Abstract
Accumulating evidence indicates that pesticide exposure is associated with an increased risk for developing Parkinson's disease (PD). Several pesticides known to damage dopaminergic (DA) neurons, such as paraquat, rotenone, lindane, and dieldrin also demonstrate the ability to activate microglia, the resident innate immune cell in the brain. While each of these environmental toxicants may impact microglia through unique mechanisms, they all appear to converge on a common final pathway of microglial activation: NADPH oxidase 2 (NOX2) activation. This review will detail the role of microglia in selective DA neurotoxicity, highlight what is currently known about the mechanism of microglial NOX2 activation in these key pesticides, and describe the importance for DA neuron survival and PD etiology.
Keywords: Microglia, NOX2, Oxidative stress, Dopaminergic neurotoxicity, Paraquat, Rotenone, Dieldrin, Parkinson's disease
I. Introduction
Microglia are the resident innate immune cells in the brain and have been implicated in the progressive nature of neurodegenerative diseases, particularly Parkinson's disease (PD). Accumulating evidence indicates that many environmental toxicants linked to PD are not only capable of directly activating microglia to cause dopaminergic (DA) neurotoxicity, but many of these compounds trigger microglial reactive oxygen species (ROS) production. Here, we define the current list of PD-linked pesticides that activate microglial NADPH Oxidase 2 (NOX2) to produce ROS and discuss the proposed mechanisms responsible for how these compounds activate microglial NOX2.
II. Microglia
Microglia, the resident central nervous system (CNS) macrophages, are distributed throughout the brain, comprising ∼12% of all cells, with variable density by brain region (1). Microglia arise from primitive myeloid progenitors of the hematopoietic system during pre-natal development, making them ontogenetically distinct from other CNS cells, such as neurons, astrocytes, and oligodendrocytes that are derived from the neuroectoderm (2,3). Given their common myeloid lineage, both microglia and peripheral macrophages express proteins essential for the innate immune response, including cell surface proteins (e.g. major histocompatibility complex proteins, pattern recognition receptors, cytokine receptors and complement receptors), chemokine and prostanoid receptors, cytokines, cyclooxygenases, inducible nitric oxide synthase (iNOS), and NOX2 (4,5). Like other macrophages, microglia are phagocytes that internalize cellular debris/microbes and employ the respiratory burst to fight invading pathogens (6). Thus, while parenchymal microglia are capable of cell division and consequent intrinsic repopulation in the adult CNS, it is not surprising that the microglial population is also affected by recruitment of circulating monocytes to varying extents in the healthy and injured CNS (3,7,8). As a consequence of these striking similarities, a single protein marker capable of distinguishing peripheral infiltrating monocytes from resident parenchymal microglia is currently unavailable.
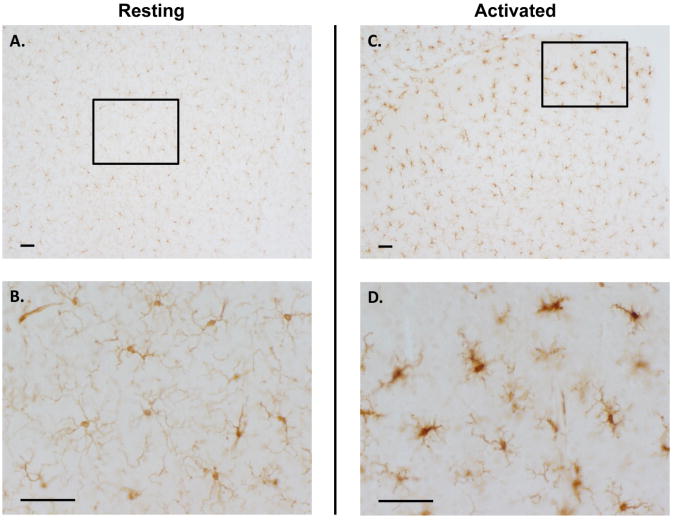
Despite these similarities, microglia are phenotypically distinct from peripheral monocytes and macrophages. The most obvious difference is the three-dimensional and branched resting morphology of microglial cells in the CNS (Figure 1 A & B). This ramified, resting morphology is characterized by a small cell body that dynamically extends highly branched fillipodia-like processes to continuously survey the CNS environment (9). It is proposed that the neuron-microglia communication in physiological conditions in addition to the occlusion of serum proteins from the brain parenchyma underlie this unique resting microglial phenotype when compared to other macrophage cell types (8,10). Interestingly, microglia express a ratio of innate immune cell proteins/surface markers that distinguish microglia from other myeloid cells (11). In addition, microglia express elevated levels of neurotransmitter receptors and certain purinergic receptors, which confer the ability to communicate with both neurons and glia, as well as enhancing sensitivity to neuronal damage (12-14). Further, only parenchymal microglia are known to express miR-124, a unique microRNA believed to regulate microglial quiescence (15). Evidence also indicates that microglia have unique signaling mechanisms in response to classic pro-inflammatory stimuli. For example, while TLR4 signaling is known to regulate lipopolysaccharide (LPS)-induced superoxide production in most macrophages, extracellular superoxide production from microglia in response to LPS is instead mediated by the MAC1 receptor (16,17). These distinctions also translate to functional differences in the pro-inflammatory response. For example, research has shown that a single perturbation with a peripheral injection of LPS can result in a short peripheral immune response that transfers to the brain to cause chronic microglial activation that persists for several months after the peripheral immune response has resolved (18). Thus, microglia are unique and their specific response to environmental exposures may be a critical component to understanding CNS pathology.
Figure 1. The Unique Morphology of Resting & Activated Microglia.
Resting microglia stained with the ionized calcium binding adaptor molecule 1 (IBA-1) microglial marker in the mouse substantia nigra are shown in Panel A at 10× magnification. Panel B focuses on the boxed in segment of Panel A at a higher magnification of 40×. The ramified morphology depicted by extensive branching is readily visible. In panels C and D, activated microglia from mice injected with 5mg/kg lipopolysaccharide were stained with the IBA-1 microglial marker. Activated microglia in the mouse substantia nigra are shown in Panel C at 10× magnification. Panel D focuses on the boxed in segment of Panel C at a higher magnification of 40×. Note that the microglia from mice treated with lipopolysaccharide have thickened cell bodies and the cell has retracted its processes, a characteristic of microglial activation in response to general neuroinflammation triggers, such as lipopolysaccharide and pesticides. The scale bar depicts 50μm.
Microglia actively monitor the CNS at rest, allowing them to quickly detect and respond to damage, disease, and external stimuli that may reach the brain (5), a process called activation. Microglial activation is a fluid, dynamic event that is stimulus dependent, where activation can be triggered by a host of signals, including classic pro-inflammatory stimuli (e.g. LPS and interferon γ), environmental toxins (e.g. pesticides, heavy metals, and air pollution), neurodegenerative pathology (e.g. α synuclein and β-amyloid plaques), and neuronal injury (19-23). These triggers elicit a change in morphology depicted by the enlarging of the cell body along with a thickening and retraction of processes (24) (Figure 1 C & D). This morphology change occurs for an entire spectrum of activation phenotypes that include both the classic proinflammatory response (M1) and the alternative (M2) forms of macrophage activation (25-27). As an innate immune cell, microglia can become activated to produce several pro-inflammatory factors (e.g. tumor necrosis factor α, interleukin-1β, and reactive oxygen species) in an M1 response (11), where many of these factors are neurotoxic (28). In physiological conditions, M1 activation proceeds to the M2 response, where M2a activation results in the production of anti-inflammatory factors necessary for resolution of inflammation and the M2b activation state regulates the wound healing response (11,27). Although the pro-inflammatory phenotype is best characterized in microglia, especially with regard to the deleterious effects of chronic inflammation in the CNS (5,29), activation of microglia and their normal function is necessary for maintenance of a healthy CNS. For example, microglial activation plays a key role in embryonic development, as microglia engage in synaptic pruning (30), initiate necessary neuronal apoptosis (31), and clear apoptotic cellular debris (4) in the developing CNS. In addition to their role in innate immunity and host defense (26,32), microglia also serve many regulatory functions in the adult CNS, where they guide neural stem cells to sites of injury (33), influence stem cell differentiation (33), regulate synaptic plasticity (34), and provide neurotrophic support (35). Thus, in addition to an excessive and chronic M1 pro-inflammatory response that is implicated in the initiation of neuron damage (5), microglial pathology undoubtedly includes the loss of the beneficial functions listed here and the impairment of the M2 healing and resolution phase of activation (27,36).
III. Parkinson's disease
Parkinson's disease is a devastating movement disorder characterized by the progressive and selective degeneration of DA neurons, whose cell bodies reside in the substantia nigra (SN) pars compacta and project to the striatum (37). PD is the second most prevalent neurodegenerative disorder, affecting approximately 4.1 million people worldwide (38). Age, gender (males have increased risk), and exposure to environmental toxins have been identified as risk factors for PD (39,40). The four classic motor disturbances of PD include bradykinesia (slowing of movement), resting tremor, muscle rigidity, and postural instability, which do not become manifest until approximately 60-70% of nigrostriatal DA neurons are lost (41). At present, therapeutic treatment is unable to halt disease progression and only addresses disease symptoms. While the cause of PD is largely unknown, some single gene mutations have been identified in familial PD (42), including genes related to α synuclein, protein degradation, mitochondrial stress response, and mitochondrial degradation (43,44), supporting a potential role for oxidative stress in the disease process. However, roughly 90% of documented PD cases are sporadic (45), indicating the significant potential for environmental impact on the disease.
III. Microglia Activation and Parkinson's disease
Microglia have been implicated in the progression of a number of neurodegenerative disorders, including Alzheimer's disease (46), amyotrophic lateral sclerosis (47), multiple sclerosis (48), Huntington's disease (49), and PD (50). In fact, neuroinflammation/microglial activation and the peripheral immune system are intricately linked and PD has been associated with peripheral immune dysregulation (51). More specifically, pro-inflammatory cytokines are elevated in PD patient blood (52) and upregulated by circulating white blood cells, both at basal levels and in response to pro-inflammatory stimuli (51), indicating that these peripheral cells are biologically altered during the process of CNS pathology. Postmortem analysis also reveals that the immunopathology includes the PD brain, as pro-inflammatory cytokine levels are elevated in the SN of PD patients (50,51,53). Following the initial post-mortem studies in the 1980's that revealed increased numbers of HLA-DR positive microglia in the SN of PD patients (54), the field of research dedicated to understanding the involvement of microglia in disease has mushroomed. Subsequent studies employing positron emission tomography (PET) imaging have confirmed activation of microglia in the SN of living PD patients (55) as well as identified an inverse relationship between microglial activation and levels of nigrostriatal DA neuron terminals (56). Thus, evidence supports that not only are microglia activated, but there is an ongoing M1 pro-inflammatory process occurring during PD that correlates with DA neuron cell damage. Importantly, PET studies have also revealed that microglial activation in the SN is reported early in the disease process of both PD and Lewy body dementia (PD-related disease) patients (57), supporting an active role of microglia throughout the disease process. However, it remains unclear at this time, precisely how the CNS immune system is perturbed in PD, pointing to a potential opportunity for pro-inflammatory environmental toxicants in the etiology of PD.
IV. Microglia & Inflammation Initiate Dopaminergic Neuron Damage
Several lines of evidence indicate that M1 activation of microglia can actively induce DA neuron damage. LPS is a major component of gram-negative bacteria, a potent pro-inflammatory stimulus, and as discussed below, is selectively toxic to DA neurons through microglial activation. In vitro studies have revealed that LPS fails to directly damage DA neurons when cultured without the presence of glia (58). Deletion of microglia from mesencephalic neuron-glia cultures (leaving astrocytes and neurons) results in complete protection from LPS-induced DA neurotoxicity, emphasizing that microglia are culpable in this neurotoxicity (59,60). Specifically, LPS activates microglia to produce tumor necrosis factor α (TNFα), superoxide, and nitric oxide (NO) that precedes selective DA neuron death in mesencephalic neuron-glia cultures (61). Importantly, in vitro evidence supports that microglia-mediated neurotoxicity is delayed and progressive, where LPS- induced DA neurotoxicity is delayed for 3 days in vitro and accumulates across time for up to 9 days (61). This premise is consistent with in vivo studies, where stimulation of microglia by LPS administered either directly to the brain (61,62), intraperitoneal injection (18), or in utero(63) results in a delayed and progressive loss of nigral DA neurons that persists well after the initial inflammatory stimulus. Other toxins have since been discovered that are capable of directly activating microglia to selectively kill DA neurons, including endogenous disease proteins and environmental toxins (5).
In addition to pro-inflammatory triggers mentioned above, microglia respond to and are activated by neuronal death, a process called reactive microgliosis. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was discovered as a neurotoxic contaminant in synthetic heroin (64), where it's active metabolite 1-methyl-4-phenylpyridinium (MPP+) is known to be selectively taken up by DA neurons to impair mitochondrial function and induce cell death (65). Importantly, MPTP and MPP+ are unable to directly activate microglia alone without the presence of neurons (66), presenting an ideal tool to study reactive microgliosis and how microglia contribute to ongoing neuron damage. Indeed, clusters of activated microglia near degenerating DA neurons of the SN in humans exposed to MPTP are readily identifiable postmortem analyses (67). In fact, microglial activation and chronic DA neuron damage continues for years after the initial exposure in humans (67) and monkeys (68), indicating that active pathology continues long after the toxin has been metabolized and eliminated. Neuroinflammation has been implicated as a key component of the ongoing neurotoxic pathology following neuronal death, where genetic deletion of key microglial inflammatory mediators including the TNFα receptor (69), cyclooxygenase 2 (COX2) (70), interferon γ (IFNγ) (71), iNOS (72), and MAC1 (73)(74) significantly reduced MPTP-induced DA neuron loss. Soluble signals released by damaged DA neurons that activate microglia (22,23,75) to selectively kill DA neurons have been identified, including α synuclein (76), neuromelanin (77,78), calpain (22), and matrix metalloproteinase 3 (MMP3) (79). Together, this indicates that microglia activation can be triggered by neuronal damage to propagate ongoing DA neurotoxicity.
Thus, microglia can become chronically activated by an instigating pro-inflammatory trigger or in response to neuronal death (reactive microgliosis) to produce neurotoxic cytokines and reactive oxygen species (ROS), a process believed to underlie the progressive nature of diverse neurodegenerative diseases, particularly PD.
V. The Vulnerable Dopaminergic Neuron
DA neurons are reported to be selectively vulnerable to microglial activation (14,60,61,80), where it takes less of a microglial response to damage this cell type. This selective vulnerability is most commonly attributed to a characteristic state of oxidative stress unique to DA neurons in the SN and the poor ability of this cell type to compensate for additional oxidative stimuli (81). Indeed, DA neurons of the SN have significantly elevated markers of oxidative stress when compared to other regions of the brain (82), as do mitochondria isolated from PD patients (83). As projection neurons that synthesize dopamine, DA neurons have expanded energy requirements of the electron transport chain, which is one of the largest sources of intracellular ROS, placing additional strain on the cell's antioxidant defenses (84). Further, the production of H2O2 resulting from the metabolism of dopamine by monoamine oxidase (85) and elevated levels of hydroxyl radicals that are formed by Fenton reaction with a rich iron content found in SN DA neurons (78,86,87) diminish the cell's battery of anti-oxidants (e.g. superoxide dismutase, glutathione, thioredoxin, catalase, and others), making these cells especially vulnerable to additional perturbation. In fact, several studies have shown that DA neurons in the SN fail to upregulate antioxidants in response to additional triggers of oxidative stress (81). Furthermore, implicit in the cellular process of aging is a reduced ability to manage redox equilibrium, the detrimental effects of which are enhanced in post-mitotic cells such as DA neurons that accumulate oxidative damage to DNA, lipids and protein over the course of a human's lifetime (88). Finally, there is regional vulnerability, where not only is the SN rich in iron that enhances the Fenton reaction (89,90), but the density of microglia has been reported to be higher (1). Thus, DA neurons in the SN have a cell-specific and brain region-specific vulnerability to oxidative stress.
VI. NOX2
NOX2, also known as gp91PHOX, is the catalytic subunit of the NADPH oxidase enzyme complex (91), is largely responsible for the production of extracellular superoxide from microglia, and has been widely implicated as a significant source of oxidative stress for DA neurons (20,59,60,80,92,93). As a member of the NOX family of NADPH oxidases, NOX2 is expressed in diverse cell types throughout the brain (91). However, NOX2 was named the phagocytic oxidase (PHOX) due to its discovery and particularly high expression in phagocytic cells. As such, microglia express all of the NOX2 subunits (NOX2, p22PHOX, p40PHOX, p47PHOX, and p67PHOX) both in vivo and in vitro(94-96), where microglial NOX2 has documented roles in host defense (97), proliferation (98), and regulation of cell signaling via redox signaling mechanisms (99-101). Traditional activation of microglial NOX2 requires a pro-inflammatory stimulus to trigger the cytosolic subunits to translocate to the membrane, interact with NOX2, and initiate superoxide production (102). As we will discuss below, recent studies with pesticides suggest that there may be additional mechanisms of NOX2 activation.
Microglial NOX2 is activated by several selective DA neurotoxins including endogenous disease proteins (29), neuronal damage (22,73,74,103), LPS (59), and pesticides (60,104,105). Importantly, microglial NOX2 is activated in PD and is implicated in the ongoing pathology (106). More specifically, the NOX2 protein is located at the cellular membrane in microglia, where ROS generated outside of the cell has been implicated in damage in surrounding tissues, particularly neurons (107). While many neurons can compensate for perturbation in ROS levels, DA neurons may be especially vulnerable, which is implicated in PD (81). As such, extracellular ROS derived from microglial NOX2 activation is believed to overwhelm compensatory anti-oxidant levels in DA neurons, leading to dysfunction of proteins, nucleic acids, lipids, and neurotoxicity (81,107).
Together, these findings support that microglial NOX2 is a common pathway for selective DA neurotoxicity. In fact, recent studies reveal that diverse pesticides shown to increase the risk of developing PD (ex. paraquat, rotenone, dieldrin, and lindane) activate microglial NOX2 (80,104,106,108). While the final end pathway of microglial NOX2 activation may be consistent across these unique toxicants, the precise triggering mechanisms of each compound in microglia may be distinct, as discussed below.
VII. Paraquat
Paraquat (N,N′ -dimethyl-4,4′-bipyridinium dichloride) is one of the most widely used herbicides and extended exposure has been linked to increased PD risk (109-112). Paraquat is a positively charged molecule that is capable of crossing the blood brain barrier through the neutral amino acid transporter following systemic exposure (113,114) and causing selective toxicity to DA neurons of the SN (115). Like other environmental neurotoxins, oxidative stress appears to be the key mechanism of paraquat-induced cell death (116). Indeed, several studies have implicated that paraquat is capable of directly damaging neurons (117), while lower concentrations are predicted to exert neurotoxic effects predominantly through microglial activation (60). Although paraquat bears structural similarity to MPP+, an extensively studied mitochondrial complex I inhibitor and DA neurotoxin, paraquat is actually a weak inhibitor of mitochondrial complex I (117). Rather, paraquat is reported to cause oxidative stress through redox cycling with molecules such as nitric oxide synthase (118), in addition to recent reports implicating activation of microglial NOX2 (60,119).
Paraquat & Animal studies
Animal studies demonstrate that repeated exposure to paraquat in vivo causes impairment of motor behavior (113), increased levels of α-synuclein deposits (120), lewy body-like structures in the SN (120), insoluble parkin (121) and dose dependent DA neuron loss (122) that is exacerbated by aging (115). While a single systemic administration of environmentally relevant doses of paraquat is not overtly neurotoxic, evidence supports that it serves to prime microglia (123). Rather, a single paraquat injection was shown to cause activated microglial morphology and significantly increased NOX2 expression, resulting in a more robust pro-inflammatory response to additional exposures, including paraquat (123), maneb (122), or LPS (123). Consistent with the premise that neuroinflammation mediates paraquat effects, inhibition of microglial activation by minocycline (123) or genetic deletion of IFNγ (124) attenuates DA neuron loss in paraquat models. Finally, depletion of microglia from neuron-glia cultures reverses paraquat-induced DA neurotoxicity (60) implicating microglia as important mediators of neurotoxicity triggered by repeated environmental paraquat exposure.
Paraquat-induced Direct Dopaminergic Neurotoxicity & Microglia
Paraquat-induced toxicity is complex and current theories hold that paraquat may exert DA neurotoxicity through: 1) a microglia- mediated mechanism and 2) direct neuronal damage mediated though both a DAT receptor- dependent and DAT receptor-independent mechanism. Regarding the direct DA neurotoxicity of paraquat, the mechanism of paraquat entry to the DA neuron and the consequences for neuron damage has been a point of some debate. While a structurally similar compound MPP+ enters DA neurons through the dopamine active transport (DAT) receptor and toxicity is dependent upon DAT expression, toxicity due to paraquat in cultures comprised of only neurons has been shown to be independent of the DAT receptor (117). Recent reports have suggested that the presence of microglial NOX2 may reduce paraquat to its radical form extracellularly and that the paraquat radical is a substrate for the DAT receptor, which is then responsible for internalization of the radical form and direct DA neurotoxicity (125). The study specifically showed that paraquat in combination with the reductant sodium dithionite (which reduces paraquat to the radical form) enhanced paraquat uptake into DA neurons, which was dependent on the DAT receptor (125). Importantly, the data also demonstrated that cells co-cultured with microglia showed enhanced paraquat accumulation and paraquat-induced toxicity, which was attenuated by the NOX2 inhibitor apocynin and not the NOS inhibitor N-Nitro-L-Arginine Methyl Ester (125). Thus, data support that microglial NOX2 may contribute to the direct DA neurotoxicity induced by paraquat. Given these intriguing findings, more inquiry is necessary to understand the mechanism of paraquat redox cycling in microglia and the localization of the paraquat radical.
Microglia Activation & Neurotoxicity
Importantly, independent of any direct neurotoxic effects, microglial NOX2 is directly activated by paraquat, where paraquat induces dose-dependent superoxide production in primary microglia cultures (60,119) and genetic deletion of NOX2 blocks paraquat-induced neurotoxicity both in vitro(60) and in vivo(123). Notably, paraquat does not appear to initiate the full spectrum of M1 microglial activation, as microglia cultures fail to produce cytokines in response to paraquat (60,126). However, inhibition of iNOS (127) and NOX2 (60,123,128), as well as co-treatment with a superoxide dismutase mimetic (128), decreases paraquat-induced microglial activation. While microglial activation appears to be key for paraquat-induced effects in vivo and in vitro, the molecular mechanisms triggering this process are largely unknown.
In the process of redox cycling, paraquat exchanges electrons with a reductant (ex. NADPH of NOX2) and molecular oxygen to continuously generate the paraquat radical and superoxide (129) . More specifically, the paraquat dication (PQ2+) accepts an electron from a reductant to form a paraquat radical cation (PQ+•), which in turn reacts with O2 to create the superoxide radical (O2−•) and regenerate PQ2+. This cycle repeats continuously in the presence of a reductant and molecular oxygen (130). As a flavoprotein, NOX2 is predicted to redox cycle with paraquat (119,125). In normal physiology, NOX2 functions to transfer electrons across biological membranes (102), where traditional activation of the enzyme complex results in the electron from NADPH being shuttled to molecular oxygen across the membrane and outside of the cell to produce extracellular superoxide. As such, NADPH is sequestered inside of the cell and is located on the COOH tail of NOX2 (102). One hypothesis holds that paraquat can gain entry to microglia, presumably through transport, to directly interact with NADPH on NOX2 to cause redox cycling and initiate the production of superoxide. However, this reaction would predict intracellular formation of both the paraquat radical and superoxide, which contradicts numerous reports that paraquat causes extracellular superoxide production from microglia (60,119). To further test this hypothesis, it will be important to discern whether the paraquat radical forms inside microglia and whether this paraquat radical is somehow shuttled out of the cell. Notably, functional interaction of the radical form of paraquat with a transporter may be difficult in normal physiology, as anaerobic conditions are mandatory to prevent immediate oxidation of the radical to the parent paraquat dication (130,131). Another redox-cycling hypothesis is that paraquat remains extracellular to microglia and takes the electron transferred across the membrane from NOX2 before it interacts with molecular oxygen, producing superoxide at more efficient and faster rate with redox cycling when compared to the NOX2 enzyme complex alone. In this case, rather than initiating superoxide production, paraquat would be a means of amplifying a low grade basal activation of NOX2. At this time, none of the proposed redox cycling hypotheses have been directly tested or confirmed.
Finally, a third hypothesis is that NOX2 activation occurs through traditional intracellular signaling and assembly of the entire NOX2 enzyme complex in response to paraquat. In fact, paraquat has been shown to cause translocation of p67PHOX to the membrane in microglia treated with paraquat, which was inhibited by rottlerin, a selective PKC delta inhibitor, implicating PKC in the assembly of the NOX2 complex (132). This is particularly intriguing, as this signal may be an initiating trigger for paraquat-induced NOX2 activation that could serve as base signal to be amplified by the extracellular redox signaling proposed in the previous hypothesis. Thus, while it is clear that paraquat activates microglia to produce extracellular superoxide and that NOX2 plays a key role in DA neurotoxicity, the mechanisms initiating NOX2 activation are likely complex, remain poorly understood, and significant additional research is necessary.
VIII. Rotenone
Rotenone is an extensively used plant-derived pesticide and chronic exposure has been associated with an increased risk for PD (133,134). Rotenone is lipophilic and readily traverses the blood-brain barrier and cellular membranes (135). Once inside cells, rotenone impedes mitochondrial function (136) by inhibiting the transfer of electrons from iron-sulfur centers in complex I to ubiquinone (Coenzyme Q10), resulting in intracellular oxidative stress (137). These rotenone-induced deficits in the mitochondrial respiratory chain are linked to decreased ATP synthesis, mitochondrial depolarization, generation of ROS, and neurotoxicity (137) which is thought to be essential to neurodegenerative disorders, such as PD. However, several reports indicate that rotenone can also activate microglia to mediate DA neuron damage (80,138).
Rotenone & Animal Studies
Animal research suggests that the ability of rotenone to repeatedly cause a PD-like phenotype with consistent lesions in the SN is closely linked to the route of exposure and the chronic nature of the exposure (135,139). For example, oral rotenone administration has resulted in both failure and success with SN lesions with evidence of α synuclein pathology (140). However, chronic systemic administration of rotenone at lower concentrations using osmotic pumps has been one of the most popular delivery regimens inducing both DA neurodegeneration and the formation of cytoplasmic inclusions in nigral DA neurons, but not all animals exposed demonstrate SN effects (141-143). Chronic intraperitoneal rotenone injections have since been modified to produce consistent behavioral deficits, decline in DA neurochemistry (144), and lewy body like inclusions (145). Aging has been shown to increase vulnerability to rotenone (146) and prenatal rotenone exposure has been shown to potentiate DA neuron damage in response to LPS with adult animals (147). Importantly, rotenone has been shown to cause a PD-like phenotype across several species, including mice, rat, zebrafish, Drosophila, and C. elegans, models (139).
Microglia & Rotenone
Rotenone activates microglia in vivo(148) and in vitro evidence demonstrates that at low concentrations, rotenone is selectively toxic to DA neurons, but only in the presence of microglia (138)(149). Further analysis showed that microglia are activated by rotenone to produce superoxide, which is selectively toxic to DA neurons, and dependent on NOX2, as genetic deletion of NOX2 (80) was neuroprotective against rotenone (80). Supporting the importance of microglial activation in rotenone-induced DA neuron damage, IFNγ receptor deficient microglia failed to cause DA cell loss when exposed to rotenone (71). Consistent with these findings, the anti-inflammitory compounds iptakalim (150) and minocycline (151) attenuate microglia activation and protect against rotenone neurotoxicity in vivo. In addition, rotenone has also been implicated in microglial priming, where pretreatment enhances the microglial M1 response to pro-inflammatory stimuli such as LPS and associated DA neurotoxicity (105). Thus, while neuroinflammation, microglial activation, and NOX2 actvation appear to be important for rotenone-induced neurotoxicity, the mechanisms responsible are poorly understood.
There are several potential mechanisms through which rotenone can activate microglia. The ability of rotenone to activate microglia supports a potential role for the importance of mitochondrial function in microglial activation. In fact, recent reports reveal that mito-K(ATP) channels participate in the regulation of the morphology changes linked to rotenone-induced microglial activation (152). Several recent studies have also specifically targeted mitochondrial dysfunction in microglia with multiple toxins including rotenone and have shown an inhibition of the IL-4-induced M2 response (153). Interestingly, a study performed in human lymphoblasts and whole blood demonstrated that several mitochondrial inhibitors impaired NOX2 activation through a PKC dependent pathway (80). Importantly, rotenone has been shown to cause a dose dependent increase in extracellular superoxide production from microglia, which is dependent on NOX2 (138), where NOX2 activation is implicated in both rotenone microglial priming and synergistic DA neurotoxicity (105).
Mechanistically, a recent report performing binding studies using membrane preparations from cells suggests that rotenone may directly interact with NOX2, the membrane bound catalytic subunit of the NOX2 enzyme complex, to affect the production of superoxide (154). Rotenone binding to NOX2 was inhibited by diphenyleneiodonium, a non-specific PHOX inhibitor with a binding site on gp91phox(154). Interestingly, further functional assays revealed that both the membrane catalytic subunit (NOX2) and the cytosolic subunits, with the exception of p47PHOX, were required for rotenone-induced superoxide production in cell-free systems (154). This is surprising, as p47 PHOX is considered to be an organizing subunit that recruits the activator subunit p67PHOX during the classical assembly of the NOX2 enzyme complex (155). Previously, both p47PHOX and p67PHOX were believed to be mandatory for microglial activation of NOX2 (156). While rotenone binding is hypothesized to facilitate the interaction of all of the subunits (except p47PHOX) with NOX2 to produce a functional enzyme that produces superoxide, it is unclear how binding of rotenone to NOX2 would signal/initiate translocation of the cytosolic proteins to the membrane in response to rotenone. Thus, these findings support the possibility of another additional intracellular signaling pathway caused by rotenone that would be responsible for the classical translocation of the cytosolic subunits to the microglial membrane. While the potential signaling pathways in microglia induced by rotenone have been largely overlooked, rotenone has been shown to cause p38 MAPK and ERK signaling in THP-1 monocytes, where blocking these pathways inhibited neurotoxicity in co-cultures. Clearly, the mechanisms of rotenone-induced microglial activation are complex and additional inquiry is warranted.
IX. Organochlorine Pesticides
Organochlorine pesticides are highly lipophilic compounds that readily cross the blood brain barrier (157), are persistently available in the environment (158), and are associated with DA neurotoxicity and oxidative stress (159,160). Postmortem analysis of PD brains have discovered the presence of organochlorine pesticides in the brain (161-163), particularly dieldrin (161,162). Importantly, case control studies indicate that serum levels of the organochlorine pesticide beta-HCH (158) and dieldrin (164) are associated with an increased risk of PD diagnosis.
Animal studies demonstrate that organophosphate pesticides impair striatal DA activity (160,165-168) and promote α-synuclein aggregation (160,168). However, specific lesions in the SN combined with motor behavior deficits have yet to be reported with organophosphate murine models in vivo(157). Dieldrin exposure early in life was found to amplify the neurotoxic effects of MPTP in adult animals, indicating that organochlorine pesticides have the ability to developmentally enhance DA neuron cell death in adult animals (169).
Mechanistically, organochlorine pesticides are known to induce oxidative damage to DA neurons, where many potential pathways including mitochondrial complex III dysfunction (170)(171) and ubiquitin-proteasome dysfunction (168) have been implicated. Recent studies have also shown that both dieldrin (104,108) and lindane (104) activate microglia to produce H2O2, which is inhibitable by NOX2 inhibitors. Further, combination of the pesticides results in synergistic amplification of H2O2(104). At this time, how dieldrin and lindane activate microglial NOX2 and the role of microglial NOX2 in DA neurotoxicity is unknown. However, consistent with rotenone, the organochlorine pesticides support the possibility of mitochondrial function and consequent intracellular ROS production regulating NOX2 and microglial activation.
X. Conclusions & Implications
Increasing evidence supports that both microglial activation and environmental toxicants may play a critical role in PD etiology as chronic sources of oxidative stress. Several pesticides associated with an elevated PD risk have been shown to directly damage DA neurons at high concentrations and activate microglial NOX2 at lower concentrations to cause DA neurotoxicity. More specifically, lindane, dieldrin, paraquat, and rotenone converge on a final pathway of NOX2-mediated ROS production in microglia. While current works suggests mitochondrial function may potentially modulate microglial NOX2 activation in some fashion, the mechanisms initiating activation of the NOX2 enzyme complex appears to be unique for each pesticide. Additional research centered on understanding how these pesticides activate microglial NOX2 will be necessary to effectively halt these environmentally-induced neurotoxic processes, as the anti-inflammatory and neuroprotective function of NOX2 inhibitors will depend on the mechanistic target in each of these unique triggers of microglial activation.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences/the National Institute of health [Grant number1R01ES016951].
List of Abbreviations
- CNS
Central Nervous System
- DA
Dopamine
- DAT
Dopamine Active Transporter
- H2O2
Hydrogen Peroxide
- iNOS
Inducible Nitric Oxide Synthase
- IFNγ
Interferon gamma
- LPS
Lipopolysaccharide
- MPP+
1-methyl-4-phenylpyridinium
- MMP3
Matrix Metalloproteinase 3
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NOX2
NADPH Oxidase 2
- NO
Nitric Oxide
- PD
Parkinson's disease
- PHOX
Phagocytic Oxidase
- PET
Positron Emission Tomography
- ROS
Reactive Oxygen Species
- SN
Substantia Nigra
- TNFα
Tumor Necrosis Factor α
References
- 1.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–70. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau I, Finsen B, Tonder N, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the prenatal rat hippocampus. J Comp Neurol. 1997;377(1):70–84. [PubMed] [Google Scholar]
- 3.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 5.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 6.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53(2):344–54. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–62. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 9.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 10.Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204(3):571–82. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 12.Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48(2):133–43. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 14.Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J. 2006;20(2):251–8. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- 15.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17(1):64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei Z, Pang H, Qian L, Yang S, Wang T, Zhang W, Wu X, Dallas S, Wilson B, Reece JM, et al. MAC1 mediates LPS-induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55(13):1362–73. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- 17.Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52(1):78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 18.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Choi DH, Block ML, Lorenzl S, Yang L, Kim YJ, Sugama S, Cho BP, Hwang O, Browne SE, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21(1):179–87. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 21.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23(6):2150–60. doi: 10.1523/JNEUROSCI.23-06-02150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML. Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain. 2010;133(Pt 3):808–21. doi: 10.1093/brain/awp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta G, Barber DS, Zhang P, Doperalski NJ, Liu B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J Neurochem. 2012;122(4):752–63. doi: 10.1111/j.1471-4159.2012.07826.x. [DOI] [PubMed] [Google Scholar]
- 24.Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. J Neurotrauma. 2011;28(9):1783–801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia. 2012 doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 26.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGeer EG, McGeer PL. Neuroinflammation in Alzheimer's disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19(1):355–61. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- 29.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35(Pt 5):1127–32. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 30.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 31.Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41(4):535–47. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 32.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 33.Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100(26):15983–8. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, Liu XG. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain Behav Immun. 2010;24(6):874–80. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90(1):89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- 36.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119(1):89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 37.Kish SJ, Shannak K, Rajput A, Deck JH, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson's disease. J Neurochem. 1992;58(2):642–8. doi: 10.1111/j.1471-4159.1992.tb09766.x. [DOI] [PubMed] [Google Scholar]
- 38.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 39.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 40.de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5(6):525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 41.Schapira AH. Science, medicine, and the future: Parkinson's disease. BMJ. 1999;318(7179):311–4. doi: 10.1136/bmj.318.7179.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson's disease: Specific evidence in humans and mammalian models. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Fallon L, Lashuel HA, Liu Z, Lansbury PT., Jr The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson's disease susceptibility. Cell. 2002;111(2):209–18. doi: 10.1016/s0092-8674(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson's disease? Ann Neurol. 2008;64(2):S3–15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesage S, Brice A. Role of Mendelian genes in “sporadic” Parkinson's disease. Parkinsonism Relat Disord. 2012;18(1):S66–70. doi: 10.1016/S1353-8020(11)70022-0. [DOI] [PubMed] [Google Scholar]
- 46.Naert G, Rivest S. The role of microglial cell subsets in Alzheimer's disease. Curr Alzheimer Res. 2011;8(2):151–5. doi: 10.2174/156720511795256035. [DOI] [PubMed] [Google Scholar]
- 47.Phani S, Re DB, Przedborski S. The Role of the Innate Immune System in ALS. Front Pharmacol. 2012;3:150. doi: 10.3389/fphar.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doring A, Yong VW. The good, the bad and the ugly. Macrophages/microglia with a focus on myelin repair. Front Biosci (Schol Ed) 2011;3:846–56. doi: 10.2741/191. [DOI] [PubMed] [Google Scholar]
- 49.Moller T. Neuroinflammation in Huntington's disease. J Neural Transm. 2010;117(8):1001–8. doi: 10.1007/s00702-010-0430-7. [DOI] [PubMed] [Google Scholar]
- 50.German DC, Eagar T, Sonsalla PK. Parkinson's Disease: A Role for the Immune System. Curr Mol Pharmacol. 2011 [PubMed] [Google Scholar]
- 51.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson's disease. Brain Behav Immun. 2009;23(1):55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2008;167(1):90–5. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 53.Panaro MA, Cianciulli A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson's disease. Curr Pharm Des. 2012;18(2):200–8. doi: 10.2174/138161212799040574. [DOI] [PubMed] [Google Scholar]
- 54.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 55.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21(2):404–12. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57(2):168–75. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 57.Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson's disease. Parkinsonism Relat Disord. 2012 doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704(1):112–6. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- 59.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–21. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 60.Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7(5-6):654–61. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 61.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81(6):1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 62.Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70(4):1584–92. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- 63.Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front Biosci. 2003;8:s826–37. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- 64.Langston J, Ballard P, Tetrud J, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 65.Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson's disease. Parkinsonism Relat Disord. 2012;18(1):S183–5. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- 66.Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. FASEB J. 2003;17(13):1957–9. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- 67.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46(4):598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 68.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54(5):599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 69.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16(11):1474–6. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 70.Hoang T, Choi DK, Nagai M, Wu DC, Nagata T, Prou D, Wilson GL, Vila M, Jackson-Lewis V, Dawson VL, et al. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radic Biol Med. 2009;47(7):1049–56. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27(12):3328–37. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5(12):1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 73.Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, Liu Y, Qian L, Wilson B, Di Monte DA, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Immunol. 2008;181(10):7194–204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Wang Y, Kovacs M, Jin J, Zhang J. Microglial activation induced by neurodegeneration: a proteomic analysis. Mol Cell Proteomics. 2005;4(10):1471–9. doi: 10.1074/mcp.M500114-MCP200. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, McGeer PL, Hong JS, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55(11):1178–88. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 77.Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson's disease. FASEB J. 2003;17(3):500–2. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- 78.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O, Shin DH, Chun HS, Beal MF, Joh TH. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25(14):3701–11. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23(15):6181–7. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515–7. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 83.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, Davis RE, Parker WD., Jr Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol. 2000;162(1):37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 84.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27(12):639–45. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Cohen G. Oxidative stress, mitochondrial respiration, and Parkinson's disease. Ann N Y Acad Sci. 2000;899:112–20. doi: 10.1111/j.1749-6632.2000.tb06180.x. [DOI] [PubMed] [Google Scholar]
- 86.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(3):S26–36. doi: 10.1002/ana.10483. discussion S36-8. [DOI] [PubMed] [Google Scholar]
- 87.Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- 88.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269(8):1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 89.Youdim MB, Ben-Shachar D, Riederer P. Iron in brain function and dysfunction with emphasis on Parkinson's disease. Eur Neurol. 1991;31(1):34–40. doi: 10.1159/000116719. [DOI] [PubMed] [Google Scholar]
- 90.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 91.Sorce S, Krause KH. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11(10):2481–504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- 92.Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS. Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J. 2005;19(6):550–7. doi: 10.1096/fj.04-2857com. [DOI] [PubMed] [Google Scholar]
- 93.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 94.Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21(4):374–84. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S, Aruga T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8(9-10):1583–96. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 97.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298(12):659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 98.Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77(4):540–51. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 100.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28(7):1347–54. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 101.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11(6):1249–63. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 103.Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J. 2003;17(13):1954–6. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 104.Mao H, Liu B. Synergistic microglial reactive oxygen species generation induced by pesticides lindane and dieldrin. Neuroreport. 2008;19(13):1317–20. doi: 10.1097/WNR.0b013e32830b3677. [DOI] [PubMed] [Google Scholar]
- 105.Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci. 2003;23(4):1228–36. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A. 2003;100(10):6145–50. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41(2-3):242–7. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 108.Mao H, Fang X, Floyd KM, Polcz JE, Zhang P, Liu B. Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res. 2007;1186:267–74. doi: 10.1016/j.brainres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 109.Hertzman C, Wiens M, Bowering D, Snow B, Calne D. Parkinson's disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17(3):349–55. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- 110.Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. 1997;48(6):1583–8. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 111.Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26(7):547–55. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169(8):919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chanyachukul T, Yoovathaworn K, Thongsaard W, Chongthammakun S, Navasumrit P, Satayavivad J. Attenuation of paraquat-induced motor behavior and neurochemical disturbances by L-valine in vivo. Toxicol Lett. 2004;150(3):259–69. doi: 10.1016/j.toxlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, et al. Carrier-mediated processes in blood--brain barrier penetration and neural uptake of paraquat. Brain Res. 2001;906(1-2):135–42. doi: 10.1016/s0006-8993(01)02577-x. [DOI] [PubMed] [Google Scholar]
- 115.McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10(2):119–27. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 116.Fei Q, McCormack AL, Di Monte DA, Ethell DW. Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J Biol Chem. 2008;283(6):3357–64. doi: 10.1074/jbc.M708451200. [DOI] [PubMed] [Google Scholar]
- 117.Richardson JR, Quan Y, Sherer TB, Greenamyre JT, Miller GW. Paraquat neurotoxicity is distinct from that of MPTP and rotenone. Toxicol Sci. 2005;88(1):193–201. doi: 10.1093/toxsci/kfi304. [DOI] [PubMed] [Google Scholar]
- 118.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96(22):12760–5. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134(1):52–6. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23(8):3095–9. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet. 2005;14(24):3885–97. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 122.Cicchetti F, Lapointe N, Roberge-Tremblay A, Saint-Pierre M, Jimenez L, Ficke BW, Gross RE. Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiol Dis. 2005;20(2):360–71. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 123.Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25(2):392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mangano EN, Litteljohn D, So R, Nelson E, Peters S, Bethune C, Bobyn J, Hayley S. Interferon-gamma plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging. 2012;33(7):1411–26. doi: 10.1016/j.neurobiolaging.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 125.Rappold PM, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, Sen N, Javitch JA, Tieu K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci U S A. 2011;108(51):20766–71. doi: 10.1073/pnas.1115141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klintworth H, Garden G, Xia Z. Rotenone and paraquat do not directly activate microglia or induce inflammatory cytokine release. Neurosci Lett. 2009;462(1):1–5. doi: 10.1016/j.neulet.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yadav S, Gupta SP, Srivastava G, Srivastava PK, Singh MP. Role of secondary mediators in caffeine-mediated neuroprotection in maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. Neurochem Res. 2012;37(4):875–84. doi: 10.1007/s11064-011-0682-0. [DOI] [PubMed] [Google Scholar]
- 128.Peng J, Stevenson FF, Oo ML, Andersen JK. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic Biol Med. 2009;46(2):312–20. doi: 10.1016/j.freeradbiomed.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fussell KC, Udasin RG, Gray JP, Mishin V, Smith PJ, Heck DE, Laskin JD. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic Biol Med. 2011;50(7):874–82. doi: 10.1016/j.freeradbiomed.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fisher HK, Clements JA, Wright RR. Enhancement of oxygen toxicity by the herbicide paraquat. Am Rev Respir Dis. 1973;107(2):246–52. doi: 10.1164/arrd.1973.107.2.246. [DOI] [PubMed] [Google Scholar]
- 132.Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–39. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119(6):866–72. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dhillon AS, Tarbutton GL, Levin JL, Plotkin GM, Lowry LK, Nalbone JT, Shepherd S. Pesticide/environmental exposures and Parkinson's disease in East Texas. J Agromedicine. 2008;13(1):37–48. doi: 10.1080/10599240801986215. [DOI] [PubMed] [Google Scholar]
- 135.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and New Animal Models of Parkinson's Disease. Journal of Biomedicine and Biotechnology. 2012 doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ravanel P, Tissut M, Douce R. Effects of rotenoids on isolated plant mitochondria. Plant Physiol. 1984;75(2):414–20. doi: 10.1104/pp.75.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med. 2008;44(11):1873–86. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22(3):782–90. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson's disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 140.Inden M, Kitamura Y, Abe M, Tamaki A, Takata K, Taniguchi T. Parkinsonian rotenone mouse model: reevaluation of long-term administration of rotenone in C57BL/6 mice. Biol Pharm Bull. 2011;34(1):92–6. doi: 10.1248/bpb.34.92. [DOI] [PubMed] [Google Scholar]
- 141.Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem. 2003;84(3):491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- 142.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179(1):9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 143.Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187(2):418–29. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 144.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34(2):279–90. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136(1):317–24. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 146.Phinney AL, Andringa G, Bol JG, Wolters E, van Muiswinkel FL, van Dam AM, Drukarch B. Enhanced sensitivity of dopaminergic neurons to rotenone-induced toxicity with aging. Parkinsonism Relat Disord. 2006;12(4):228–38. doi: 10.1016/j.parkreldis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 147.Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol. 2004;190(2):373–83. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 148.Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson's disease. Neurosci Lett. 2003;341(2):87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 149.Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57(6):645–56. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G. Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology. 2007;32(12):2570–80. doi: 10.1038/sj.npp.1301381. [DOI] [PubMed] [Google Scholar]
- 151.Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem. 2006;97(4):934–46. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- 152.Zhou F, Yao HH, Wu JY, Ding JH, Sun T, Hu G. Opening of microglial K(ATP) channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12(5A):1559–70. doi: 10.1111/j.1582-4934.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferger AI, Campanelli L, Reimer V, Muth KN, Merdian I, Ludolph AC, Witting A. Effects of mitochondrial dysfunction on the immunological properties of microglia. J Neuroinflammation. 2010;7:45. doi: 10.1186/1742-2094-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou H, Zhang F, Chen SH, Zhang D, Wilson B, Hong JS, Gao HM. Rotenone activates phagocyte NADPH oxidase by binding to its membrane subunit gp91phox. Free Radic Biol Med. 2012;52(2):303–13. doi: 10.1016/j.freeradbiomed.2011.10.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163(12):6732–40. [PubMed] [Google Scholar]
- 156.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic requirement of p47phox for superoxide production by murine microglia. FASEB J. 2001;15(2):285–7. doi: 10.1096/fj.00-0608fje. [DOI] [PubMed] [Google Scholar]
- 157.Moretto A, Colosio C. Biochemical and toxicological evidence of neurological effects of pesticides: the example of Parkinson's disease. Neurotoxicology. 2011;32(4):383–91. doi: 10.1016/j.neuro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 158.Richardson JR, Shalat SL, Buckley B, Winnik B, O'Suilleabhain P, Diaz-Arrastia R, Reisch J, German DC. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol. 2009;66(7):870–5. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol. 1998;150(2):263–71. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- 160.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology. 2005;26(4):701–19. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 161.Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36(1):100–3. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 162.Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp Neurol. 1998;150(2):339–42. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- 163.Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health A. 2000;59(4):229–34. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 164.Weisskopf MG, Knekt P, O'Reilly EJ, Lyytinen J, Reunanen A, Laden F, Altshul L, Ascherio A. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74(13):1055–61. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bloomquist JR, Barlow RL, Gillette JS, Li W, Kirby ML. Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. Neurotoxicology. 2002;23(4-5):537–44. doi: 10.1016/s0161-813x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 166.Karen DJ, Li W, Harp PR, Gillette JS, Bloomquist JR. Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology. 2001;22(6):811–7. doi: 10.1016/s0161-813x(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 167.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20(10):424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 168.Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315(1):69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- 169.Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. FASEB J. 2006;20(10):1695–7. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- 170.Hatcher JM, Richardson JR, Guillot TS, McCormack AL, Di Monte DA, Jones DP, Pennell KD, Miller GW. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204(2):619–30. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31(11):1473–85. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]