivalent from either MR (Gly161–Cys209), PLA2R (Gly174–Cys222), or DEC-205 (Gly162–Cys209) (Genscript, Piscataway, NJ), and synthetic DEC-205 N-terminal cDNA with FN-II domain sequence (Gly162–Cys209) replaced by equivalent from uPARAP (Gly179–Cys227) (BlueHeron, Bothell, WA). MR protein family FN-II domain borders were predicted with the EMBL Smart protein online tool, and the amino acid region that represents the core of the second FN-II domain found in murine MMP-2 (Gly284–Cys332) was used as a reference sequence for comparison.
Expression and Purification of Recombinant Proteins from Insect Cells
For each receptor, expression vectors were constructed to encode the native murine signal peptide followed by the first three N-terminal domains (Cys-Rich, FN-II, and CTLD-1) and a common C-terminal tag for affinity purification (Fig. 1A). These vectors encoding uPARAP (Met1–Val377), MR (Met1–Gly361), PLA2R (Met1–His379), and DEC-205 (Met1–Glu349) were designed for expression in an insect cell-based expression system (Drosophila S2 cells, Invitrogen) as follows: PCR was performed with specific cDNA templates and 5′- and 3′-primers specific for the terminal sequences of the DNA encoding the first three N-terminal domains from each receptor (amino acids included are specified above). XbaI- and SpeI- specific recognition sequences were included in the 5′- and 3′-primer overhangs, respectively. The resulting PCR fragment for each receptor was inserted in the insect cell pMTC-X expression plasmid in frame with the suPAR-DIII protein epitope tag (47) using XbaI/SpeI cloning. Correct fragment insertion was confirmed by DNA sequencing. Transfection of insect cells with expression vectors, large scale insect cell culturing for production, and affinity chromatography for purification of recombinant receptor constructs using mAb R2 directed against the suPAR-DIII protein tag was performed as previously described (47).
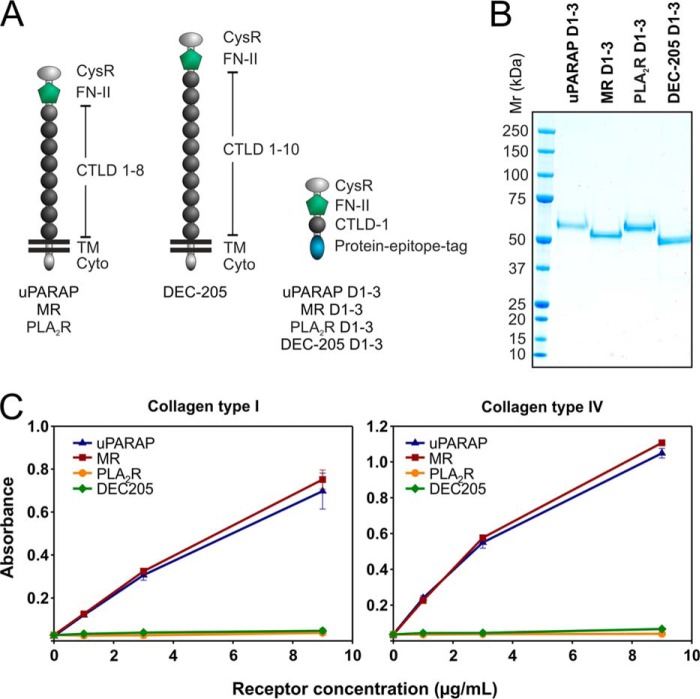
FIGURE 1.
Recombinant uPARAP and MR, but not PLA2R and DEC-205, bind collagens type I and IV. A, overview of domains in the full-length receptors belonging to the MR protein family and in soluble recombinant constructs. uPARAP, MR, PLA2R, and DEC-205 consist of a large ectodomain (including a Cys-rich domain, an FN-II domain, and 8–10 CTLDs), a transmembrane (TM) region, and a short cytoplasmic domain (Cyto). Soluble recombinant proteins (uPARAP D1–3, MR D1–3, PLA2R D1–3, DEC-205 D1–3) consist of the Cys-Rich domain, the FN-II domain, and the first CTLD fused to a protein-epitope tag. B, SDS-PAGE analysis and Coomassie Brilliant Blue staining of recombinant proteins (1.5 μg samples). The theoretical molecular masses are 53.3, 51.5, 54.6, and 50.5 kDa for uPARAP D1–3, MR D1–3, PLA2R D1–3, and DEC-205 D1–3, respectively. C, ELISA analysis of the binding of soluble recombinant protein (0, 1, 3, and 9 μg/ml) to immobilized, heat-denatured collagen type I (5 μg/ml, left panel) and IV (5 μg/ml, right panel). Recombinant protein binding was detected with an anti-tag mAb and a secondary HRP-coupled antibody. Error bars represent S.D. of duplicate samples.
Collagen Binding Assay
Binding of recombinant MR-family proteins to immobilized collagen types I and IV was analyzed in an ELISA-based setup as previously described (42). Collagens were immobilized using a concentration of 5 μg/ml. Recombinant receptors were tested in concentration series from 0–9 μg/ml.
Generation of MR Family Member and Chimera Expression Plasmids
cDNAs encoding murine uPARAP, MR, PLA2R, and DEC-205 were cloned into expression vector pcDNA5/FRT/TO by USER cloning using PfuX7 polymerase PCR (48) and deoxyuridine containing primers specific for uPARAP, MR, PLA2R, or DEC-205 cDNA template. USER enzyme mix was used according to the manufacturer's instructions (New England Biolabs). Generated expression plasmid constructs were confirmed by sequencing. uPARAP-MR-FN-II, uPARAP-PLA2R-FN-II, and uPARAP-DEC-205-FN-II chimera DNAs (Fig. 6A) were generated by SbfI/HindIII cloning of synthetic cDNAs (see above) into pcDNA5/FRT/TO/uPARAP expression plasmid. DEC-205-uPARAP-FN-II chimera (Fig. 7A) DNA was generated by XhoI/XmaI cloning of synthetic DNA (see above) into pcDNA5/FRT/TO/DEC-205 expression plasmid. DEC-205-uPARAP-D1–4 chimera (See Fig. 7A) was generated by USER cloning as described above by mixing PfuX7 polymerase PCR generated uPARAP D1–4 (Met1–Lys504) DNA fragment with pcDNA5/FRT/TO/DEC-205 (DEC-205 Lys486–Asp1723) DNA fragment in the USER enzyme reaction.
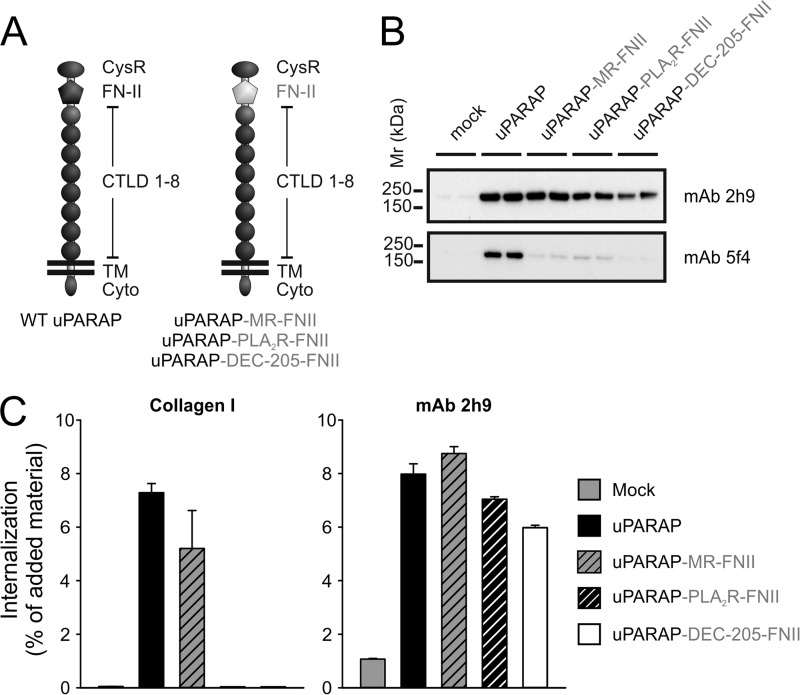
FIGURE 6.
Loss of collagen internalizing function in uPARAP chimeras with PLA2R and DEC-205 FN-II domains. A, schematic overview of domain-swaps in uPARAP chimeras (uPARAP-MR-FN-II, uPARAP-PLA2R-FN-II, uPARAP-DEC-205-FN-II). Inserted domains from MR, PLA2R, and DEC-205 (gray) are highlighted to distinguish these from remaining uPARAP domains (black). B, Western blot analysis of uPARAP chimera expression in HEK-293T cells. Anti-uPARAP mAb 2h9 or 5f4 were used as primary antibodies for detection. Note that the epitope of mAb 5f4 is situated in the FN-II domain of uPARAP whereas mAb 2h9 with an epitope outside this domain detects all uPARAP chimeras. Experimental conditions were as described in Fig. 2. C, internalization of radiolabeled collagen type I (left panel) or control ligand (mAb 2h9, right panel) by HEK-293T cells transfected with uPARAP chimeras. Cells were incubated with radiolabeled ligands (100 ng/ml) in the presence of E64d (20 μm) for 4 h after which the fraction of intracellular ligand was determined. Data are presented as radioactive ligand in intracellular fraction in percent of total radioactive ligand added to cells. Error bars represent S.D. of triplicate samples.
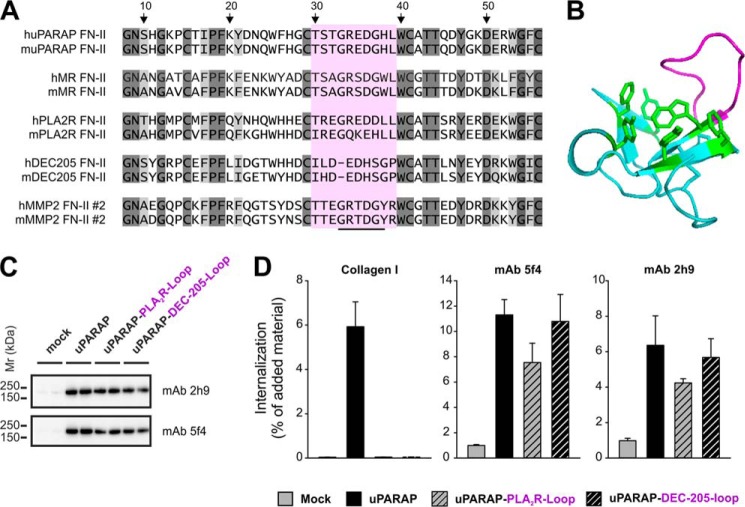
FIGURE 7.
Loss of collagen internalizing function in uPARAP FN-II mutants. A, list of sequences representing the second FN-II domain from human and mouse MMP-2 and the single FN-II domain from human and mouse uPARAP, MR, PLA2R, and DEC-205. Dark gray: amino acids completely retained in all sequences. Light gray: amino acid positions where only conservative changes have occurred. The stretch of amino acids (Gly33–Tyr38) suggested to be involved in collagen binding is underlined in the MMP-2 sequence. The sequence marked by purple (Thr30–Arg39) constitutes a loop protruding from the core of the second FN-II domain from MMP-2. The amino acid numbering refers to the position in the second FN-II domain sequence from MMP-2 following published FN-II domain annotation (38). B, crystal structure of the ligand free form of the second FN-II domain from MMP-2 (PDB ID: 1CK7)(53). The protruding loop including Gly33–Tyr38 is marked by purple. The aromatic side chains of residues forming a hydrophobic groove, also suggested to be important in ligand binding, are marked by green. C, Western blot analysis of uPARAP loop mutants (uPARAP-PLA2R-Loop and uPARAP-DEC-205-Loop) expression in HEK-293T cells. Anti-uPARAP mAb 2h9 or 5f4 were used as primary antibodies for detection. Experimental conditions were as described in Fig. 2. D, internalization of radiolabeled collagen type I (100 ng/ml, left panel) or control ligands (100 ng/ml mAb 5f4 or 2h9, center and right panel, respectively) by HEK-293T cells transfected with uPARAP loop mutants. Experimental conditions and data presentation were as described for Fig. 6.
Transfection of HEK-293T and HeLa Cells
To transfect HEK-293T cells (Thermo Fisher Scientific, Waltham, MA), the cells were seeded in 12- or 6-well plates and grown overnight in DMEM with 10% FCS. Transfection medium was prepared by carefully mixing a ratio of 2 μg of expression plasmid DNA to 3 μg of Lipofectamine 2000 in 200 μl of DMEM, incubating for 20 min at room temperature and then adding 1300 μl of DMEM with 10% FCS. Transfection medium was added to cells in place of seeding medium, and cells were incubated for 24 h before harvest for Western blotting analysis or before performing endocytosis assay (see below). Mock transfection was performed with the pcDNA5/FRT/TO/CAT plasmid. For transfection of HeLa cells (CCL-2, ATCC, Manassas, VA) for fluorescent ligand endocytosis assays, coverslips were coated with rat tail collagen type I (20 μg/ml in 20 mm acetic acid) (BD Biosciences) for 2 h at room temperature followed by 3 washes with PBS. HeLa cells were then seeded overnight on the collagen-coated coverslips. Transfection was performed as described for HEK-293T cells.
Western Blotting
Transfected HEK-293T cells were homogenized in 50 μl of lysis buffer (1% Triton X-100, Tris-HCl 10 mm, 150 mm NaCl, pH 7.4, 0.5% Protease Inhibitor cocktail III (CalBioChem)) per 600,000 transfected cells. Equal amounts of cell lysates were analyzed by Western blotting under non-reducing conditions using the following primary antibodies: mouse anti-uPARAP mAb 2h9 (0,5 μg/ml) (40) and 5f4 (2 μg/ml) (33), rat anti-MR mAb (1 μg/ml) (clone MR5D3 AbD Serotec), rabbit anti-PLA2R pAb (1:1000, Novus Biologicals), rat anti-DEC-205 mAb (1:500, eBioscience), and rabbit anti-DEC-205 mAb (1:5000, epitomics). Primary antibodies were detected with matching HRP-coupled secondary antibodies (1:6000, Dako).
Endocytosis Assays
Labeling of proteins with 125I and assay for internalization of labeled proteins was performed with 300,000 transfected HEK-293T cells as previously described for newborn mouse skin fibroblasts (9) with the following modifications. Following a 4-h incubation with 125I-labeled ligand (150 ng/ml in DMEM, 20 mm HEPES, 15 mg/ml BSA), the medium was removed, and cells gently harvested by incubation for 5 min in ice cold PBS with 5 mm EDTA and transfer to an Eppendorf tube. Cells were then washed twice in PBS by centrifugation for 2 min at 200 × g and resuspension of pellet in fresh PBS. Finally, cells were trypsinized in suspension for 2 min and centrifuged for 1 min at 1000 × g to remove surface bound radiolabeled ligand from intracellular ligand. The amount of internalized radiolabeled ligand was determined using a gamma counter. Experiments, in which the effect of mannose on ligand internalization was examined, were conducted in low-glucose DMEM with or without 50 mm mannose.
Endocytosis of fluorescent ligand was assayed with transfected HeLa cells. Fluorescent ligand (OG-gelatin, 20 μg/ml) was added to HeLa cells in DMEM with 10% FCS, 1% penicillin/streptomycin and 20 μm E64d, and incubated for 16 h. 3 drops of NucBlue® Live ReadyProbesTM Reagent (Life Technologies) per ml medium was added and incubation continued for 20 min. After 3 washes in PBS, 5 μg/ml WGA-Alexa647 was added for 5 min followed by another 4 washes in PBS and 3 washes in water. Finally, coverslips were mounted on microscope slides using prolong gold antifade mounting medium (Invitrogen).
RESULTS
Collagen Binding Activity of Recombinant Receptor Constructs
To initiate a comparative analysis of the four members of the MR protein family with respect to collagen interactions, we initially utilized a purified system with recombinant, truncated receptor variants. Since previous work has shown that the isolated, recombinant FN-II domain from uPARAP is unstable (43), we included the flanking domains in our molecular constructs. Thus, we made constructs comprising the Cys-rich domain, the FN-II domain and the first CTLD domain from each receptor (uPARAP D1–3, MR D1–3, PLA2R D1–3, and DEC-205 D1–3; Fig. 1A), including a common protein epitope tag for affinity purification (47). The affinity purified recombinant proteins were homogenous and migrated with the predicted electrophoretic mobility in SDS-PAGE (Fig. 1B). These proteins were analyzed for their ability to bind immobilized collagen in an ELISA type setup (42) (Fig. 1C). Strikingly, both recombinant uPARAP and MR showed strong binding toward collagen types I and IV, whereas recombinant PLA2R and DEC-205 were completely devoid of binding activity. Although restricted to isolated binding studies using purified components, these results point to functional distinctions within the MR family with respect to collagen-related functions.
Only uPARAP and MR Mediate Collagen Endocytosis and Degradation
To further investigate the abilities of the four receptors to engage in collagen turnover, we next created a system with specific expression of each receptor in a functional cellular setting. In this system, HEK-293T cells were transiently transfected with cDNA encoding full-length uPARAP, MR, PLA2R, or DEC-205, respectively. Western blotting performed on whole cell lysates collected 24 h post transfection revealed a strong specific expression of each receptor in the transfected cells (Fig. 2, A–D). A very low endogenous expression of uPARAP was noted in mock-transfected HEK-293T cells (Fig. 2A) but this was subsequently shown to be irrelevant in the functional analysis (see Figs. 3 to 7, below). No expression of MR, PLA2R, or DEC-205 could be detected in mock-transfected cells.
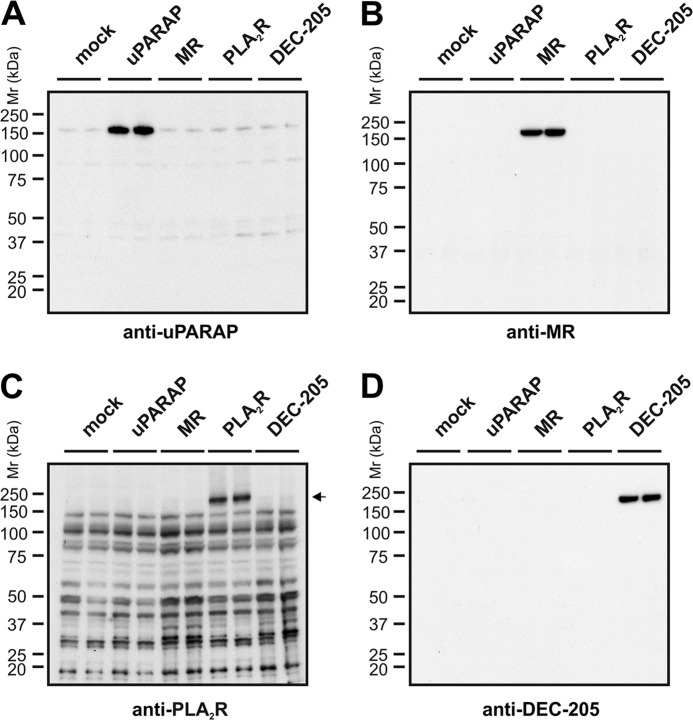
FIGURE 2.
Transfected HEK-293T cells express members of the MR protein family. Western blot analysis of uPARAP (A), MR (B), PLA2R (C), and DEC-205 (D) expression in whole cell lysates from HEK-293T cells 24 h post transfection. In each panel, duplicate samples from separate transfections with each receptor were analyzed in parallel lanes as indicated. A mouse mAb (2h9) against uPARAP (A), a rat mAb against MR (B), a rabbit pAb against PLA2R (C), or a rat mAb against DEC-205 (D), were used as primary antibodies for detection. In each case, the assay detected a protein with the theoretical molecular mass (∼180 kDa for uPARAP, MR, and PLA2R; 205 kDa for DEC-205) in cells transfected with the relevant receptor. No endogenous expression of these receptors was noted except for uPARAP, where a very weak band could be detected by mAb 2h9 in non-uPARAP transfected cells (A). Although the polyclonal anti-PLA2R antibody gave rise to several unspecific bands (C), these were clearly distinguishable from the specific PLA2R signal around 180 kDa (marked by black arrowhead).
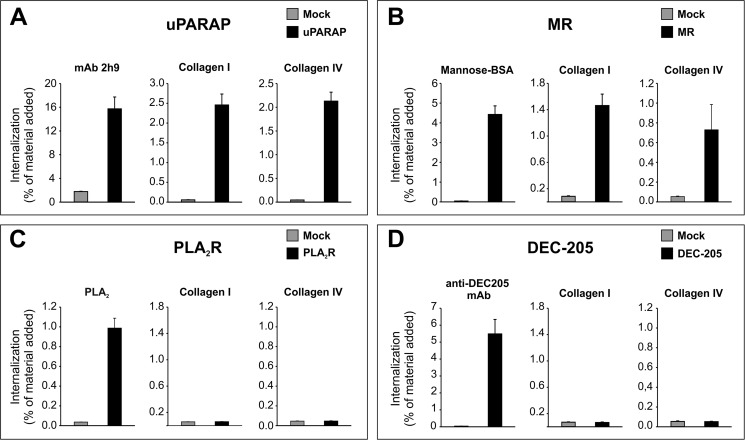
FIGURE 3.
Endocytosis of radiolabeled ligands mediated by uPARAP, MR, PLA2R, and DEC-205. HEK-293T cells were transfected with uPARAP (A), MR (B), PLA2R (C), and DEC-205 (D). In each panel, black columns show the internalization of a radiolabeled positive control ligand (left), collagen type I (center) and collagen type IV (right), with mock transfected cells shown for comparison (gray columns). Cells were incubated with radiolabeled ligands (100 ng/ml) for 4 h after which the fraction of intracellular ligand was determined. Positive control ligands were anti-uPARAP mAb (2h9), mannose-BSA, porcine pancreatic PLA2, and anti-DEC-205 mAb, for uPARAP, MR, PLA2R, and DEC-205, respectively. Data are presented as radioactive ligand in the intracellular fraction in percent of the total radioactive ligand added to cells. Error bars represent S.D. of triplicate samples.
To ensure receptor functionality and expression on the plasma membrane, the transfected cells were evaluated for their ability to internalize radiolabeled positive control ligands specific for each receptor (Fig. 3, A–D, left panels), i.e. using monoclonal antibodies against uPARAP (mAb 2h9, Fig. 3A) and DEC-205 (Fig. 3D), the MR ligand mannose-BSA (33) (Fig. 3B), and the PLA2R ligand, pancreatic PLA2 homologue (Fig. 3C) (28, 49). These ligands were efficiently taken up by the respective receptors (black columns) with no uptake in mock-transfected cells (gray columns). Together, these results demonstrate that the transfected HEK-293T cells expressed the four receptors and that these receptors possessed a strong endocytic capacity.
Having established these tools, we went on to analyze the endocytic capacity of each receptor for labeled collagen types I and IV (Fig. 3, A–D, center and right panels). In complete accordance with the binding studies above, uPARAP (Fig. 3A) and MR (Fig. 3B) transfected cells demonstrated a strong ability to internalize collagens type I and IV, whereas no collagen internalization could be detected in PLA2R (Fig. 3C) and DEC-205 (Fig. 3D) transfected cells, despite the successful internalization of positive control ligands, noted above.
To further substantiate this pattern of collagen internalization in the MR protein family, we next employed a different assay to directly visualize internalized collagen microscopically in transfected cells. In this assay, fluorescently labeled gelatin (a denatured collagen known to interact strongly with uPARAP (40, 42)) was added to transfected HeLa cells, which due to a superior ability to adhere, could be grown and transfected directly on coverslips. Following incubation with the fluorescent ligand, cells were examined by confocal microscopy. In this assay, a strong intracellular signal was detected from the labeled gelatin when HeLa cells had been transfected with uPARAP or MR (Fig. 4, B and C, respectively). In contrast, mock, PLA2R and DEC-205 transfected cells did not accumulate fluorescent gelatin (Fig. 4, A, D, and E), in accordance with the results found using radiolabeled ligands.
FIGURE 4.
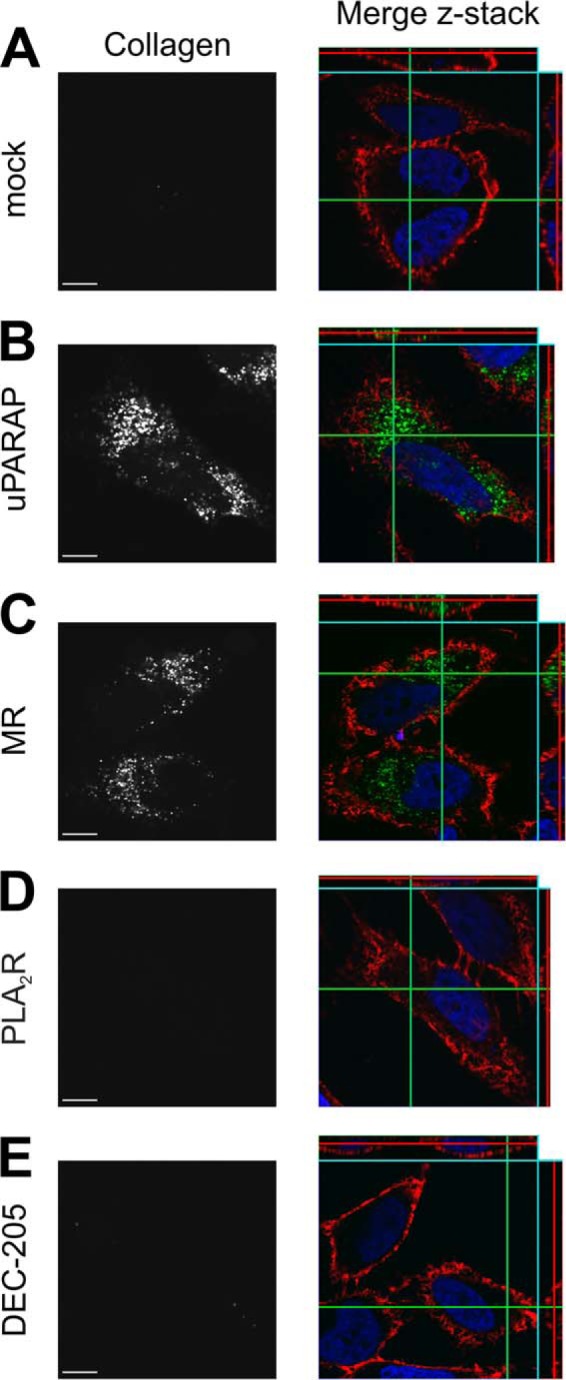
Endocytosis of fluorescent gelatin in uPARAP and MR-transfected cells. Internalization of fluorescently labeled gelatin by HeLa cells transfected with empty vector (mock, A), uPARAP (B), MR (C), PLA2R (D), and DEC-205 (E). Cells were incubated for 16 h with fluorescent collagen ligand (20 μg/ml, right panels, green). Cell nuclei were stained with Hoechst (right panels, blue) and cell membranes with wheat germ agglutinin (right panels, red). Z-stacks were collected using a confocal microscope. Left panels show the signal from fluorescent collagen in gray scale in a single plane. Size bar:10 μm.
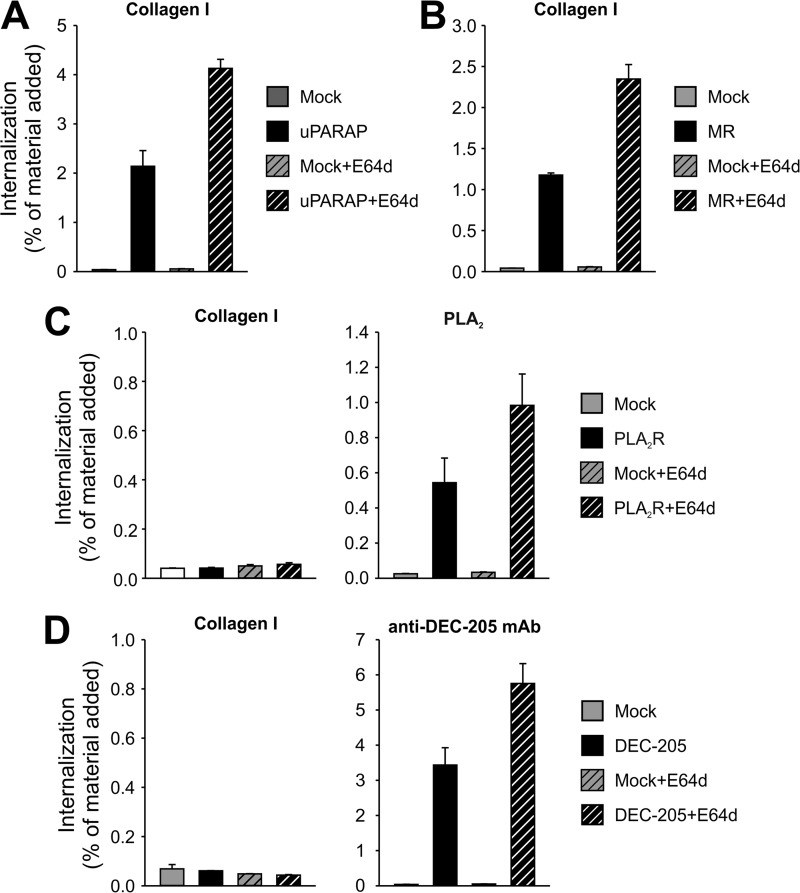
To investigate the fate of internalized ligands in the transfected cells, we next performed internalization experiments with radiolabeled ligands in the presence of E64d, a cysteine protease inhibitor known to reduce the degradation of internalized proteins in the lysosomal compartment (50), ultimately leading to an increased accumulation of internalized protein. In accordance with the expected lysosomal routing of cargo taken up by these receptors (29, 31, 32, 43, 51), uPARAP or MR transfected cells displayed an increase in intracellular accumulation of radiolabeled collagen in the presence of E64d, compared with conditions without the inhibitor (Fig. 5, A and B). This observation was in accordance with the pattern by which fluorescent gelatin accumulated in perinuclear vesicles consistent with endosomes and lysosomes in uPARAP or MR transfected HeLa cells (Fig. 4). Notably, the inclusion of E64d did not lead to any emergence of detectable intracellular collagen in PLA2R and DEC-205 transfected cells, although the positive control ligands for these receptors were indeed accumulated in increased amounts under these conditions (Fig. 5, C and D).
FIGURE 5.
Ligands internalized by each receptor are degraded lysosomally. The intracellular accumulation of radiolabeled collagen type I (100 ng/ml) in HEK-293T cells transfected with uPARAP (A), MR (B), PLA2R (C, left panel), and DEC-205 (D, left panel) is shown in the absence or presence of lysosomal protease inhibitor E64d (20 μm). Each sample set includes mock transfected cells for comparison. For PLA2R (C) and DEC-205 (D), control ligands were also included (100 ng/ml Pancreatic PLA2 and anti-DEC-205 mAb, respectively; right panels). All other experimental conditions and data presentation were as described for Fig. 3.
Together, the results above show that uPARAP and MR both display collagen binding and have the capacity to internalize solubilized collagen for degradation, whereas the remaining two members of the protein family are completely devoid of this activity. This was found to be the case although the latter proteins remained active endocytic receptors.
PLA2R and DEC-205 FN-II Domains Lack Collagen Binding Activity
The finding that PLA2R and DEC-205 lack the ability to interact with collagens, is surprising in light of their possession of a well-conserved FN-II domain (Fig. 7A and “Discussion”). Therefore, this observation prompted us to investigate the importance of the molecular context of this domain within the MR protein family. We first wanted to examine whether any potential collagen binding activity resided in the FN-II domain of PLA2R and DEC-205, which would just be insufficient in the context of the full-length PLA2R and DEC-205 receptors. Therefore, we designed receptor chimeras of uPARAP in which the entire 49 amino acid FN-II domain was replaced with the counterpart from PLA2R or DEC-205. uPARAP in which the FN-II domain was replaced with the FN-II domain from MR served as a positive control (Fig. 6A, uPARAP-PLA2R-FN-II, uPARAP-DEC-205-FN-II, and uPARAP-MR-FN-II, respectively).
The chimeric constructs were transiently transfected into HEK-293T cells and expression was confirmed by Western blotting. The uPARAP-specific mAb, 2h9 (40), which has an epitope situated outside the FN-II domain of uPARAP,3 detected expression of wt uPARAP as well as each of the three chimeras (Fig. 6B, upper panel). Another uPARAP specific mAb, 5f4 (16), with an epitope located inside the FN-II domain (results below),3 detected wt uPARAP but not the chimeras, thereby verifying that the FN-II domain of uPARAP had been successfully replaced (Fig. 6B, lower panel). Next, each chimera was evaluated for the ability to mediate collagen internalization. Strikingly, uPARAP-MR-FN-II transfected cells were nearly as efficient in internalizing labeled collagen as cells transfected with wt uPARAP, whereas cells transfected with uPARAP-PLA2R-FN-II or uPARAP-DEC-205-FN-II were completely unable to do so (Fig. 6C, left panel). Importantly, all chimeras had retained their endocytic properties since they all efficiently internalized the common uPARAP mAb, 2h9 (Fig. 6C. right panel). This finding indicates that the FN-II domains of PLA2R and DEC-205 by themselves are completely devoid of collagen binding capability. At the same time it demonstrates a high level of similarity in the collagen binding mechanism of uPARAP and MR, allowing for exchange of the FN-II domain of uPARAP with the counterpart of MR, without any apparent consequences for the ability to internalize collagen.
A Short Protruding Loop in the FN-II Domain of uPARAP Is Required for Collagen Binding
The evident difference in activity between otherwise well conserved FN-II domains within the MR family incited us to investigate the structural elements involved in collagen binding by uPARAP and MR FN-II domains in more detail. To do this, we performed a comparison of the amino acid sequences of the FN-II domains within the MR family along with a well-characterized active collagen binding FN-II domain from MMP-2 (Fig. 7A and “Discussion”). This comparison demonstrated the high level of homology within this domain in the MR family (52) but also revealed a distinct difference: A stretch of 10 amino acids (Thr30–Leu39, uPARAP sequence, Fig. 7A, purple marking) was clearly more conserved in the active collagen binding FN-II domains of uPARAP, MR, and MMP-2 as compared with the inactive counterparts from PLA2R and DEC-205. Importantly, in the collagen binding second FN-II domain of MMP-2, these 10 amino acid residues were previously demonstrated to constitute a loop protruding from the central core (53) (Fig. 7B, purple loop), and in addition, several of these amino acids were proposed to be involved in collagen binding by this domain (54) (Fig. 7A and “Discussion”). To analyze the role of this candidate collagen binding loop in uPARAP, we constructed two uPARAP mutant receptors (uPARAP-PLA2R-Loop and uPARAP-DEC-205-Loop), wherein residues Thr30–Leu39 from murine uPARAP FN-II were replaced by residues Ile30–Leu39 or Ile30–Pro38 from murine PLA2R or DEC-205 FN-II domains, respectively. The uPARAP mutants were transiently expressed in HEK-293T cells and Western blotting with both mAb 2h9 and 5f4 confirmed expression (Fig. 7C). When the mutants were subsequently analyzed for their ability to internalize collagens, we found that both mutants had completely lost all activity toward collagen, despite the fact that both of the positive control ligands, mAb 5f4 and 2h9, were efficiently internalized (Fig. 7D). Particularly, uptake of mAb 5f4, with an epitope situated in the FN-II domain, indicated proper folding of this domain in the mutants. These results effectively demonstrate that Thr30–Leu39 is actively involved in collagen binding by uPARAP.
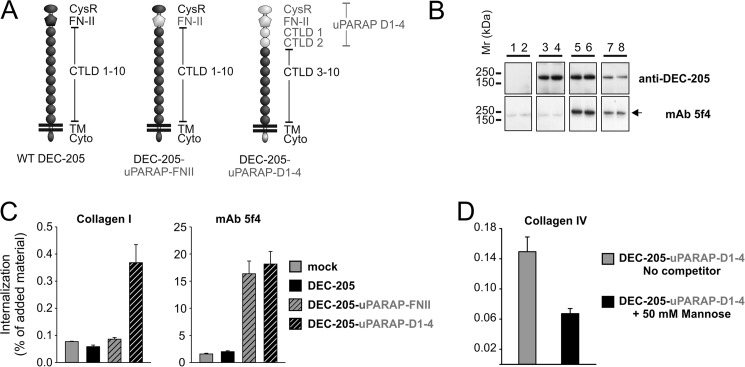
FN-II Flanking Domains Are Required for Collagen Internalization
The findings above pointed toward two different models that might explain the difference in collagen interactions within the receptor family: Either the FN-II domain from the active collagen-binding receptors would confer collagen internalization capacity to any receptor within the family, or a more complicated interplay with the flanking domains of each specific receptor could be involved. To study these two possibilities, two different chimeras were designed (Fig. 8A). In this set, different domains from uPARAP were inserted into DEC-205 to investigate the requirements to transform a non-collagen binding receptor into an active collagen receptor. The first chimera, DEC-205-uPARAP-FN-II, contained the FN-II domain of uPARAP inserted into DEC-205 in place of its native FN-II. The second chimera, DEC-205-uPARAP-D1–4, contained the first four N-terminal domains of uPARAP (Cys-rich, FN-II, CTLD-1, and CTLD-2) in place of the corresponding four N-terminal domains of DEC-205. These chimeras were transiently transfected into HEK-293T cells and receptor expression was confirmed by Western blotting (Fig. 8B). An antibody directed against the C-terminal region of DEC-205 successfully detected expression of wt DEC-205 (lanes 3 and 4) along with DEC-205-uPARAP-FN-II (lanes 5 and 6) and DEC-205-uPARAP-D1–4 (lanes 7 and 8), whereas the uPARAP specific mAb, 5f4, detected DEC-205-uPARAP-FN-II (lanes 5 and 6), and DEC-205-uPARAP-D1–4 (lanes 7 and 8) only, thereby confirming the presence of elements from uPARAP in the DEC-205 chimeras. Next, the collagen internalization ability of the chimeras was investigated. Surprisingly, only DEC-205-uPARAP-D1–4 transfected HEK-293T cells internalized collagen. This was the case, even though both chimeras facilitated a strong uptake of mAb 5f4 compared with mock and wt DEC-205 (Fig. 8C), confirming an intact, functioning uPARAP FN-II domain in the chimeras. Evidently, the FN-II domain from uPARAP on its own does not confer collagen internalizing capabilities to DEC-205.
FIGURE 8.
Gain of collagen internalization function in DEC-205 chimera with uPARAP D1–4 domain cassette. A, schematic overview of domain-swaps in DEC-205 chimeras (DEC-205-uPARAP-FN-II, DEC-205-uPARAP-D1-4). Inserted domains from uPARAP (gray) are highlighted to distinguish them from remaining DEC-205 domains (black). B, Western blot analysis of HEK-293T cells transfected with receptor chimeras using rabbit mAb against C-terminal DEC-205 or mAb 5f4 as primary antibody. Lanes 1 and 2: mock, lanes 3 and 4: DEC-205, lanes 5 and 6: DEC-205-uPARAP-FN-II, lanes 7 and 8: DEC-205-uPARAP-D1–4. Note that mAb 5f4 detects the FN-II domain from uPARAP in the two DEC-205 chimeras of ∼205 kDa (marked by a black arrowhead). Experimental conditions were as described in Fig. 2. C, internalization of radiolabeled collagen type I (100 ng/ml, left panel) and control ligand (100 ng/ml mAb 5f4, right panel) by HEK-293T cells transfected with DEC-205 wt and chimeras in the presence of E64d (20 μm). D, internalization of radiolabeled collagen type IV (100 ng/ml) by HEK-293T cells transfected with DEC-205-uPARAP-D1-4 in the absence or presence of 50 mm mannose. Experimental conditions and data presentation were as described for Fig. 6 (C and D).
We have previously shown that the single functional lectin domain in uPARAP, CTLD-2, is important for uptake of glycosylated collagens through its lectin ativity (42). We therefore wanted to investigate if this function of uPARAP could be transferred to DEC-205 along with the general ability to internalize collagen. To this end we examined uptake of collagen IV, a highly glycosylated collagen, by DEC-205-uPARAP-D1-4 in the presence or absence of a potential competitor monosaccharide, mannose (42). The addition of mannose partially inhibited internalization of collagen IV by DEC-205-uPARAP-D1-4 transfected HEK-293T cells (Fig. 8D), as was the case for uPARAP (42), showing that the lectin function of uPARAP was indeed acquired by the DEC-205 chimera.
Taken together, these chimera studies demonstrate how the functions of uPARAP in intracellular collagen degradation can be adapted by an inactive collagen receptor, DEC-205, simply by transferring the regions of uPARAP necessary for collagen binding. Furthermore, they illustrate for the first time how the single FN-II from uPARAP is insufficient to ensure collagen internalization in the scaffold of a related endocytic receptor.
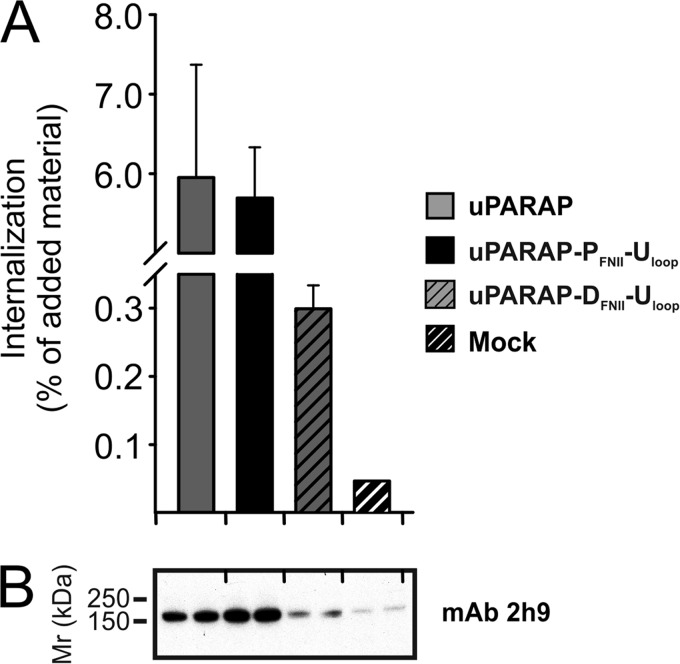
The Collagen Binding Loop from uPARAP Re-activates FN-II Domains from PLA2R and DEC-205
In a final experiment, we wanted to investigate the structural properties driving the interaction between FN-II domains and collagens in more detail. Specifically we wanted to investigate whether or not the proposed binding loop (Thr30–Leu39) of uPARAP's FN-II domain alone was sufficient to enable collagen binding by the inactive FN-II domains from PLA2R and DEC-205. To do this, we capitalized on the fact that uPARAP chimeras with PLA2R and DEC-205 FN-II domains (Fig. 6A) constituted receptors with all necessary elements required for collagen binding except for the active FN-II domain. Consequently, we constructed two additional mutant receptors. In these, the binding loop (Thr30–Leu39) from uPARAP's FN-II domain was re-introduced into the FN-II domains of PLA2R and DEC-205, positioned in the uPARAP chimeras. These mutant constructs were denoted uPARAP-PFNII-Uloop and uPARAP-DFNII-Uloop, respectively. Both mutants were transiently expressed in HEK-293T cells and compared with wt uPARAP with respect to collagen internalization. Strikingly, uPARAP-PFNII-Uloop completely regained the ability to internalize collagen. uPARAP-DFNII-Uloop also regained a pronounced ability to internalize collagen despite the fact that it was expressed at a lower level (Fig. 9, A and B).
FIGURE 9.
A critical FN-II domain loop from uPARAP reconstitutes collagen internalization function in uPARAP chimeras with PLA2R and DEC-205 FN-II domains. A, internalization of radiolabeled collagen type I (100 ng/ml) by HEK-293T cells transfected with uPARAP FN-II chimeras modified to re-introduce the protruding loop from uPARAP (uPARAP-PFNII-Uloop and uPARAP-DFNII-Uloop). Experimental conditions and data presentation were as described for Fig. 6. B, Western blot analysis of the HEK-293T cells expressing the receptor chimeras shown in A. Anti-uPARAP mAb 2h9 was used as primary antibody for detection. Experimental conditions were as described in Fig. 2. Note the weak expression of uPARAP-DFNII-Uloop, which is still above background expression of uPARAP in mock-transfected cells.
These results confirm the active role of residues Thr30–Leu39 in collagen binding by the FN-II domain of uPARAP and suggest that these residues constitute an externally protruding binding loop, which has effectively been altered in the otherwise homologous FN-II domains of PLA2R and DEC-205.
DISCUSSION
This work is the first comparative functional study of the entire MR family with respect to the ability to interact with collagens. By examining recombinant soluble MR-family proteins and characterizing cells expressing full-length receptors, we show conclusively that collagen internalization is not a common functional property but is limited to uPARAP and MR.
The lack of collagen internalization in PLA2R and DEC-205 was partly due to a lack of collagen binding by the FN-II domain of these two receptors. This is surprising, since sequence comparisons of the FN-II domain in this family has led to the suggestion that they might all be active in collagen binding (8). Indeed, the FN-II domain is the most highly conserved domain within the MR protein family (52). Furthermore, individual amino acid residues with proposed structural and ligand binding function are conserved. Thus, cysteine residues along with invariant aromatic residues in the FN-II domains of the collagen-binding proteins MMP-2, MMP-9, and fibronectin are completely retained in the MR protein family and most of the additional aromatic residues shown to affect ligand binding (38, 39, 54, 55) display only very conservative changes. However, careful sequence inspection does reveal interesting differences in the FN-II domains of PLA2R and DEC-205 when compared with uPARAP, MR, fibronectin and MMP-2 and -9 (Fig. 7A). One of the most prominent differences is found within the Gly33–Tyr38 amino acid stretch (Fig. 7A, MMP-2 FN-II domain sequence underlined), which has been implicated in collagen binding by MMP-2 (39, 54, 56) and furthermore has been demonstrated to be part of a loop which extends from the central core structure of this domain (53). The positively charged Arg34, the negatively charged Asp36, and Gly33 and Gly37 are conserved in uPARAP and MR and in addition a bulky or aromatic residue has been conserved at position 38. In the FN-II domain of PLA2R, Arg34 and Asp36 have only changed conservatively but importantly, in all examined species the Gly37 residue has been replaced by bulky or charged residues. In addition the aromatic Tyr38 has been replaced by a leucine. The FN-II domain of DEC-205 comprises numerous changes in this stretch of amino acids, including substitution of Gly33 and Tyr38. Indeed, through mutational studies we were able to demonstrate that this stretch of amino acid residues is crucial for the binding of collagen by uPARAP's FN-II domain and that changes in this sequence account for the lack of collagen binding by PLA2R and DEC-205 FN-II domains. Our comparative study should, therefore, aid further refined studies to pinpoint the structural requirements for collagen binding in this domain type.
We also show that an active FN-II domain from uPARAP is insufficient to transform DEC-205 into a collagen internalizing receptor. The presence of all four N-terminal uPARAP domains did, however, accomplish this. Importantly, this finding demonstrates that the FN-II domain in uPARAP is not an independent collagen-binding unit and that adjacent domains directly modulate the binding between uPARAP and collagen. This is consistent with observations reported for fibronectin and MMP-2, where, albeit a single FN-II domain does exhibit low affinity binding toward collagen, additional adjacent FN-II domains or other domain types serve to modulate the interaction (37, 57). Furthermore, this is supported by two studies reporting that in a purified system a combination of the Cys-rich and the FN-II domain from uPARAP or MR alone is unstable and that at least one CTLD is required for stabilization of the protein region (41, 43). Importantly, in our work no instability was observed for the FN-II domain from uPARAP in the context of the DEC-205-uPARAP-FN-II chimera, since the protein efficiently internalized the 5f4 antibody against the FN-II domain. Nonetheless, no internalization of collagen was observed with this chimeric construct.
It is presently unclear how the FN-II-adjacent domains stimulate collagen binding in uPARAP. One possibility is that the Cys-rich domain and the first CTLD are involved in a multimerization process of the receptor, which could lead to the formation of a complex with high avidity for collagen ligands. This suggestion is intriguing, considering the multiple adjacent binding sites, which must be located in a single collagen I α-chain (40) and the general complex multimerization and packaging of collagen molecules in collagen structures. This concept is supported by our unpublished results, which demonstrate that artificial antibody-mediated multimerization of recombinant uPARAP produces a complex with stronger binding toward collagen than the monomeric receptor.4 Furthermore, a similar phenomenon has been reported for artificial multimers of MR (41). Such a multimerization process on the cell surface, dictated by the Cys-rich domain and/or one or more CTLDs of uPARAP, could explain why the DEC-205-uPARAP-FN-II chimera lacks the ability to internalize collagen while the DEC-205-uPARAP-D1–4 is able do so.
CTLDs may also be directly involved in the modulation of collagen binding by other means. We and others have previously shown that CTLD-2 from uPARAP is an active lectin (58) that directly binds collagen carbohydrate moieties (42). In this work we show that this function can be transferred to DEC-205 along with the general collagen binding ability by including CTLD-2 in domains transferred from uPARAP. CTLD-2 from uPARAP as well as CTLD-4 from MR, has also been proposed to play a role in the formation of a pH-dependent globular, bent N-terminal structure driven by an intra-protein interaction between the Cys-rich and the CTLD, as demonstrated by single particle electron microscopy (45, 46). This may represent a built-in mechanism that allows for regulation of collagen binding on the cell surface and subsequent transport to endosomes and lysosomes, with a low pH environment.
Functional studies have suggested that PLA2R can mediate increased cellular adhesion to collagen types I and IV coated surfaces, dependent on the Cys-rich and FN-II domains (44), and also an increased cellular invasion through a collagen type IV rich matrix (49). Both of these observations indirectly indicate a PLA2R-collagen interaction. However, it is unclear whether this represents any discrepancy with our present findings. In the invasion study, the reported effect was dependent on cellular stimulation with other PLA2R ligands, which could suggest that the PLA2R-mediated invasion is a receptor signaling-dependent mechanism rather than a direct collagen interaction. We cannot rule out that PLA2R indirectly mediates adhesion to, or invasion through, collagen structures, but our results demonstrate that there is no direct interaction between either PLA2R or DEC-205 and collagen. These receptors are likely to have other important functions, which for PLA2R have been found to include PLA2 enzyme regulation during inflammation (59–62) and a pathogenic role in the autoimmune disease membranous nephropathy (63), whereas for DEC-205 they include antigen presentation (29, 64).
uPARAP-mediated intracellular collagen degradation has been thoroughly documented to be an important ECM remodeling mechanism in development and pathological processes (13–20). The role of MR in intracellular collagen degradation in vivo, however, has only recently started to become clear. In one study, MR was shown to be responsible for liver specific clearance of collagen fragments from the blood by endothelial cells (65) and very recently we have been able to show that both MR and uPARAP is involved in collagen internalization in the skin (66). The latter work, in which intracellular collagen degradation was visualized in vivo using confocal microscopy, demonstrated that both receptors can be active in the same tissue at the same time, but that each receptor appears to be confined to specific cell types. Based on this in vivo work, our work presented here, and results demonstrating a large overlap in uPARAP and MR tissue expression (14)3,4, we speculate that the impact of intracellular collagen degradation on development and disease may be even stronger than demonstrated so far in studies with each receptor alone, due to a pronounced functional overlap. For instance, MR may protect against fibrosis in parallel with uPARAP (16, 18) or participate in the matrix destruction seen in cancer progression (19), which could imply an even greater impact of the current collagen degradation mechanism in these diseases than previously recognized. In addition, both, uPARAP and MR might contribute to the characteristic excessive matrix destruction observed in diseases such as arthritis and osteoporosis (1, 25, 67). Altogether, the data presented here constitute important new findings in the ongoing process of deducing the elusive collagen degradation mechanism employed by uPARAP and MR, the now identified principal collagen-internalizing receptors.
Acknowledgment
We thank Katharina H. Stegmann for excellent technical assistance.
This work was supported by the NIDCR Intramural Research Program (D. M. and T. H. B.), the Danish Cancer Society, the Danish Medical Research Council, the Danish Cancer Research Foundation, the Novo Nordisk Foundation, the Danish National Research Foundation (Danish-Chinese Center for Proteases and Cancer) and the European Community's Seventh Framework Programme FP7/2007-2011 under Grant agreement n°201279 (to M. C. M. and N. B.), by the Lundbeck Foundation (to D. H. M. and L. H. E.), the “Grosserer Alfred Nielsen og Hustrus” foundation (to L. H. E.). The work was also supported by personal grants from Rigshospitalet/Copenhagen University Hospital (to H. J. J. and A. P.).
H. J. Jürgensen, unpublished data.
D. H. Madsen, unpublished data.
- ECM
- extracellular matrix
- MMP
- matrix metalloproteinase
- MR
- mannose receptor
- uPARAP
- urokinase plasminogen activator receptor-associated protein
- PLA2R
- phospholipase A2 receptor
- Cys-rich
- cysteine-rich
- FN-II
- fibronectin type-II
- CTLD
- C-type lectin-like domain
- WGA
- wheat germ agglutinin
- PLA2
- phospholipase A2
- USER
- uracil specific excision reagent
- mAb
- monoclonal antibody.
REFERENCES
- 1. Holmbeck K., Szabova L. (2006) Aspects of extracellular matrix remodeling in development and disease. Birth Defects Res C Embryo Today 78, 11–23 [DOI] [PubMed] [Google Scholar]
- 2. Rowe R. G., Weiss S. J. (2009) Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 25, 567–595 [DOI] [PubMed] [Google Scholar]
- 3. Bugge T. H., Behrendt N. (2011) Cooperation between extracellular proteolysis and endocytosis in collagen turnover in Extracellular Matrix Degradation, (Parks W. C., Mecham R. P., eds), pp. 53–74, Springer, Heidelberg [Google Scholar]
- 4. Stamenkovic I. (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J. Pathol. 200, 448–464 [DOI] [PubMed] [Google Scholar]
- 5. Everts V., van der Zee E., Creemers L., Beertsen W. (1996) Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem. J. 28, 229–245 [DOI] [PubMed] [Google Scholar]
- 6. Lauer-Fields J. L., Juska D., Fields G. B. (2002) Matrix metalloproteinases and collagen catabolism. Biopolymers 66, 19–32 [DOI] [PubMed] [Google Scholar]
- 7. Segal G., Lee W., Arora P. D., McKee M., Downey G., McCulloch C. A. (2001) Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J. Cell Sci. 114, 119–129 [DOI] [PubMed] [Google Scholar]
- 8. East L., Isacke C. M. (2002) The mannose receptor family. Biochim. Biophys. Acta 1572, 364–386 [DOI] [PubMed] [Google Scholar]
- 9. Engelholm L. H., List K., Netzel-Arnett S., Cukierman E., Mitola D. J., Aaronson H., Kjøller L., Larsen J. K., Yamada K. M., Strickland D. K., Holmbeck K., Danø K., Birkedal-Hansen H., Behrendt N., Bugge T. H. (2003) uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 160, 1009–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. East L., McCarthy A., Wienke D., Sturge J., Ashworth A., Isacke C. M. (2003) A targeted deletion in the endocytic receptor gene Endo180 results in a defect in collagen uptake. EMBO Rep. 4, 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behrendt N., Jensen O. N., Engelholm L. H., Mørtz E., Mann M., Danø K. (2000) A urokinase receptor-associated protein with specific collagen binding properties. J. Biol. Chem. 275, 1993–2002 [DOI] [PubMed] [Google Scholar]
- 12. Sheikh H., Yarwood H., Ashworth A., Isacke C. M. (2000) Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 113, 1021–1032 [DOI] [PubMed] [Google Scholar]
- 13. Fasquelle C., Sartelet A., Li W., Dive M., Tamma N., Michaux C., Druet T., Huijbers I. J., Isacke C. M., Coppieters W., Georges M., Charlier C. (2009) Balancing selection of a frame-shift mutation in the MRC2 gene accounts for the outbreak of the Crooked Tail Syndrome in Belgian Blue Cattle. PLoS Genet. 5, e1000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madsen D. H., Jürgensen H. J., Ingvarsen S., Melander M. C., Albrechtsen R., Hald A., Holmbeck K., Bugge T. H., Behrendt N., Engelholm L. H. (2013) Differential Actions of the Endocytic Collagen Receptor uPARAP/Endo180 and the Collagenase MMP-2 in Bone Homeostasis. PLoS One 8, e71261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagenaar-Miller R. A., Engelholm L. H., Gavard J., Yamada S. S., Gutkind J. S., Behrendt N., Bugge T. H., Holmbeck K. (2007) Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol. Cell. Biol. 27, 6309–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madsen D. H., Jurgensen H. J., Ingvarsen S., Melander M. C., Vainer B., Egerod K. L., Hald A., Rønø B., Madsen C. A., Bugge T. H., Engelholm L. H., Behrendt N. (2012) Endocytic collagen degradation: a novel mechanism involved in protection against liver fibrosis. J. Pathol. 227, 94–105 [DOI] [PubMed] [Google Scholar]
- 17. Bundesmann M. M., Wagner T. E., Chow Y. H., Altemeier W. A., Steinbach T., Schnapp L. M. (2012) Role of urokinase plasminogen activator receptor-associated protein in mouse lung. Am. J. Respir. Cell Mol. Biol. 46, 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. López-Guisa J. M., Cai X., Collins S. J., Yamaguchi I., Okamura D. M., Bugge T. H., Isacke C. M., Emson C. L., Turner S. M., Shankland S. J., Eddy A. A. (2012) Mannose receptor 2 attenuates renal fibrosis. J. Am. Soc. Nephrol. 23, 236–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curino A. C., Engelholm L. H., Yamada S. S., Holmbeck K., Lund L. R., Molinolo A. A., Behrendt N., Nielsen B. S., Bugge T. H. (2005) Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J. Cell Biol. 169, 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wienke D., Davies G. C., Johnson D. A., Sturge J., Lambros M. B., Savage K., Elsheikh S. E., Green A. R., Ellis I. O., Robertson D., Reis-Filho J. S., Isacke C. M. (2007) The collagen receptor Endo180 (CD280) Is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res. 67, 10230–10240 [DOI] [PubMed] [Google Scholar]
- 21. Schnack Nielsen B., Rank F., Engelholm L. H., Holm A., Danø K., Behrendt N. (2002) Urokinase receptor-associated protein (uPARAP) is expressed in connection with malignant as well as benign lesions of the human breast and occurs in specific populations of stromal cells. Int. J. Cancer 98, 656–664 [DOI] [PubMed] [Google Scholar]
- 22. Sulek J., Wagenaar-Miller R. A., Shireman J., Molinolo A., Madsen D. H., Engelholm L. H., Behrendt N., Bugge T. H. (2007) Increased expression of the collagen internalization receptor uPARAP/Endo180 in the stroma of head and neck cancer. J. Histochem. Cytochem. 55, 347–353 [DOI] [PubMed] [Google Scholar]
- 23. Kogianni G., Walker M. M., Waxman J., Sturge J. (2009) Endo180 expression with cofunctional partners MT1-MMP and uPAR-uPA is correlated with prostate cancer progression. Eur. J. Cancer 45, 685–693 [DOI] [PubMed] [Google Scholar]
- 24. Huijbers I. J., Iravani M., Popov S., Robertson D., Al-Sarraj S., Jones C., Isacke C. M. (2010) A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One 5, e9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamaguchi T., Nakaoka H., Yamamoto K., Fujikawa T., Kim Y. I., Yano K., Haga S., Katayama K., Shibusawa T., Park S., Maki K., Kimura R., Inoue I. (2013) Genome-wide association study of degenerative bony changes of the temporomandibular joint. Oral Dis 10.1111/odi.12140 [DOI] [PubMed] [Google Scholar]
- 26. Jiang W., Swiggard W. J., Heufler C., Peng M., Mirza A., Steinman R. M., Nussenzweig M. C. (1995) The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375, 151–155 [DOI] [PubMed] [Google Scholar]
- 27. Ezekowitz R. A., Sastry K., Bailly P., Warner A. (1990) Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in COS-1 cells. J. Exp. Med. 172, 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lambeau G., Ancian P., Barhanin J., Lazdunski M. (1994) Cloning and expression of a membrane receptor for secretory phospholipases A2. J. Biol. Chem. 269, 1575–1578 [PubMed] [Google Scholar]
- 29. Mahnke K., Guo M., Lee S., Sepulveda H., Swain S. L., Nussenzweig M., Steinman R. M. (2000) The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151, 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howard M. J., Isacke C. M. (2002) The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J. Biol. Chem. 277, 32320–32331 [DOI] [PubMed] [Google Scholar]
- 31. Zvaritch E., Lambeau G., Lazdunski M. (1996) Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J. Biol. Chem. 271, 250–257 [DOI] [PubMed] [Google Scholar]
- 32. Schweizer A., Stahl P. D., Rohrer J. (2000) A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J. Biol. Chem. 275, 29694–29700 [DOI] [PubMed] [Google Scholar]
- 33. Madsen D. H., Ingvarsen S., Jürgensen H. J., Melander M. C., Kjøller L., Moyer A., Honoré C., Madsen C. A., Garred P., Burgdorf S., Bugge T. H., Behrendt N., Engelholm L. H. (2011) The non-phagocytic route of collagen uptake: a distinct degradation pathway. J. Biol. Chem. 286, 26996–27010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Llorca O. (2008) Extended and bent conformations of the mannose receptor family. Cell Mol. Life Sci. 65, 1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez-Pomares L. (2012) The mannose receptor. J. Leukoc. Biol. 92, 1177–1186 [DOI] [PubMed] [Google Scholar]
- 36. Napper C. E., Drickamer K., Taylor M. E. (2006) Collagen binding by the mannose receptor mediated through the fibronectin type II domain. Biochem. J. 395, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bányai L., Tordai H., Patthy L. (1994) The gelatin-binding site of human 72 kDa type IV collagenase (gelatinase A). Biochem. J. 298, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pickford A. R., Potts J. R., Bright J. R., Phan I., Campbell I. D. (1997) Solution structure of a type 2 module from fibronectin: implications for the structure and function of the gelatin-binding domain. Structure 5, 359–370 [DOI] [PubMed] [Google Scholar]
- 39. Collier I. E., Krasnov P. A., Strongin A. Y., Birkedal-Hansen H., Goldberg G. I. (1992) Alanine scanning mutagenesis and functional analysis of the fibronectin-like collagen-binding domain from human 92-kDa type IV collagenase. J. Biol. Chem. 267, 6776–6781 [PubMed] [Google Scholar]
- 40. Madsen D. H., Engelholm L. H., Ingvarsen S., Hillig T., Wagenaar-Miller R. A., Kjøller L., Gårdsvoll H., Høyer-Hansen G., Holmbeck K., Bugge T. H., Behrendt N. (2007) Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J. Biol. Chem. 282, 27037–27045 [DOI] [PubMed] [Google Scholar]
- 41. Martinez-Pomares L., Wienke D., Stillion R., McKenzie E. J., Arnold J. N., Harris J., McGreal E., Sim R. B., Isacke C. M., Gordon S. (2006) Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur. J. Immunol. 36, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 42. Jürgensen H. J., Madsen D. H., Ingvarsen S., Melander M. C., Gårdsvoll H., Patthy L., Engelholm L. H., Behrendt N. (2011) A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uPARAP/ENDO180. J. Biol. Chem. 286, 32736–32748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wienke D., MacFadyen J. R., Isacke C. M. (2003) Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol. Biol. Cell 14, 3592–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ancian P., Lambeau G., Lazdunski M. (1995) Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry 34, 13146–13151 [DOI] [PubMed] [Google Scholar]
- 45. Rivera-Calzada A., Robertson D., MacFadyen J. R., Boskovic J., Isacke C. M., Llorca O. (2003) Three-dimensional interplay among the ligand-binding domains of the urokinase-plasminogen-activator-receptor-associated protein, Endo180. EMBO Rep. 4, 807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boskovic J., Arnold J. N., Stilion R., Gordon S., Sim R. B., Rivera-Calzada A., Wienke D., Isacke C. M., Martinez-Pomares L., Llorca O. (2006) Structural model for the mannose receptor family uncovered by electron microscopy of Endo180 and the mannose receptor. J. Biol. Chem. 281, 8780–8787 [DOI] [PubMed] [Google Scholar]
- 47. Gårdsvoll H., Hansen L. V., Jørgensen T. J., Ploug M. (2007) A new tagging system for production of recombinant proteins in Drosophila S2 cells using the third domain of the urokinase receptor. Protein Expr. Purif. 52, 384–394 [DOI] [PubMed] [Google Scholar]
- 48. Nørholm M. H. (2010) A mutant Pfu DNA polymerase designed for advanced uracil-excision DNA engineering. BMC Biotechnol. 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kundu G. C., Mukherjee A. B. (1997) Evidence that porcine pancreatic phospholipase A2 via its high affinity receptor stimulates extracellular matrix invasion by normal and cancer cells. J. Biol. Chem. 272, 2346–2353 [PubMed] [Google Scholar]
- 50. Mehdi S. (1991) Cell-penetrating inhibitors of calpain. Trends Biochem. Sci 16, 150–153 [DOI] [PubMed] [Google Scholar]
- 51. Kjøller L., Engelholm L. H., Høyer-Hansen M., Danø K., Bugge T. H., Behrendt N. (2004) uPARAP/endo180 directs lysosomal delivery and degradation of collagen IV. Exp. Cell Res. 293, 106–116 [DOI] [PubMed] [Google Scholar]
- 52. Wu K., Yuan J., Lasky L. A. (1996) Characterization of a novel member of the macrophage mannose receptor type C lectin family. J. Biol. Chem. 271, 21323–21330 [DOI] [PubMed] [Google Scholar]
- 53. Morgunova E., Tuuttila A., Bergmann U., Isupov M., Lindqvist Y., Schneider G., Tryggvason K. (1999) Structure of human pro-matrix metalloproteinase-2: activation mechanism revealed. Science 284, 1667–1670 [DOI] [PubMed] [Google Scholar]
- 54. Briknarová K., Grishaev A., Bányai L., Tordai H., Patthy L., Llinás M. (1999) The second type II module from human matrix metalloproteinase 2: structure, function and dynamics. Structure 7, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 55. Xu X., Mikhailova M., Ilangovan U., Chen Z., Yu A., Pal S., Hinck A. P., Steffensen B. (2009) Nuclear magnetic resonance mapping and functional confirmation of the collagen binding sites of matrix metalloproteinase-2. Biochemistry 48, 5822–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tordai H., Patthy L. (1999) The gelatin-binding site of the second type-II domain of gelatinase A/MMP-2. Eur. J. Biochem. 259, 513–518 [DOI] [PubMed] [Google Scholar]
- 57. Katagiri Y., Brew S. A., Ingham K. C. (2003) All six modules of the gelatin-binding domain of fibronectin are required for full affinity. J. Biol. Chem. 278, 11897–11902 [DOI] [PubMed] [Google Scholar]
- 58. East L., Rushton S., Taylor M. E., Isacke C. M. (2002) Characterization of sugar binding by the mannose receptor family member, Endo180. J. Biol. Chem. 277, 50469–50475 [DOI] [PubMed] [Google Scholar]
- 59. Hanasaki K., Arita H. (2002) Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat. 68–69, 71–82 [DOI] [PubMed] [Google Scholar]
- 60. Rouault M., Le Calvez C., Boilard E., Surrel F., Singer A., Ghomashchi F., Bezzine S., Scarzello S., Bollinger J., Gelb M. H., Lambeau G. (2007) Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry 46, 1647–1662 [DOI] [PubMed] [Google Scholar]
- 61. Hanasaki K., Yokota Y., Ishizaki J., Itoh T., Arita H. (1997) Resistance to endotoxic shock in phospholipase A2 receptor-deficient mice. J. Biol. Chem. 272, 32792–32797 [DOI] [PubMed] [Google Scholar]
- 62. Tamaru S., Mishina H., Watanabe Y., Watanabe K., Fujioka D., Takahashi S., Suzuki K., Nakamura T., Obata J. E., Kawabata K., Yokota Y., Murakami M., Hanasaki K., Kugiyama K. (2013) Deficiency of phospholipase A2 receptor exacerbates ovalbumin-induced lung inflammation. J. Immunol. 191, 1021–1028 [DOI] [PubMed] [Google Scholar]
- 63. Beck L. H., Jr., Bonegio R. G., Lambeau G., Beck D. M., Powell D. W., Cummins T. D., Klein J. B., Salant D. J. (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kamphorst A. O., Guermonprez P., Dudziak D., Nussenzweig M. C. (2010) Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J. Immunol. 185, 3426–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Malovic I., Sørensen K. K., Elvevold K. H., Nedredal G. I., Paulsen S., Erofeev A. V., Smedsrød B. H., McCourt P. A. (2007) The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology 45, 1454–1461 [DOI] [PubMed] [Google Scholar]
- 66. Madsen D. H., Leonard D., Masedunskas A., Moyer A., Jürgensen H. J., Peters D. E., Amornphimoltham P., Selvaraj A., Yamada S. S., Brenner D. A., Burgdorf S., Engelholm L. H., Behrendt N., Holmbeck K., Weigert R., Bugge T. H. (2013) M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 202, 951–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gravallese E. M. (2002) Bone destruction in arthritis. Ann. Rheum. Dis. 61, Suppl. 2, ii84-ii86 [DOI] [PMC free article] [PubMed] [Google Scholar]