Abstract
Lung cancer is a complex, multifactorial disease which is the leading cause of cancer death in both men and women. NF-κB is a transcription factor which is known to affect the expression of more than 150 genes related to inflammation, lymphocyte activation, cell proliferation, differentiation, and apoptosis, as well as contributing to cell apoptosis and survival. However, NF-κBIA (IκBα) is the inhibitor of the transcription factor. The -94ins/delATTG polymorphism of the NF-κB1 gene promoter region which causes a functional effect and NF-κBIA 3′UTR A → G polymorphism has been shown to be related to various inflammatory diseases and cancer. Ninety-five NSCLC patients and 99 healthy controls were included in study. The NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G polymorphism have been studied by using PCR-RFLP method. It was found that the NF-κB1 -94ins/delATTG DD genotype and D allele frequencies were higher in patients than healthy controls and the presence of the DD genotype has a 3.5-fold increased risk of the disease (P: 0.014). This study is the first to investigate the NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G polymorphism together in the Turkish population. According to the results, the NF-κB1 -94ins/del ATTG promoter polymorphism may have a role in lung carcinogenesis and prognosis.
1. Introduction
Non-small cell lung cancer (NSCLC), which includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma, is the most common lung cancer possessing approximately 80–85% rates in the prevalence of lung cancer case [1]. Despite all advances in the current treatments including surgical resection, chemotherapy, and radiation therapy alone or in combination, the disease is rarely curable prognosis and remains poor [2]. As all of these facts are considered, recent researches are tending to understand molecular, biological, and genetic factors and to find other prognostic factors providing long-term survival and intending new targeted therapies [3, 4]. Lung cancer cells manage to escape from the signal transduction pathways to facilitate their own survival and proliferation by using multiple mechanisms [5]. Carcinogens and inflammatory cytokines contributing substantially to cancer development are involved in activation of common cell survival signaling pathways. The one of this cell survival signal is nuclear factor-kappaB (NF-κB) which is involved in multiple steps in carcinogenesis and in cancer cell's resistance to chemo- and radiotherapy [6]. Recently, many studies with animal models and cell culture systems indicate the interplay between NF-κB and lung carcinogenesis, which emphasizes the importance of targeting the NF-κB signaling pathway for lung cancer treatment and chemoprevention [7]. NF-κB, a nuclear transcription factor [8], was first identified in 1986 by Sen and Baltimore [9]. It was initially observed to be a transcription factor binding to the intronic enhancer of the kappa light chain gene (the κB site) in B cells, but it was later shown to be present in every cell type [10]. Afterwards, NF-κB emerged as a major regulator of more than 200 genes involved in diverse process such as cell survival and cell adhesion, inflammation, differentiation, and growth [11]. NF-κB is activated by phosphorylation of IκBα which is catalyzed by an IκBα Kinase (IKK) complex consisting of IkK-α, IkK-β, IkK-γ (also called NEMO), and other proteins yet to be identified [12, 13]. After activation of NF-κB, IκBα is degraded and p50–p65 heterodimer is translocated to the nucleus, binds to the DNA (at the promoter region), and activates gene [14, 15]. Currently, studies show that activation of the transcription factor nuclear factor (NF) κB is a novel mechanism of chemoresistance in NSCLC and other tumors [16, 17]. NF-κB1 is inhibited by IkB proteins (e.g., NF-κBIA) [18]. Phosphorylation of serine residues on the I-kappa-B proteins, by kinases and marks them for degradation, thereby allowing activation of the NF-κB complex [19]. As the first potential functional NF-κB1 genetic variation was identified 94 insertion/deletion ATTG located between two received key promoter regulatory elements in the NF-κB1 gene, ATTG deletion causes the loss of binding to nuclear proteins, which leads to reduced promoter activity [20]. The NF-κB -94ins/delATTG polymorphism of the NF-κB1 gene promoter region which causes a functional effect and NF-κBIA 3′UTR A → G polymorphism have been shown to be associated with various inflammatory diseases and cancers [21]. The aim of this study is firstly to investigate the NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G polymorphism together in the Turkish population.
2. Materials and Methods
2.1. Study Groups
Ninety-five primary non-small cell lung cancer (NSCLC) patients and 99 healthy individuals were included in the study. NSCLC patients were recruited from the Yedikule Chest Diseases and Thoracic Surgery Training Research Hospital, Istanbul. The diagnosis of NSCLC was made by the pathologist based on histopathological examination. In NSCLC group, all subjects were diagnosed and confirmed with histopathological examination. They were all newly diagnosed without a history of prior radiotherapy and/or chemotherapy. Exclusion criteria included primary extra pulmonary malignancy, small cell lung cancer, a history of malignant disease, and withdrawal of consent and patient aged less than 18. Pathological staging information on all NSCLC cases was confirmed by manual review of the pathology reports and clinical charts. Nodal status was categorized as no regional lymph nodes affected (N0) or at least one nodal metastasis. The mean ages of the patients and controls were 61.2 ± 9.83 years and 57.49 ± 10.83 years, respectively. The percentage of females was 6.3% for patients and 32.3% for controls, and percentage of males was 93.7% for patients and 67.7% for controls. 99 healthy subjects without any malignancy were selected for the control group that is comprised only of individuals with a negative family history of cancer. The patient and control groups were matched for age. All participants signed an informed consent before enrollment and Institutional Ethical committee approval was obtained for the study.
2.2. Polymorphism Analysis
Blood samples from all study participants were collected in EDTA-containing tubes. Genomic DNA was extracted from peripheral whole blood according to kit protocol (High Pure PCR Template Preparation Kit, REF 11796828001 (Roche, Diagnostics GmbH, Mannheim, Germany). Genotyping was performed by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP); the procedures of PCR-RFLP are given in Table 1. Two separate PCR reactions were used to detect the two types of polymorphism in NF-κB gene, namely, NF-κB1 -94ins/delATTG polymorphism and NF-κBIA 3′UTR A → G polymorphism. The appropriate primers were used to amplify the corresponding gene of the subjects by PCR and the reaction products were digested by using the appropriate enzyme and incubated at 37°C overnight. The digested products were analyzed on 3% agarose gel, stained with ethidium bromide, and examined under transillumination (Figures 1 and 2). Each gel was read by two observers, unaware of the subject's status. In order to verify our PCR-RFLP results, we repeated PCR-RFLP stage 2 times for each of selected subject. The expected results after restriction for each gene were also given in Table 1.
Table 1.
PCR and RFLP procedures and products of NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A→G.
| Primers (forward and reverse) | PCR product | Restriction enzyme | Restriction products | |
|---|---|---|---|---|
| NF-κB1 | 5′-TGGGCACAAGTCGTTTATGA-3′ | 285 bp | Van91l (PflMI) | ATTG2/ATTG2 (ins/ins): 240 bp, 45 bp |
| 5′-CTGGAGCCGGTAGGGAAG-3′ | ATTG1/ATTG1 (del/del): 281 | |||
| ATTG2/ATTG1 (ins/del): 281, 240, 45 | ||||
| NF-κBIA | 5′-GGCTGAAAGAACATGGACTTG-3′ | 424 bp | HaeIII | AA wild type: 424 |
| 5′-GTACACCATTTACAGGAGGG-3′ | AG heterozygous: 316, 108 | |||
| GG mutant: 424, 316, 108 |
Figure 1.
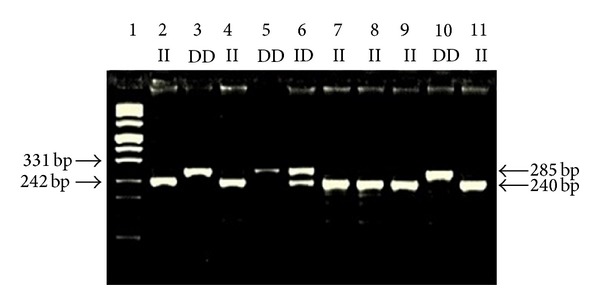
Representative genotypes of NF-κB1 -94ins/delATTG polymorphism. Lane 1: marker DNA ladder; Lanes 2, 4, 7, 8, 9, and 11: ins/ins (ATTG2/ATTG2) genotypes; Lanes 3, 5, and 10: del/del (ATTG1/ATTG1) genotypes; Lane 6: heterozygous del/ins (ATTG1/ATTG2) genotypes.
Figure 2.
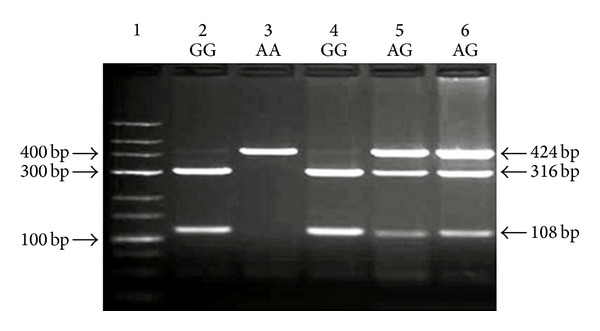
Representative genotypes of NF-κBIA 3′UTR A → G polymorphism. Lane 1: marker DNA ladder; Lanes 2 and 4: GG mutant genotypes; Lane 3: AA wild-type homozygous genotypes; Lanes 5 and 6: AG heterozygous genotypes.
2.3. Statistical Analysis
Statistical analyses were performed using the SPSS software package (revision 13.0 SPSS Inc., Chicago, IL, USA). Data were expressed as means ± SD. Differences in the distribution of NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G genotypes or alleles between cases and controls were tested using the Chi-square statistic, respectively (Table 2). Differences in characteristics between NSCLC patients and controls were assessed with Fisher's exact test, as well as disparities in genotype and allele frequencies. Relative risk at 95% confidence intervals (CI) was calculated as the odds ratio (OR). Values P < 0.05 were considered statistically significant. A multivariate logistic regression model was performed to investigate possible effects of genotypes and alleles after adjustment for age.
Table 2.
Distribution of NF-κB1 and NF-κBIA genotypes and allele in NSCLC patients and controls.
| Genotypes/alleles | Controls n (%) | Patients n (%) | O.R (95% CI) | P value |
|---|---|---|---|---|
| NF-κB1 | ||||
| II | 46 (46.47) | 35 (36.84) | Reference | |
| ID | 47 (47.47) | 44 (46.32) | 1.23 (0.67–2.25) | 0.500 |
| DD | 6 (6.06) | 16 (16.84) | 3.50 (1.24–9.87) | 0.014 |
| ID + DD | 53 (53.53) | 60 (63.16) | 1.49 (0.84–2.64) | 0.174 |
| I allele | 139 (70.2) | 114 (60) | Reference | |
| D allele | 59 (29.8) | 76 (40) | 1.57 (1.03–2.39) | 0.035 |
| NF-κBIA | ||||
| AA | 21 (21.21) | 17 (17.9) | Reference | |
| AG | 45 (45.46) | 45 (47.36) | 1.24 (0.58–2.65) | 0.587 |
| GG | 33 (33.33) | 33 (34.74) | 1.24 (0.55–2.75) | 0.605 |
| AG + GG | 78 (78.79) | 78 (82.1) | 1.24 (0.61–2.52) | 0.561 |
| A allele | 87 (43.94) | 79 (41.58) | Reference | |
| G allele | 111 (56.06) | 111 (58.42) | 1.10 (0.74–1.65) | 0.639 |
O.R: Odds ratio; CI: confidence interval.
3. Results
In this study, we examined 194 volunteers, 95 NSCLC (89 males; 6 females) patients, and 99 healthy people (67 males; 32 females) detecting any chronic disease or any evidence of malignancy. Distribution of NF-κB1 and NF-κBIA genotypes according to clinic features in NSCLC patients is shown in Table 3. The distribution of the NF-κB1 -94ins/delATTG genotypes in control and NSCLC patients was found to be significantly different (P: 0.048). It was evaluated that individuals carrying DD genotype had 3.5-fold increased risk for NSCLC (P: 0.014 χ 2: 5.605, O.R: 3.50, %95 CI: 1.24–9.87). No statistically significant differences between groups were observed when the NF-κBIA 3′UTR A → G genotypes distributions were compared (P: 0.844). A significant correlation between genotype combinations of NF-κB1 and NF-κBIA (DDAG genotype) and NSCLC risk was found compared to all other combinations (P: 0.025; O.R: 5.035; 95% CI: 1.067–24.14) and DDAG genotype had increased risk for NSCLC. The prevalence of IIAA genotype combinations versus to all other combinations was 5.3% in patients and 12.1% in the control group, but there are no statistically significant differences between groups (P: 0.091; O.R: 0.403; 95% CI: 0.136–1.191). The results of multivariate logistic regression analysis are presented in Table 4. Gender, age (<57/≥57), and NF-κB1 DD genotype were associated with NSCLC in univariate analysis, and additionally these were associated with this disease in multivariate logistic regression analysis.
Table 3.
Distribution of NF-κB1 and NF-κBIA genotypes with clinic features in NSCLC patients.
| NF-κB1 | NF-κBIA | |||||||
|---|---|---|---|---|---|---|---|---|
| II | ID | DD | P value | AA | AG | GG | P value | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Sex | ||||||||
| Men | 33 (37.10) | 41 (46.10) | 15 (16.80) | 0.980 | 15 (16.90) | 43 (48.30) | 31 (34.80) | 0.570 |
| Women | 2 (33.30) | 3 (50.00) | 1 (16.70) | 2 (33.30) | 2 (33.30) | 2 (33.30) | ||
| Age | ||||||||
| <57 | 10 (29.4) | 17 (50) | 7 (20.6) | 0.496 | 5 (14.7) | 19 (55.9) | 10 (29.4) | 0.567 |
| ≥57 | 27 (41.5) | 27 (41.5) | 11 (16.9) | 12 (18.5) | 29 (44.6) | 24 (36.9) | ||
| Smoking (box/year) | ||||||||
| <50 | 25 (48.10) | 24 (46.20) | 3 (5.80) | 0.02 | 10 (19.20) | 25 (48.10) | 17 (32.70) | 0.876 |
| ≥50 | 10 (23.30) | 20 (46.50) | 13 (30.20) | 7 (16.30) | 20 (46.50) | 16 (37.20) | ||
| Alcohol consumption | ||||||||
| No | 17 (31.50) | 28 (51.90) | 9 (16.70) | 0.405 | 6 (11.10) | 31 (57.40) | 17 (31.50) | 0.044 |
| Yes | 18 (43.90) | 16 (39.00) | 7 (17.10) | 11 (26.80) | 14 (34.10) | 16 (39.00) | ||
| Histopathology | ||||||||
| Squamous | 16 (45.70) | 16 (45.70) | 3 (8.60) | 0.179 | 7 (20.00) | 20 (57.10) | 8 (22.90) | 0.173 |
| Nonsquamous | 19 (31.70) | 28 (46.70) | 13 (21.70) | 10 (16.70) | 25 (41.70) | 25 (41.70) | ||
| Total protein | ||||||||
| <6 g/L | 2 (50.00) | 0 (0) | 2 (50.00) | 0.087 | 0 (0) | 3 (75.00) | 1 (25.00) | 0.452 |
| ≥6 g/L | 31 (38.30) | 38 (46.90) | 12 (14.80) | 16 (19.80) | 37 (45.70) | 28 (34.60) | ||
| Albumin | ||||||||
| <3 g/L | 6 (54.50) | 1 (9.10) | 4 (36.40) | 0.019 | 1 (9.10) | 6 (54.50) | 4 (36.40) | 0.635 |
| ≥3 g/L | 26 (34.70) | 39 (52.00) | 10 (13.30) | 16 (21.30) | 35 (46.70) | 24 (32.00) | ||
| Calcium | ||||||||
| <10 mg/Dl | 30 (40.00) | 30 (40.00) | 15 (20.00) | 0.001 | 16 (21.30) | 35 (46.70) | 24 (32.00) | 0.506 |
| ≥10 mg/dL | 0 (0) | 11 (100) | 0 (0) | 1 (9.10) | 7 (63.60) | 3 (27.30) | ||
| LDH | ||||||||
| <250 U/L | 22 (36.10) | 28 (45.90) | 11 (18.00) | 0.644 | 13 (21.30) | 31 (50.80) | 17 (27.90) | 0.249 |
| ≥250 U/L | 10 (47.60) | 8 (38.10) | 3 (14.30) | 3 (14.30) | 8 (38.10) | 10 (47.60) | ||
Table 4.
The results of multivariate logistic regression.
| Covariates | P value | Exp (B) | 95% C.I for Exp (B) |
|---|---|---|---|
| Gender | <0.001 | 7.866 | 2.915–21.231 |
| Age (<57/≥57) | <0.001 | 5.074 | 2.633–9.776 |
| NF-κB1 DD genotype | 0.035 | 3.167 | 1.086–9.234 |
4. Discussion
A functional polymorphism in the NF-κB1 gene promoter region (-94ins/delATTG) has been identified and associated with both chronic inflammatory diseases and malignant diseases [22]. NF-κB is inactivated in the cytoplasm by IκBα, β, or γ and the most common protein of this family is the NF-κB inhibitor α (NF-κBIA) [23]. -94ins/delATTG polymorphism has evidence from two independent functional assays, in vitro promoter activity and differential an unidentified nuclear protein binding, that the specific allele inherited likely has functional consequences [24]. NF-κBIA 3′UTR A → G polymorphism may affect mRNA stability and translational efficacy or conduces to differential nuclear RNA processing, or export also cannot be completely excluded. Many studies have been conducted to investigate a possible association between NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G polymorphism and both inflammatory diseases and various cancer types [25]. However no data are available in the English literature to report the association with NSCLC to date. Our study is the initial report on these two forms of polymorphism (both NF-κB1 -94ins/delATTG and NF-κBIA 3′UTR A → G) studied together in NSCLC patients to our knowledge. The genotypic combinations of NF-κB1 and NF-κB2 polymorphism have been shown to be associated with the development of common inflammatory diseases including ulcerative colitis (UC), Crohn's disease, and Type I diabetes, as well as susceptibility of several cancers, such as oral squamous cell carcinoma and colorectal cancer [26]. It can be concluded that previous studies have conflicting results [27]. Oliver et al. suggest that the NF-κB1 -94ins/delATTG gene variation, previously associated with UC susceptibility in North Americans, does not influence either susceptibility or phenotype of UC in the Spanish population [28]. In this study, we performed a risk association between the NF-κB1 -94ins/delATTG promoter polymorphism and NSCLC. The -94ins/delATTG polymorphism has been shown as a first potential functional NF-κB1 polymorphism by Karban et al. Nuclear proteins from normal human colon tissue showed significant binding to -94insATTG but not to -94delATTG containing oligonucleotides. NF-κB1 promoter/exon 1 luciferase reporter plasmid constructs containing the -94delATTG allele and transfected into either HeLa or HT-29 cell lines showed low promoter activity more than comparable constructs containing the -94insATTG allele. Therefore, it is known that D allele promoter activity is low and I allele promoter activity is high. Previous studies have suggested that D allele may result in decreased NF-κB1 message and hence decreased p50/p105 NF-κB protein production leads to increased inflammatory response. Otherwise, a potential explanation of decreased NF-κB1 D allele gene expression may be the resulting decreases in NF-κB p50/p65 heterodimers that are major mediators of inflammation [29].
Defects in components that regulate NF-κB release from IκBα result in constitutive or decreased NF-κB activation. These components may be any of the kinases, phosphatases, or other signal transducers, normally involved in NF-κB-activation pathways [30]. Sonenshein suggests that alterations of NF-κB1 expression play an important role in the protection of cells from apoptosis [31]. NF-κB1 activity has been observed in various types of cancer, as well as colorectal cancer and breast cancer, to contribute to tumor angiogenesis, invasion, and progression [32]. Therefore, the variants of the NF-κB1 gene could be expected to have an effect on cell death and thus carcinogenesis. NF-κB1 3′UTR A → G polymorphism has functional effects on expression of the NF-κBIA gene and altered NF-κB transcription [33]. There are many studies with different results on NF-κBIA 3′UTR polymorphism in the literature [34]. Our results suggested that NF-κBIA polymorphism has no effect on risk of NSCLC. In conclusion, we here clearly demonstrated that NF-κB1 -94ins/delATTG promoter polymorphism and the presence of the DD genotype might have a risk factor for NSCLC pathogenesis in our ethnic population. Larger trials that included different ethnic groups are necessary to define objectively the correlation between NF-κB1 -94ins/delATTG promoter and development of NSCLC as well as prognosis of disease.
Acknowledgments
The present work was supported by a Grant from the Scientific Research Projects Coordination Unit of Istanbul University (Project no. 10537). The authors would like to thank M.Sc. Allison P. Eronat, B.Sc. Nesibe Selma Guler, and Ph.D. Ayla Karimova for their understanding and suggestions in English grammar of our article and the Editor and anonymous reviewers for their valuable comments and suggestions, which were helpful in improving the paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer Journal for Clinicians. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. The Journal of Clinical Investigation. 2007;117(10):2740–2750. doi: 10.1172/JCI31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischhacker M, Beinert T, Possinger K. Molecular genetic characteristics of lung cancer—useful as “real” tumor markers? Lung Cancer. 1999;25(1):7–24. doi: 10.1016/s0169-5002(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, Liu D, Shishodia S, et al. Nuclear factor-κB (NF-κB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107(11):2637–2646. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 5.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits: a preliminary report. British Medical Journal. 1954;1(4877):1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koti M, Gooding RJ, Nuin P, et al. Identification of the IGF1/PI3K/NFκB/ERK gene signalling networks associated with chemotherapy resistance and treatment response in high-grade serous epithelial ovarian cancer. BMC Cancer. 2013;13, article 549 doi: 10.1186/1471-2407-13-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Li Z, Bai L, Lin Y. NF-κB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Frontiers in Bioscience. 2011;16(3):1172–1185. doi: 10.2741/3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salim PH, Jobim M, Bredemeier M, et al. Interleukin-10 gene promoter and NFKB1 promoter insertion/deletion polymorphisms in systemic sclerosis. Scandinavian Journal of Immunology. 2013;77(2):162–168. doi: 10.1111/sji.12020. [DOI] [PubMed] [Google Scholar]
- 9.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 10.Sethi G, Sung B, Aggarwal BB. Nuclear factor-κB activation: from bench to bedside. Experimental Biology and Medicine. 2008;233(1):21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. Signaling to NF-κB. Genes & Development. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 12.Karin M. The beginning of the end: IκB kinase (IKK) and NF-κB activation. The Journal of Biological Chemistry. 1999;274(39):27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Lopez-Dee Z, Kumar R, Ling J. Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Letters. 2013;337(1):90–95. doi: 10.1016/j.canlet.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Bharti AC, Aggarwal BB. Nuclear factor-κ B and cancer: its role in prevention and therapy. Biochemical Pharmacology. 2002;64(5-6):883–888. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 15.Datta DD, Datta A, Bhattacharjya S, Roychoudhury S. NF-κB mediated transcriptional repression of acid modifying hormone gastrin. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0073409.e73409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DR, Broad RM, Madrid LV, Baldwin AS, Jr., Mayo MW. Inhibition of NF-κB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis. The Annals of Thoracic Surgery. 2000;70(3):930–936. doi: 10.1016/s0003-4975(00)01635-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang C-Y, Cusack JC, Jr., Liu R, Baldwin AS., Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nature Medicine. 1999;5(4):412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 18.Adamzik M, Schäfer S, Frey UH, et al. The NFKB1 promoter polymorphism (−94ins/delATTG) alters nuclear translocation of NF-κB1 in monocytes after lipopolysaccharide stimulation and is associated with increased mortality in sepsis. Anesthesiology. 2013;118(1):123–133. doi: 10.1097/ALN.0b013e318277a652. [DOI] [PubMed] [Google Scholar]
- 19.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr., Sledge GW., Jr. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Molecular and Cellular Biology. 1997;17(7):3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegazy DM, O’Reilly DA, Yang BM, Hodgkinson AD, Millward BA, Demaine AG. NFκB polymorphisms and susceptibility to type 1 diabetes. Genes & Immunity. 2001;2(6):304–308. doi: 10.1038/sj.gene.6363776. [DOI] [PubMed] [Google Scholar]
- 21.Gazdar AF. DNA repair and survival in lung cancer—the two faces of Janus. The New England Journal of Medicine. 2007;356(8):771–773. doi: 10.1056/NEJMp068308. [DOI] [PubMed] [Google Scholar]
- 22.Vangsted J, Klausen TW, Abildgaard N, et al. Single nucleotide polymorphisms in the promoter region of the IL1B gene influence outcome in multiple myeloma patients treated with high-dose chemotherapy independently of relapse treatment with thalidomide and bortezomib. Annals of Hematology. 2011;90(10):1173–1181. doi: 10.1007/s00277-011-1194-3. [DOI] [PubMed] [Google Scholar]
- 23.Song S, Chen D, Lu J, et al. NFκB1 and NFκBIA polymorphisms are associated with increased risk for sporadic colorectal cancer in a southern Chinese population. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021726.e21726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang M, Xu X, Gong Y, Tang Y, Lin L. Risk association between the NF-κB1 −94ins/delATTG promoter polymorphism and inflammatory bowel diseases: a meta-analysis. Digestive Diseases and Sciences. 2012;57(9):2304–2309. doi: 10.1007/s10620-012-2164-x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng CW, Su JL, Lin CW, et al. Effects of NFKB1 and NFKBIA gene polymorphisms on hepatocellular carcinoma susceptibility and clinicopathological features. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0056130.e56130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vu D, Tellez-Corrales E, Sakharkar P, et al. Impact of NF-κB gene polymorphism on allograft outcome in Hispanic renal transplant recipients. Transplant Immunology. 2013;28(1):18–23. doi: 10.1016/j.trim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Zou Y-F, Yuan F-L, Feng X-L, et al. Association between NFKB1 −94ins/delATTG promoter polymorphism and cancer risk: a meta-analysis. Cancer Investigation. 2011;29(1):78–85. doi: 10.3109/07357907.2010.535054. [DOI] [PubMed] [Google Scholar]
- 28.Oliver J, Gómez-García M, Paco L, et al. A functional polymorphism of the NFKB1 promoter is not associated with ulcerative colitis in a Spanish population. Inflammatory Bowel Diseases. 2005;11(6):576–579. doi: 10.1097/01.mib.0000161916.20007.76. [DOI] [PubMed] [Google Scholar]
- 29.Karban AS, Okazaki T, Panhuysen CIM, et al. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Human Molecular Genetics. 2004;13(1):35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- 30.Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-κB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. The Journal of Clinical Investigation. 1997;100(12):2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonenshein GE. Rel/NF-κB transcription factors and the control of apoptosis. Seminars in Cancer Biology. 1997;8(2):113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 32.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Archiv. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 33.Glavac D, Ravnik-Glavac M, O’Brien SJ, Dean M. Polymorphisms in the 3′ untranslated region of the IκB/MAD-3 (NFKBI) gene located on chromosome 14. Human Genetics. 1994;93(6):694–696. doi: 10.1007/BF00201573. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Pfeifer D, He L-J, et al. Association of NFKBIA polymorphism with colorectal cancer risk and prognosis in Swedish and Chinese populations. Scandinavian Journal of Gastroenterology. 2007;42(3):345–350. doi: 10.1080/00365520600880856. [DOI] [PubMed] [Google Scholar]


