Abstract
Context
Depression is common in cardiac patients, especially in patients with heart failure (HF), and is associated with increased risk for adverse health outcomes. There also is a growing literature to suggest that aerobic exercise may reduce depressive symptoms, but no previous study has evaluated the effects of exercise on depression in HF patients.
Objective
To determine if exercise training in HF patients will result in greater improvements in depressive symptoms compared with usual care.
Design
Multicenter, randomized (1:1) controlled trial
Setting
Ambulatory in 82 clinical centers in the US, Canada, and France.
Participants
2,322 stable patients with a left ventricular ejection fraction (LVEF) ≤35% and NYHA class II to IV HF who completed the Beck Depression Inventory-II (BDI-II) to assess depressive symptoms (range 0-63; clinically significant ≥ 14).
Interventions
Supervised aerobic exercise (goal of 90 min/wk for months 1-3) followed by home exercise (goal of ≥120 min/wk for months 4-12), versus education and usual, guideline-based HF care conducted between April, 2003 and February, 2007.
Main Outcome Measures
Scores on the BDI-II at 3- and 12-months and the composite of death or hospitalization from any cause.
Results
789 (68%) patients died or were hospitalized in the usual care (UC) arm and 759 (66%) in the aerobic exercise (AE) arm (Hazard Ratio [HR] = 0.89, 95% CI = 0.81, 0.99; p=.03) over a median follow-up period of 30 months. The median BDI-II score at study entry was 8, with 28% of the sample obtaining BDI-II scores ≥14. Compared to UC, AE resulted in lower mean BDI-II scores at 3-months, AE = 8.95 (95% CI =8.61, 9.29) vs. 9.70 (95% CI = 9.34, 10.06) for UC (difference =−0.76,95 % CI= −1.22, −0.29, p = .002), and at 12-months, AE= 8.86 (95%CI= 8.67, 9.24) vs. 9.54 (95% CI = 9.15, 9.92) for UC (difference = −0.68, 95% CI = −1.20, −0.16; p = .01).
Conclusions
Compared to guideline-based usual care, exercise training resulted in reduced depressive symptoms and better clinical outcomes.
An estimated 5 million people in the United States have heart failure (HF), and more than 500,000 new cases are diagnosed annually (1). HF is associated with increased morbidity and mortality and compromised quality of life. Indeed, clinical depression is a common comorbidity, affecting as many as 40% of HF patients (2), with up to 75% of patients reporting elevated depressive symptoms (3). Depression also is associated with worse clinical outcomes in a variety of cardiac patient populations including myocardial infarction, unstable angina, and coronary bypass surgery (4-6). Recent reports from our group and others also have reported an association between depression and increased risk of adverse events in HF patients (7-10). Despite this increased risk, however, there have been few randomized trials to treat depression in patients with HF. In the Sertraline Against Depression and Heart Disease-Heart Failure (SADHART-CHF) trial, reductions in depressive symptoms were not greater in patients receiving sertraline compared to placebo controls, and there was no effect of treating depression on clinical outcomes (11).
Aerobic exercise has been proposed as an alternative treatment for depression (12), and may be comparable to established pharmacologic therapies (13,14). The Heart Failure - A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study reported that exercise training, when added to usual, evidence-based care, produced modest reductions in all-cause mortality and all-cause hospitalization (15). This HF-ACTION ancillary study was designed to assess the effects of exercise on depressive symptoms and to determine if reduced depressive symptoms were associated with improved clinical outcomes.
METHODS
Eligibility and Trial Overview
A description of the methods and primary results of the HF-ACTION trial has been published previously (15,16). Briefly, HF-ACTION was a multicenter, randomized clinical trial of exercise training versus usual care in patients with left ventricular ejection fraction (LVEF) ≤35% and New York Heart Association (NYHA) class II to IV symptoms despite optimal HF therapy for at least 6 weeks. Patients were recruited from 82 centers within the United States, Canada, and France. Race and ethnicity were documented by self-report (i.e., white, Hispanic, Black/African American, Native American, Asian, Hawaiian/Pacific Islander) per NIH reporting guidelines. The protocol was approved by the respective Institutional Review Boards or ethics committees for each of the clinical sites and the coordinating center. All patients voluntarily provided written informed consent. The study was conducted between April 2003 and February 2007. After an initial baseline assessment, patients were randomized 1:1 to either aerobic exercise training (AE) or usual care (UC). A permuted block randomization scheme stratified by clinical center and by HF etiology (i.e., ischemic versus non-ischemic) was used. Ischemic etiology was defined as the presence of at least one of the four following criteria: 1) angiographic evidence of ≥ 75% lesion in one or more of the three major epicardial vessels; 2) history of MI; 3) history of revascularization procedure; or 4) evidence of significant perfusion defect in the setting of ischemic symptoms. The primary medical endpoint was all-cause mortality and/or hospitalization, while the primary psychological endpoint was depressive symptoms assessed after 3 months of supervised exercise. Secondary medical endpoints included cardiovascular (CV) mortality and hospitalization and HF mortality and HF hospitalization; the secondary psychological endpoint was depressive symptoms assessed at 12 months.
Assessment procedures
Exercise testing
All patients underwent baseline exercise stress testing under continuous electrocardiographic monitoring with direct measurement of oxygen consumption to document aerobic fitness. Tests were reviewed by investigators to identify significant arrhythmias or ischemia that would prevent safe exercise training and to establish appropriate training heart rate ranges.
Assessment of depression
Depression was assessed by the Beck Depression Inventory-II (BDI-II) (17). The BDI-II is a 21-item, self-report measure of depressive symptoms using a 0-3 scale (range 0-63). Each item asks about a particular symptom of depression, as outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (18). The BDI-II has been widely studied in cardiac patients and has excellent psychometric properties including a test-retest reliability coefficient of 0.93. A score of 14 or greater is considered to reflect clinically significant depressive symptoms; a 50% reduction in clinical symptoms is generally considered to be clinically meaningful in trials of patients with major depression (19), but a clinically significant difference in BDI-II scores between two treatment groups has not been established.
Interventions
Aerobic Exercise (AE)
Patients randomized to AE participated in 3 supervised exercise sessions per week for 3 months; patients exercised on a treadmill or stationary cycle ergometer as their primary training mode. Patients were encouraged to begin home-based exercise after 18 supervised sessions and to fully transition to home exercise after 36 supervised sessions. Patients were provided home exercise equipment of their choosing (cycle or treadmill; [ICON; Logan, UT]) and heart rate monitors (Polar USA, Inc., New York, NY). The primary index of adherence was weekly volume of self-reported exercise (in minutes). Full adherence was defined a priori as ≥ 90 min/week of supervised exercise during months 1-3 and ≥ 120 min/week of home-based exercise during months 4-12.
Usual Care (UC)
Patients randomized to UC were not provided with a formal exercise prescription. All patients, regardless of treatment arm, received detailed self-management educational materials at the time of enrollment, including information on medications, fluid management, symptom exacerbation, sodium intake, and activity recommendations of 30 minutes of moderate-intensity activity on most days of the week, consistent with the American College of Cardiology/American Heart Association guidelines (20).
Follow-up
Patients were asked to return for clinic visits every 3 months for the first 2 years of participation and yearly thereafter up to 4 years. Depression was assessed by repeat administration of the BDI-II at 3-month intervals for the first year. To provide comparable levels of attention from study personnel in the AE and UC arms, patients were called every 2 weeks for the first 9 months, monthly until 24 months of follow-up, and quarterly thereafter. During these calls, patients in the AE arm were asked if they were performing the exercise training regimen as prescribed. Antidepressant medication use was recorded at baseline and 12 months. Patients in the UC arm were asked if they were exercising, but because of concern that inquiring about exercise could unintentionally promote exercise, no quantification of exercise was obtained.
Patients made their final visit at the end of the study follow-up period or at 4 years, whichever came first. Follow-up was completed on March 15, 2008. For patients lost to follow-up, searches of the Social Security Death Index and the National Death Index were performed to assess whether any of these patients had died.
Primary and Secondary Outcomes
The primary medical endpoint was a composite of all-cause mortality or all-cause hospitalization; cardiovascular (CV) mortality and hospitalization and HF mortality and hospitalization served as combined secondary medical endpoints. The primary psychological endpoint was BDI-II score at 3-months (i.e., at the completion of the supervised exercise phase of the study); BDI-II scores at 12-months served as a secondary endpoint. We also explored the relationship between exercise adherence and BDI-II scores. Patients and investigators were blinded with respect to their BDI-II scores. Deaths and hospitalizations were adjudicated by a Clinical Endpoint Committee, blinded to treatment assignment and BDI-II scores. Once the patient had an adjudicated HF hospitalization, no further hospitalizations for the patient were reviewed. Event times were calculated as the time elapsed from randomization to the first event or to censoring (time to last contact).
Statistical Analysis
Sample characteristics were described as median and interquartile range (IQR) for continuous variables and frequency and percent for categorical variables. To examine the effect of exercise on BDI-II scores at 3-and 12-months, we used two separate general linear models (GLMs), in which treatment group assignment predicted post-treatment BDI-II scores. We selected, a priori, age, gender, race, smoking, mitral valve regurgitation grade, Weber class (i.e., aerobic capacity), NYHA class, diabetes, use of antidepressant medication, and pretreatment BDI-II score as adjustment covariates. We supplemented this analysis by exploring the same model with the sample restricted to participants with clinically significant levels of depressive symptoms (BDI-II ≥ 14) at baseline. Theses analyses adhered to the intent-to-treat principle, using SAS PROC MI to impute missing data. Number needed to treat was calculated using adjusted BDI-II values generated from the above GLMs. We also used GLMs to examine the association between self-reported minutes of exercise and BDI-II scores at 3- and 12-months. Because self-reported minutes of exercise were not recorded in the UC group, the analysis was limited to only AE participants. In this model, we included the following baseline variables selected a priori: BDI-II, gender, age, race, smoking status, blood urea nitrogen, LVEF, NYHA class, hypertension, diabetes, six minute walk distance, Weber score, Kansas City Cardiomyopathy Questionnaire score (21), site, beta blockade dose, and mitral valve regurgitation, ventricular conduction status, and use of any antidepressant medication. We also estimated a Cox regression model (22) to investigate the association between self-reported minutes of exercise and the primary and secondary clinical endpoints. These latter models included the same covariates described above in the analysis of minutes of exercise and BDI-II scores.
Finally, we estimated two sets of Cox regression models that examined the association between the baseline BDI-II scores and the primary clinical endpoint and the change in BDI-II scores from baseline to 3-months with the clinical endpoints. In the latter model, we limited our analysis to clinical events that occurred after the end of the 3-month treatment period. The models included the same covariates described above in the analysis of exercise minutes and BDI-II scores. With the exception of the GLMs for the 3- and 12-month depression outcomes, adjustment covariates with missing data were imputed using the median of the variable. For key predictor variables that were measured as continuous variables (minutes of exercise, BDI-II scores) we tested for potential nonlinearity using restricted cubic splines with 3 knots (23).
The sample size for the main trial was calculated for the primary medical endpoint such that there was 90% power to detect an 11% reduction associated with treatment in 2-year all-cause mortality or all-cause hospitalization. We also calculated the detectable effect size for treatment on the BDI-II scores, given the known available sample size. With a sample size of 2322, assuming a standard deviation of 10 on the BDI-II and a two-sided significance level of .05, we estimated that we would have 80% power to detect a treatment group difference of about 1 point on the BDI-II. All analyses used a two-sided test for significance at an alpha of .05. Analyses were carried out using SAS (SAS Institute, Cary, NC) and the rms package in R (http://www.r-project.org/).
RESULTS
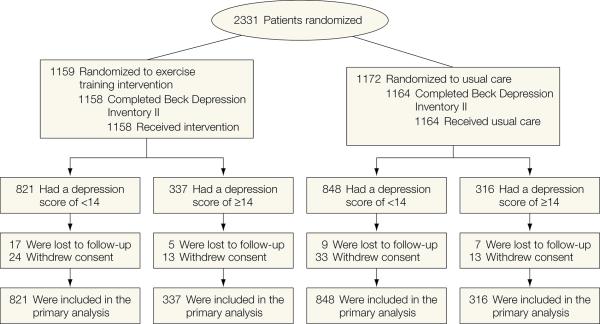
The sample was comprised of 2322 participants (99% of the original sample of 2331 patients) who completed the BDI-II at baseline (pre-treatment). Figure 1 displays the patient flow through the study. Table 1 displays the background demographic and clinical characteristics of the sample for each treatment group, stratified by baseline depressive symptom severity. The median age of the participants was 59 years (IQR= 51-68, range = 19-91). The majority of participants were white, male, married, and achieved at least a high school education (see Table 1). The median BDI-II score at study entry was 8 (IQR = 4-15, , range= 0 -59); BDI-II scores ≥14 are considered clinically significant (17), which was noted in 28% of the sample. The AE and UC groups were generally comparable with respect to baseline demographic and clinical characteristics. A slightly higher proportion of AE participants had a greater than high school education compared to UC (62% vs. 58%) and achieved a shorter distance on the six minute walk test (366 vs. 373 meters). Participants with more severe depressive symptoms at baseline were more likely to be taking an antidepressant medication, and also were more likely to have more severe HF, although these differences were comparable across treatment groups.(See eTable 1 in online supplement for a more detailed description of baseline variables).
Figure 1.
Flow of participants through trial.
Table 1.
Baseline Demographic and Clinical Characteristics of Sample
| Exercise (N = 1158) | Usual Care (N = 1164) | ||||||
|---|---|---|---|---|---|---|---|
| N with nonmissing data | BDI-II < 14 (N=821) | BDI-II ≥ 14 (N=337) | All Exercise Participants | BDI-II < 14 (N=848) | BDI-II ≥ 14 (N=316) | All Usual Care Participants | |
| Age [Years] | 2322 | 61 (52, 69) | 56 (50, 64) | 59 (51, 68) | 61 (53, 69) | 56 (47, 63) | 59 (51, 68) |
| Race : | 2288 | ||||||
| Black | 32% (262) | 35% (115) | 33% (377) | 30% (254) | 37% (116) | 33% (377) | |
| White | 62% (503) | 59% (194) | 61% (697) | 64% (539) | 59% (184) | 61% (697) | |
| Other | 5% (43) | 7% (22) | 6% (65) | 5% (45) | 4% (11) | 6% (65) | |
| Sex : Women | 2322 | 31% (252) | 28% (94) | 30% (346) | 26% (220) | 28% (90) | 30% (346) |
| Region : US | 2322 | 88% (723) | 90% (303) | 89% (1026) | 88% (744) | 92% (292) | 89% (1026) |
| Marital Status: Married | 2315 | 59% (481) | 53% (178) | 57% (659) | 61% (513) | 52% (165) | 58% (678) |
| Education: > High School | 2271 | 63% (505) | 57% (189) | 62% (694) | 61% (500) | 51% (161) | 58% (661) |
| Income: > $25K | 2067 | 62% (460) | 52% (156) | 59% (616) | 62% (459) | 50% (144) | 59% (603) |
| BDI-II | 2322 | 6 (4, 9) | 20 (16, 25) | 8 (5, 15) | 6 (3, 9) | 20 (17, 25) | 8 (4, 15) |
| Peak VO2 [mL/kg/min] | 2268 | 15 (12, 18) | 14 (11, 17) | 14 (11, 18) | 15 (12, 18) | 14 (11, 17) | 14 (12, 18) |
| Body Mass Index [kg/m2] | 2316 | 30 (26, 34) | 31 (27, 36) | 30 (26, 35) | 29 (26, 34) | 32 (27, 37) | 30 (26, 35) |
| Diabetes | 2322 | 32% (261) | 35% (117) | 33% (378) | 30% (255) | 36% (115) | 32% (370) |
| Hypertension | 2309 | 62% (504) | 61% (207) | 62% (711) | 57% (483) | 61% (190) | 58% (673) |
| Current Smoking | 2311 | 14% (116) | 20% (68) | 16% (184) | 16% (133) | 21% (66) | 17% (199) |
| NYHA Class: III or IV | 2322 | 31% (258) | 53% (177) | 38% (435) | 32% (269) | 47% (147) | 36% (416) |
| Angina class: None | 2319 | 85% (694) | 78% (261) | 83% (955) | 87% (735) | 80% (252) | 85% (987) |
| CHF Etiology: Non-Ischemic | 2322 | 49% (399) | 48% (162) | 48% (561) | 47% (396) | 55% (173) | 49% (569) |
| LVEF | 2318 | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) |
| Six Minute Walk [meters] | 2271 | 377 (300, 442) | 351 (279, 420) | 366 (296, 436) | 380 (310, 440) | 354 (266,) 420 | 373 (300, 432) |
| Mitral Valve Regurgitation: High | 2129 | 11% (81) | 14% (42) | 12% (123) | 11% (88) | 15% (44) | 12% (132) |
| Ventricular Conduction: Normal | 2263 | 44% (352) | 42% (137) | 43% (489) | 44% (360) | 41% (127) | 43% (487) |
| KCCQ Score | 2322 | 75 (61, 86) | 48 (37, 62) | 68 (50, 82) | 76 (61, 88)) | 48 (36, 58) | 69 (52, 84) |
| Weber Score | 2268 | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) |
| Blood Urea Nitrogen | 2020 | 21 (16, 28) | 18 (15, 28) | 20 (15, 28) | 19 (15, 28) | 21 (15, 29) | 21 (15, 28) |
| Beta Blockade Dose [mg/day] | 2302 | 25 (13, 50) | 50 (13, 50) | 38 (13, 50) | 28 (13, 50) | 25 (13, 50) | 25 (13, 50) |
| Loop Diuretic Dose [mg/day] | 2289 | 40 (1, 80) | 40 (20, 80) | 40 (20, 80) | 40 (20, 80) | 40 (20, 80) | 40 (20, 80) |
| Any antidepressant | 2322 | 16% (131) | 35% (117) | 21% (248) | 17% (149) | 40% (129) | 23% (278) |
Values are median (25th, 75th percentile) for continuous variables and % (N) of column for categorical variables.
BDI-II: Beck Depression Inventory II
NYHA: New York Heart Association
LVEF: Left ventricular ejection fraction
KCCQ: Kansas City Cardiomyopathy Questionnaire is a 23-item survey to assess quality of life in HF patients. Higher scores reflect better quality of life.
Weber Score: Weber scores reflect levels of aerobic capacity with A (peak VO2 > 20 mL/kg/min); B (peak VO2 > 16 and ≤ 20); C (peak VO2 > 10 and ≤ 16); D (peak VO2 ≤ 10).
Adherence to exercise protocol
As reported in the primary paper (15), patients in the AE group exercised for a median of 76 min/wk during the first 3 months of supervised exercise, increased to a median of 95 min/wk during months 4-6, and then decreased to 74 min/wk at months 10-12. During the supervised phase (i.e., months 0-3), 41% of the sample achieved full adherence (defined as ≥ 90-min/wk), while full adherence (defined as >120 min/wk) during the home-based phase was achieved in 42% of the sample in months 4-6, 41% of the sample achieved full adherence in months 7-9, and 38% of the sample achieved full adherence in months 10-12.
Cardiopulmonary changes with treatment
After 3 months, participants in the AE group increased their peak oxygen consumption (VO2peak) by 0.6 ml/kg/min compared to 0.2 ml/kg/min in the UC group (p< 0.001); AE participants further increased their VO2peak by 0.7 ml/kg/min from baseline to 12 months compared to 0.1 ml/kg/min for UC (p <0.001).
Effects of exercise on depressive symptoms
BDI-II scores were available for 2322 participants at baseline, 2019 at 3-months and 1738 at 12-months. The adjusted 3-month BDI-II mean score was 8.95 (95% CI = 8.61, 9.29) for the AE group and 9.70 (95% CI = 9.34, 10.06) for UC (difference =−0.76, 95 % CI= −1.22, −0.29, p = .002). The adjusted BDI-II score at 12-months was 8.86 (95% CI= 8.67, 9.24) for the AE group and 9.54 (95% CI = 9.15, 9.92) for UC (difference = −0.68, 95% CI = −1.20, −0.16; p = .01). The estimated number needed to treat for the BDI-II outcome at 3 months was 49 (95% CI = 20, infinity) and 35 (95% CI = 17, 4646) for the 12-month BDI-II outcome. We also examined the treatment effects within the subset of patients with clinically significant depressive symptoms (baseline BDI-II scores ≥14). In this case, BDI-II scores at 3 months were lower for patients in the AE group (Mean = 16.66; [95% CI = 15.78, 17.53]) compared to patients in UC (Mean = 17.98; [95% CI = 17.04, 18.91]); p = .04, difference= −1.31 [95% CI = −2.54, −0.09]), and also lower after 12-months for depressed patients in the AE group (Mean=15.85 [95% CI = 14.90, 16.78] compared to depressed patients in UC (Mean=17.34 [95% CI = 16.34, 18.34];p = .02, difference= −1.56 [95% CI = −2.84, −0.27]). Based on this subsetted analysis, using BDI-II ≥ 14 as the cutoff, the number needed to treat was 6 (95%CI = 4, 9) and 5 (95% CI = 4, 7) for 3- and 12-months respectively.
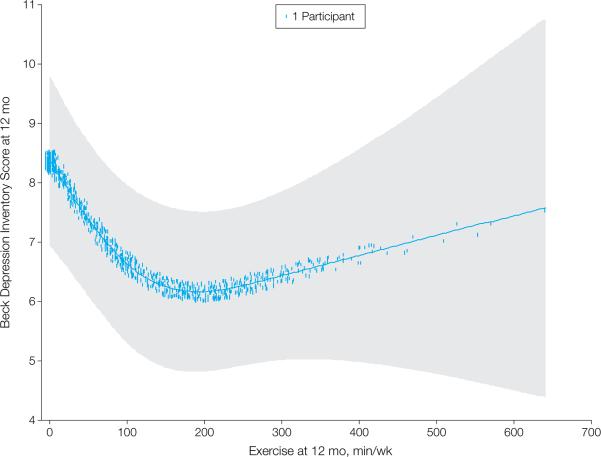
We also explored the association between self-reported minutes of exercise per week and BDI-II scores at 3-months and 12-months. Because detailed exercise information was available for only the participants in the AE group, participants in the UC group were excluded from these analyses, leaving 814 participants with complete data for the 3-month analysis and 629 for the 12-month analysis. Volume of exercise (in minutes) at 3-months was inversely related to depressive symptoms at 3-months. Compared to a participant reporting no exercise, a participant reporting 90 minutes of exercise per week could be expected to have a 1.55-point lower BDI-II score at 3 months (95% CI = −2.88, −0.33, p = 0.001) and a 1.67-point lower BDI-II score at 12-months (95% CI = −2.62, −0.73, p = 0.001). Self-reported exercise at 12- months was associated with BDI-II scores at 12-months in a nonlinear fashion (p = .003) (Figure 2.) The nonlinear effect was such that exercise time beyond 90 minutes of exercise per week appeared to provide little added benefit.
Figure 2.
Nonlinear association between self-reported weekly minutes of exercise (from months 9-12) and BDI-II scores at 12 months, adjusted for gender, age, race, smoking status, blood urea nitrogen, LVEF, NYHA class, hypertension, diabetes, six minute walk distance, Weber score, Kansas City Cardiomyopathy Questionnaire score (21), site, beta blockade dose, and mitral valve regurgitation, ventricular conduction status, use of any antidepressant medication, baseline BDI-II scores, and BDI-II scores at 3 months. The weekly minutes term in the model was fitted using a 3-knot restricted cubic spline. The analysis is limited to participants in the AE condition only and with complete BDI-II data at 12 months (N = 629). Fitted line is for a typical participant (median of continuous covariates, most prevalent class for categorical variables). Shaded area represents 95% CI and hatch marks represent case density, with each dot representing a case. Some density dots extend beyond x-axis range in order to display the cases more clearly. Comparing a participant who reported 90 minutes of exercise per week to a participant who reported 0 minutes per week revealed a regression coefficient of −1.67 (95% CI = −2.62, −0.73).
Relation of exercise and subsequent clinical events
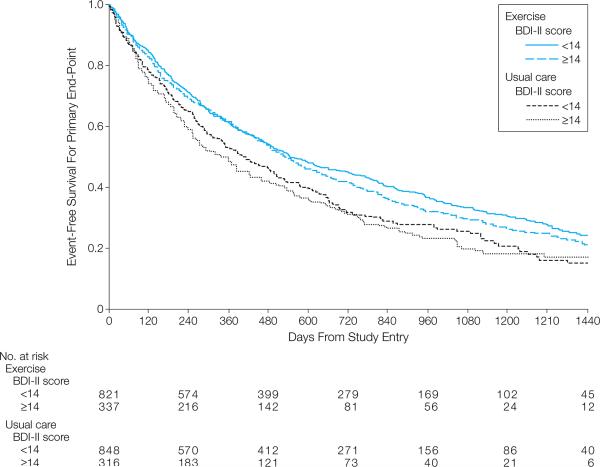
Table 2 displays the frequency for the primary endpoint and two secondary endpoints for each treatment group stratified on BDI-II severity at baseline. Among the 2322 cases available for the analysis of the primary clinical endpoint (all-cause death or first all-cause hospitalization), we observed 1548 events (386 deaths and 1162 hospitalizations) over a median follow-up time of 30 months (IQR = 5.3-27, range = 0-49). Figure 3 displays Kaplan-Meier curves for event-free survival for the primary endpoint, stratified by treatment group and BDI-II severity categories (BDI-II < 14, BDI-II ≥ 14). The Cox regression model revealed that AE was associated with a lower risk for an event compared to UC (HR = 0.89, 95%CI = 0.81, 0.99, p = .03). AE also was associated with lower risk for the secondary endpoint of HF hospitalizations and death (HR = 0.85, 95% CI = 0.73, 0.98, p = .03) compared to UC, and tended to be associated with a lower risk of CV hospitalization and death (HR = 0.91, 95% CI = 0.81, 1.01, p = .09).
Table 2.
Primary and secondary event rates by treatment assignment and depressive symptom severity
| Treatment Assignment |
||||||
|---|---|---|---|---|---|---|
| Exercise | Usual Care | |||||
| BDI-II < 14 N = 821 | BDI-II ≥ 14 N = 337 | All Exercise N = 1158 | BDI-II < 14 N = 848 | BDI-II ≥ 14 N = 316 | All Usual Care N = 1164 | |
| All-cause: | ||||||
| Deaths | 123 (15.0%) | 66 (19.6) | 189 (16.3%) | 134 (15.8%) | 63 (19.9%) | 197 (16.9%) |
| Hospitalizations | 393 (47.9%) | 177 (52.5%) | 570 (49.2%) | 423 (49.9%) | 169 (53.5%) | 592 (50.9%) |
| Total | 516 (62.9%) | 243 (72.1) | 759 (49.0) | 557 (65.7%) | 232 (73.4%) | 789 (51.0%) |
| Heart Failure-related: | ||||||
| Deaths | 8 (1.0%) | 5 (1.5%) | 13 (1.1%) | 13 (1.5%) | 7 (2.2%) | 20 (1.7%) |
| Hospitalizations | 174 (21.8%) | 105 (32.2%) | 284 (24.5%) | 214 (25.2%) | 99 (31.3%) | 313 (26.9%) |
| Total | 182 (22.2%) | 110 (32.6%) | 297 (25.7%) | 227 (26.8%) | 106 (33.5%) | 333 (28.6%) |
| Cardiovascular-related: | ||||||
| Deaths | 40 (4.9%) | 9 (2.7%) | 49 (4.2%) | 38 (4.5%) | 15 (4.8%) | 53 (4.6%) |
| Hospitalizations | 382 (46.5%) | 202 (60.0%) | 584 (50.4%) | 433 (51.1%) | 181 (57.3%) | 619 (53.2%) |
| Total | 422 (51.4%) | 211 (62.6%) | 633 (54.7%) | 471 (55.5%) | 196 (62.0%) | 672 (57.7%) |
Note: Heart failure-related and cardiovascular-related deaths include deaths of undetermined cause.
Figure 3.
Unadjusted Kaplan-Meier curves for time to composite endpoint (all-cause death or first all-cause hospitalization). Curves represent sample stratified on treatment group assignment (blue = Aerobic Exercise, red = Usual Care) and depressive symptom severity category (solid line = BDI-II < 14, dashed line = BDI-II ≥ 14). The number at risk for each group is displayed across the bottom of the plot.
Relation of depressive symptoms and subsequent clinical events
Higher baseline BDI-II was associated with increased event risk. Because BDI-II scores were modeled as a continuous variable, we elected to scale it in the Cox model such that the HR compared a non-depressed participant (BDI-II score of 4, the value at the 25th percentile) with a participant with mild clinical depression (BDI-II ≥ 14). The HR for this BDI-II scaling was 1.16 (95% CI = 1.05, 1.29, p = .01). Participants with higher levels of depression also were at increased risk for HF death and HF hospitalizations. Again, comparing a participant with a BDI-II score of 14 with a participant with score of 4 resulted in a HR of 1.20 (95% CI = 1.03, 1.40, p = .02). Similarly, for the combined secondary CVD endpoint, comparing a typical participant with a BDI-II score of 14 at baseline to a participant with a score of 4, the HR was 1.17 (95% CI = 1.04, 1.31, p = .003).
We also examined the association of change in BDI-II from baseline to three months and the time to all-cause death or first hospitalization by adding the raw change in BDI-II (from baseline to three months) to the primary model, maintaining baseline BDI-II in the model. After excluding persons who had no BDI-II data at 3 months, 2009 patients were available for analysis, 1289 of whom suffered an event during the subsequent follow-up period. The treatment group by BDI-II change interaction was not significant (p = 0.73), suggesting that the relationship between change in depressive symptoms and the primary endpoint was similar for the AE and UC groups. After adjusting for covariates (including baseline BDI-II scores and antidepressant medication use), we observed a significant association between BDI-II change and all cause death or hospitalization (p =.02) (eFigure 1 in supplement). We examined two local HRs (and 95% CIs) using predicted values from the Cox model; one HR reflecting a comparison of a participant who improved on the BDI-II versus no change (−10 point change vs 0 change), and a second comparing a participant who worsened versus no change (+10 points vs. 0 change). The HR for improvement in BDI-II was 0.92 (95% CI= 0.79, 1.06), while the HR for BDI-II worsening was 1.21 (95% CI= 1.03, 1.43). A similar pattern was seen for BDI-II change and the two secondary endpoints, combined CV death and hospitalization (p = .003) and HF death and hospitalization (p = .001). For the combined CV endpoints, the HR for improvement was 0.87 (95% CI= 0.74, 1.02), while the HR for worsening was 1.27 (95%CI= 1.06, 1.51). For the combined HF endpoints, the HR for improvement was 0.88, (95%CI= 0.72, 1.09), while the HR for worsening was 1.43 (95% CI= 1.15, 1.78).
DISCUSSION
The results of this HF-ACTION ancillary study confirm and extend previous research by demonstrating that exercise training may be effective in reducing depressive symptoms and by further documenting the prognostic significance of depression in HF patients. While previous studies have reported that exercise is associated with reduced symptoms of depression in patients with clinical depression (12,24), to our knowledge, this is the first randomized trial to show that exercise resulted in a significant reduction in depressive symptoms in HF patients. After 3 months of supervised exercise, patients in the AE condition achieved a 1.75-point reduction in BDI-II scores compared to 0.98-points in UC controls; differences were even larger for patients with BDI-II scores ≥14. Patients in the AE condition continued to exhibit greater reductions in depressive symptoms during months 4-12, in which they engaged in home-based exercise, showing a 2.2-point reduction from baseline compared to 1.3-points in UC. The difference between exercise and control is modest and its clinical significance is not known. However, because the difference was consistent over 12 months suggests that the difference is robust and does not simply reflect daily fluctuations in symptoms but is likely to be associated with better social functioning and higher quality of life. It also should be noted that among patients with BDI-II scores ≥ 14 at baseline, the difference between AE and UC was 1.3 and 1.6 points at three and twelve months, respectively, which is comparable to placebo-control trials of patients with MDD. In the SADHART trial, the difference between placebo and sertraline was 0.8 points on the HAM-D (25) and in the SADHART-CHF the difference was 0.3 points (11). In the ENRICHD trial (26), patients receiving CBT showed a 2.7-point greater reduction in BDI scores compared to usual care controls. However, ENRICHD patients were clinically depressed and received treatment (CBT and anti-depressant medication) to reduce their depression. Participants in the HF-ACTION trial were not diagnosed with MDD and most were not clinically depressed. Furthermore, participants in UC received biweekly phone calls from study staff and 23% of UC participants received antidepressant medication and 48% indicated that they engaged in regular (up to twice per week) exercise during the trial.
Within the AE group, patients who reported greater adherence to the exercise prescription achieved even larger reductions in depressive symptoms. Compared to no exercise, 90 minutes of exercise per week, the equivalent of 3, 30-minute sessions, was associated with more than a 1.5 point reduction in BDI-II scores. These differences, albeit relatively modest, are not insignificant, particularly in light of the fact that relatively few patients reported elevated depressive symptoms to begin with. It should be noted, however, that people who did not exercise may have been more depressed, so that it is not clear if exercise resulted in lower depression, or if depression resulted in less exercise.
Several prior studies have shown that exercise training may be comparable to antidepressant medication in reducing depressive symptoms (13,14). However, to date, the benefits of antidepressant medication in cardiac patients have provided mixed results. In the SADHART study, only the subgroup of patients with more severe depression appeared to benefit from sertraline (25). Results from the SADHART-CHF trial showed that sertraline performed no better than placebo after 12 weeks of treatment, and did not improve clinical outcomes (11). In the present study, 90 minutes of exercise per week was sufficient to reduce depressive symptoms; greater volumes of exercise were not necessarily associated with greater improvements in depressive symptoms. The optimal dose of exercise to achieve benefit remains uncertain. Prior studies in patients with major depression reported that 90 minutes of exercise per week was sufficient to reduce depressive symptoms (13,14) and maintenance of 60 min/week of exercise reduced the risk of relapse over a 1-year follow up period (27). Data from the present study must be interpreted with caution, since patients self-selected their exercise dose; however, a small study of depressed, non-cardiac patients suggested that 150 min/wk of exercise was most effective in reducing depressive symptoms (28). The optimal dose of exercise needed to achieve the maximal therapeutic benefit in reducing depressive symptoms in cardiac patients still needs to be determined.
We also observed that elevated depressive symptoms were associated with more than a 20% increase in risk for all-cause mortality and hospitalizations, and that the increased risk was independent of antidepressant use and established risk factors in HF patients including age and disease severity. These data add to a growing body of evidence that elevated depressive symptoms, without necessarily meeting diagnostic criteria for major depressive disorder, are associated with increased risk for adverse clinical events (29,30). These findings also support the recent recommendations of the American Heart Association Scientific Advisory Board, which recommended that depression be routinely assessed in cardiac patients (31).
Examination of the relationship of changes in depression and clinical outcomes revealed that patients whose depression worsened over time were at particularly increased risk. These findings are consistent with our prior study of 147 HF patients in which patients who had an increase in BDI-I scores of 3 points or more after 1 year were at more than twice the risk of adverse outcomes then patients whose BDI score remained relatively stable (32). A recent substudy from SADHART-CHF (33) found that those depressed HF patients who were considered remitted after 12 weeks of treatment had fewer cardiovascular events compared to non-remitted patients, independent of treatment. While it is possible that reduced depressive symptoms were responsible for improved clinical outcomes, it is also possible that worsening of depression may identify a subset of depressed patients who may be vulnerable to adverse events. Thus, efforts not only to reduce depressive symptoms but to prevent worsening of depressive symptoms may be especially important. In addition to its cardiopulmonary benefits, exercise may be effective in reducing depressive symptoms and preventing worsening of symptoms in HF patients.
Limitations
Patients enrolled in HF-ACTION had to been willing and able to engage in aerobic exercise. Because patients who either were already exercising or who were unwilling to be randomized to an exercise condition were excluded from the trial, the generalizability of the results may be limited. Second, although this was a planned secondary analysis of HF-ACTION, the design was a retrospective analysis of prospectively collected data and participants were not randomized to different pre-specified exercise volumes. Thus, it is possible that patients who were healthier or more motivated to adhere to treatment, including medications, might have been better able to engage in exercise. The observed association between minutes of exercise and reduced depressive symptoms could be a result of the beneficial effects of greater volumes of exercise in reducing depressive symptoms, but also could reflect that patients with more severe depressive symptoms may be less likely to engage in exercise. An adequately powered and appropriately designed dose-response trial would be needed to determine the optimal dose of exercise needed to reduce depressive symptoms to clinically significant levels. Third, due to missing data for some of the clinical parameters measured in HF-ACTION, we used simple median imputation for the time-to-event analyses, which allowed us to include a greater number of cases. The results of these analyses differed very little compared to simply omitting variables for which complete data were unavailable, however. Fourth, we used the BDI-II to assess depressive symptoms. While the BDI-II is widely recognized as a valid and reliable psychometric instrument for assessing depressive symptoms, and has been associated with increased risk of morbidity and mortality in a number of studies in cardiac patient populations (4,5,7), it should not be used to diagnose clinical depression. The extent to which exercise could improve depressive symptoms in patients with major depression was not assessed in this trial. BDI-II scores were also somewhat lower than expected. Using the BDI-I, Sherwood et al. (32) reported that more than half of the sample exhibited significant levels of depressive symptoms, compared to only 28% in the present sample. Moreover, Sherwood reported that the level of risk associated with elevated BDI scores was greater than the risk we observed in HF-ACTION. The reasons for this discrepancy are not obvious. Sixth, unplanned crossover may have affected our findings. Only about 40% of patients assigned to the AE condition were fully compliant with the exercise program in achieving pre-specified target exercise volumes (i.e., 90 min/week for months 1-3 and 120 min/wk for months 4-12), and 40-50% of UC patients reported that they had engaged in at least some exercise during the first year of the trial. Finally, while we suggested that worsening depressive symptoms may contribute to deteriorating HF, it also is possible that increasing depressive symptoms may reflect worsening HF. Whether worsening depression is a cause or consequence of increased HF symptoms could not be determined.
Supplementary Material
Acknowledgements
Dr. James Blumenthal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Michael Babyak was primarily responsible for the data analysis. He was compensated for his work by a grant from the National Heart, Lung and Blood Institute awarded to Dr. Blumenthal (Grant No. HL 080664). HF ACTION was funded by 13 grants from the National Heart Lung and Blood Institute, Bethesda, MD (Coordinating center/C. O'Connor, Principal Investigator: 5U01-HL063747; Economic and Quality of Life/K. Schulman, Principal Investigator: 5U01-HL066461; U grant enrolling centers—Boston Medical Center/W. Colucci, Principal Investigator: HL068973; Case Western Reserve University/ I. Piña, Principal Investigator: HL066501; Emory University/A. Smith, Principal Investigator: HL066482; Henry Ford Hospital/S. Keteyian, Principal Investigator: HL064250; Ohio State University/W. Abraham, Principal Investigator: HL066494; Oregon Health Science University/R. Hershberger, Principal Investigator: HL064257; University of Alabama/V. Bittner, Principal Investigator: HL066497; University of California–Los Angeles/G. Fonarow, Principal Investigator: HL068980; University of Colorado/E. Wolfel, Principal Investigator: HL064265; Wake Forest University/D. Kitzman, Principal Investigator: HL066491; Washington University of St Louis/G. Ewald, Principal Investigator: HL064264). This research also was supported by Grant No. HL 080664 from the National Institutes of Health, Bethesda, Maryland to Dr. Blumenthal. We especially wish to thank Stephen J. Ellis, Ph.D. from the Duke Clinical Research Institute (DCRI) for assembling the dataset used in this analysis and for providing statistical advice, for which he was compensated as part of his employment at the coordinating center, Yanhong Li, MA, also from the DCRI who provided endpoint data for this analysis, and Andrew Sherwood, Ph.D., from the Department of Psychiatry and Behavioral Sciences at Duke University for his comments and suggestions on an earlier version of this manuscript. The complete list of collaborators in HF-ACTION has been reported previously (15).
Role of the Sponsor: The National Heart, Lung and Blood Institute was involved in the design and conduct of the study; and in the collection and management and oversight of the data analysis. However, the funding source had no formal role in the interpretation of the data or in the preparation, review, or approval of the manuscript.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT00047437
References
- 1.Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Guck TP, Elsasser GN, Kavan MG, Barone EJ. Depression and congestive heart failure. Congestive Heart Failure. 2003;9(3):163–169. doi: 10.1111/j.1527-5299.2003.01356.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Col Cardiol. 2001;38(1):199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 4.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 5.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Internal Med. 2000;160:1354–1360. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal JA, Lett HS, Babyak MA, White W, Smith PK, Mark DB, Jones R, Mathew JP, Newman MF, for the NORG Investigators Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood A, Blumenthal JA, Trivedi R, et al. Relationship of depression to death or hospitalization in patients with heart failure. Arch Int Med. 2007;167(4):367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, et al. Congestive heart failure - Relationship between depressive symptoms and long-term mortality in patients with heart failure. A Heart J. 2007;154(1):102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Caridol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. 17. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev. 2009:CD004366. doi: 10.1002/14651858.CD004366.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano M, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan R. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal JA, Babyak MA, Doraiswamy M, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whellan D, O'Connor CM, Lee KL, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 18.American Psychiatric Association . Task Force on DSM-IV Diagnostic and statistical manual of mental disorders (DSM-IV-TR) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 19.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder:remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 20.Haskell WL, Lee I-M, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 22.Cox D. Regression models and life-tables. J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 23.Harrell FE. Regression modeling strategies. Springer; NY: 2001. [Google Scholar]
- 24.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–7. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr., Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM. Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- 26.Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, Coons MJ, Blumenthal JA. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med. 2011;73:127–133. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Bush DE, Ziegelstein RC, Tayback M, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol. 2001;88:337–41. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 30.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 31.Lichtman JH, Bigger JT, Jr, Blumenthal JA, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 32.Sherwood A, Blumenthal JA, Hinderliter AL, et al. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2011;57(4):418–423. doi: 10.1016/j.jacc.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Krishnan R, Kuchibhatla M, et al. Characteristics of depression remission and its relation with cardiovascular outcome among patients with chronic heart failure (from the SADHART-CHF Study). Am J Cardiol. 2011;107(4):545–551. doi: 10.1016/j.amjcard.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.