Abstract
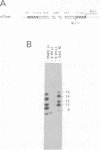
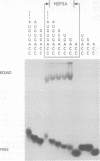
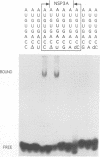
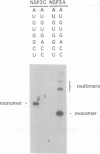
The interaction of the group A rotavirus non-structural protein NSP3 (NSP3A) with RNA has been studied in vitro. Using semi-purified NSP3A protein expressed by a recombinant baculovirus and in vitro synthesized RNA, we determined by UV cross-linking and gel retardation assays that NSP3A binds, in a sequence-specific manner, the consensus sequence (AUGUGACC) present on the 3' ends of all group A rotavirus mRNAs. Using short oligoribonucleotides, we established that the minimal RNA sequence required for binding of NSP3A is GACC. Modifications of the UGACC oligonucleotide sequence impaired binding of the protein to the RNA. Furthermore, the recombinant NSP3 protein from rotavirus group C showed specificity for the 3' end consensus sequence (AUGUGGCU) of only group C mRNAs. Sequence analysis of the NSP3 proteins did not reveal significant homologies with other RNA binding proteins, thus the NSP3 proteins of rotaviruses are the prototypes of a new kind of sequence-specific RNA binding protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aponte C., Mattion N. M., Estes M. K., Charpilienne A., Cohen J. Expression of two bovine rotavirus non-structural proteins (NSP2, NSP3) in the baculovirus system and production of monoclonal antibodies directed against the expressed proteins. Arch Virol. 1993;133(1-2):85–95. doi: 10.1007/BF01309746. [DOI] [PubMed] [Google Scholar]
- Bardwell V. J., Wickens M., Bienroth S., Keller W., Sproat B. S., Lamond A. I. Site-directed ribose methylation identifies 2'-OH groups in polyadenylation substrates critical for AAUAAA recognition and poly(A) addition. Cell. 1991 Apr 5;65(1):125–133. doi: 10.1016/0092-8674(91)90414-t. [DOI] [PubMed] [Google Scholar]
- Birney E., Kumar S., Krainer A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993 Dec 25;21(25):5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremont M., Chabanne-Vautherot D., Vannier P., McCrae M. A., Cohen J. Sequence analysis of the gene (6) encoding the major capsid protein (VP6) of group C rotavirus: higher than expected homology to the corresponding protein from group A virus. Virology. 1990 Oct;178(2):579–583. doi: 10.1016/0042-6822(90)90357-w. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Abdurashidova G. G. Polynucleotide-protein cross-links induced by ultraviolet light and their use for structural investigation of nucleoproteins. Prog Nucleic Acid Res Mol Biol. 1989;37:1–65. doi: 10.1016/s0079-6603(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Chastain M., Tinoco I., Jr Structural elements in RNA. Prog Nucleic Acid Res Mol Biol. 1991;41:131–177. doi: 10.1016/S0079-6603(08)60008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K. R., Wolf V., McBryant S. J., Zhang P., Liao X., Wright P. E., Gottesfeld J. M. Molecular basis for specific recognition of both RNA and DNA by a zinc finger protein. Science. 1993 Apr 23;260(5107):530–533. doi: 10.1126/science.8475383. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Gorziglia M. I., Collins P. L. Intracellular amplification and expression of a synthetic analog of rotavirus genomic RNA bearing a foreign marker gene: mapping cis-acting nucleotides in the 3'-noncoding region. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5784–5788. doi: 10.1073/pnas.89.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamy F., Asseline U., Grasby J., Iwai S., Pritchard C., Slim G., Butler P. J., Karn J., Gait M. J. Hydrogen-bonding contacts in the major groove are required for human immunodeficiency virus type-1 tat protein recognition of TAR RNA. J Mol Biol. 1993 Mar 5;230(1):111–123. doi: 10.1006/jmbi.1993.1129. [DOI] [PubMed] [Google Scholar]
- Hua J., Mansell E. A., Patton J. T. Comparative analysis of the rotavirus NS53 gene: conservation of basic and cysteine-rich regions in the protein and possible stem-loop structures in the RNA. Virology. 1993 Sep;196(1):372–378. doi: 10.1006/viro.1993.1492. [DOI] [PubMed] [Google Scholar]
- Keller W., Bienroth S., Lang K. M., Christofori G. Cleavage and polyadenylation factor CPF specifically interacts with the pre-mRNA 3' processing signal AAUAAA. EMBO J. 1991 Dec;10(13):4241–4249. doi: 10.1002/j.1460-2075.1991.tb05002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kim B., Little J. W. Dimerization of a specific DNA-binding protein on the DNA. Science. 1992 Jan 10;255(5041):203–206. doi: 10.1126/science.1553548. [DOI] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. RNA recognition: a family matter? Cell. 1993 Jun 4;73(5):837–840. doi: 10.1016/0092-8674(93)90265-r. [DOI] [PubMed] [Google Scholar]
- Mattion N. M., Cohen J., Aponte C., Estes M. K. Characterization of an oligomerization domain and RNA-binding properties on rotavirus nonstructural protein NS34. Virology. 1992 Sep;190(1):68–83. doi: 10.1016/0042-6822(92)91193-x. [DOI] [PubMed] [Google Scholar]
- Olsen H. S., Nelbock P., Cochrane A. W., Rosen C. A. Secondary structure is the major determinant for interaction of HIV rev protein with RNA. Science. 1990 Feb 16;247(4944):845–848. doi: 10.1126/science.2406903. [DOI] [PubMed] [Google Scholar]
- Pashev I. G., Dimitrov S. I., Angelov D. Crosslinking proteins to nucleic acids by ultraviolet laser irradiation. Trends Biochem Sci. 1991 Sep;16(9):323–326. doi: 10.1016/0968-0004(91)90133-g. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Gallegos C. O. Rotavirus RNA replication: single-stranded RNA extends from the replicase particle. J Gen Virol. 1990 May;71(Pt 5):1087–1094. doi: 10.1099/0022-1317-71-5-1087. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Salter-Cid L., Kalbach A., Mansell E. A., Kattoura M. Nucleotide and amino acid sequence analysis of the rotavirus nonstructural RNA-binding protein NS35. Virology. 1993 Feb;192(2):438–446. doi: 10.1006/viro.1993.1059. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Greenberg H. B., Graham D. Y., Estes M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1(2):133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Poncet D., Aponte C., Cohen J. Rotavirus protein NSP3 (NS34) is bound to the 3' end consensus sequence of viral mRNAs in infected cells. J Virol. 1993 Jun;67(6):3159–3165. doi: 10.1128/jvi.67.6.3159-3165.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Puglisi J. D., Tan R., Calnan B. J., Frankel A. D., Williamson J. R. Conformation of the TAR RNA-arginine complex by NMR spectroscopy. Science. 1992 Jul 3;257(5066):76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- Qian Y. A., Jiang B. M., Saif L. J., Kang S. Y., Ishimaru Y., Yamashita Y., Oseto M., Green K. Y. Sequence conservation of gene 8 between human and porcine group C rotaviruses and its relationship to the VP7 gene of group A rotaviruses. Virology. 1991 Jun;182(2):562–569. doi: 10.1016/0042-6822(91)90597-5. [DOI] [PubMed] [Google Scholar]
- Qian Y. A., Jiang B. M., Saif L. J., Kang S. Y., Ojeh C. K., Green K. Y. Molecular analysis of the gene 6 from a porcine group C rotavirus that encodes the NS34 equivalent of group A rotaviruses. Virology. 1991 Oct;184(2):752–757. doi: 10.1016/0042-6822(91)90446-i. [DOI] [PubMed] [Google Scholar]
- Ramig R. F., Ward R. L. Genomic segment reassortment in rotaviruses and other reoviridae. Adv Virus Res. 1991;39:163–207. doi: 10.1016/s0065-3527(08)60795-2. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Furuichi Y. Synthesis in Escherichia coli of the reovirus nonstructural protein sigma NS. J Virol. 1985 Nov;56(2):527–533. doi: 10.1128/jvi.56.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A., Wahle E. Poly(A) tail metabolism and function in eucaryotes. J Biol Chem. 1993 Nov 5;268(31):22955–22958. [PubMed] [Google Scholar]
- Senear D. F., Brenowitz M. Determination of binding constants for cooperative site-specific protein-DNA interactions using the gel mobility-shift assay. J Biol Chem. 1991 Jul 25;266(21):13661–13671. [PubMed] [Google Scholar]
- Siomi H., Matunis M. J., Michael W. M., Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993 Mar 11;21(5):1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N. M., Gomatos P. J. Binding to selected regions of reovirus mRNAs by a nonstructural reovirus protein. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3457–3461. doi: 10.1073/pnas.79.11.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Steitz T. A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990 Aug;23(3):205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Yolken R., Arango-Jaramillo S., Eiden J., Vonderfecht S. Lack of genomic reassortment following infection of infant rats with group A and group B rotaviruses. J Infect Dis. 1988 Nov;158(5):1120–1123. doi: 10.1093/infdis/158.5.1120. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Thomas C., Bremer C., Roy P. Deletion and mutational analyses of bluetongue virus NS2 protein indicate that the amino but not the carboxy terminus of the protein is critical for RNA-protein interactions. J Virol. 1994 Apr;68(4):2179–2185. doi: 10.1128/jvi.68.4.2179-2185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staden V., Theron J., Greyling B. J., Huismans H., Nel L. H. A comparison of the nucleotide sequences of cognate NS2 genes of three different orbiviruses. Virology. 1991 Nov;185(1):500–504. doi: 10.1016/0042-6822(91)90808-o. [DOI] [PubMed] [Google Scholar]