Abstract
Background
Fabry disease (FD) is a rare lysosomal storage disorder also affecting the heart. The aims of this study were to determine the frequency of cardiac troponin I (cTNI) elevation, a sensitive parameter reflecting myocardial damage, in a smaller cohort of FD-patients, and to analyze whether persistent cTNI can be a suitable biomarker to assess cardiac dysfunction in FD.
Methods
cTNI values were determined at least twice per year in 14 FD-patients (6 males and 8 females) regularly followed-up in our centre. The data were related to other parameters of heart function including cardiac magnetic resonance imaging (cMRI).
Results
Three patients (21%) without specific vascular risk factors other than FD had persistent cTNI-elevations (range 0.05–0.71 ng/ml, normal: <0.01). cMRI disclosed late gadolinium enhancement (LGE) in all three individuals with cTNI values ≥0.01, while none of the 11 patients with cTNI <0.01 showed a pathological enhancement (p<0.01). Two subjects with increased cTNI-values underwent coronary angiography, excluding relevant stenoses. A myocardial biopsy performed in one during this procedure demonstrated substantial accumulation of globotriaosylceramide (Gb3) in cardiomyocytes.
Conclusion
Continuous cTNI elevation seems to occur in a substantial proportion of patients with FD. The high accordance with LGE, reflecting cardiac dysfunction, suggests that cTNI-elevation can be a useful laboratory parameter for assessing myocardial damage in FD.
Introduction
Fabry disease (FD) is a rare X-linked disorder affecting hemizygous males and heterozygous females. Deficiency of the lysosomal enzyme alpha-galactosidase A (α-GAL-A) results in accumulation of glycosphingolipids with terminal alpha-galactosyl residues in various organs and tissues, in particular in the central and peripheral nervous system, vessels and kidneys. Approximately 60% of patients with FD have cardiac involvement such as ventricular hypertrophy, valvular heart disease, conduction defects, or coronary artery disease [1], [2]. These alterations predispose to congestive heart failure, arrhythmia, and myocardial infarction, and can determine the prognosis of the disease [3]–[5]. In FD, assessment of disease burden by specific biomarkers is crucial, since this can disclose preclinical organ involvement, and can help to make therapeutic decisions timely [6]–[8].
Cardiac troponin I (cTNI) is a laboratory parameter well known to reflect acute and chronic cardiac muscular damage [9]. Recently, we noticed a continuously elevated cTNI in a FD patient with cardiac involvement, but without coronary artery disease [10]. The purpose of this study was to investigate whether cTNI can be a suitable biomarker for assessment of the cardiac status in FD. To accomplish this, we determined cTNI levels in a smaller cohort of patients with FD and related the data to other parameters of cardiac function.
Patients and Methods
Patients
The study cohort included 14 patients with FD treated and followed-up in our centre (6 males and 8 females). Specific parameters such as enzyme activity (α-GAL-A reference range 33.2–109 nmol MU/h/mg protein) and levels of Lyso-globotriaosylceramide in serum (Lyso-Gb3, values >0.5 ng/ml were interpreted as increased) were determined in all patients. The definite diagnosis of FD had been confirmed by molecular genetic analysis demonstrating a heterozygous or hemizygous mutation in the α-GAL-A-gene [11]. Specific signs and symptoms as characteristic for FD such as angioceratoma, cornea verticillata, gastrointestinal symptoms, and any other clinical signs related to FD pathology were recorded systematically.
Age at last follow-up ranged from 21–80 years (median 50 years). 7 subjects received enzyme replacement therapy with recombinant alpha galactosidase A. In all patients the routine follow-up protocol encompassed laboratory testing including cTNI determination at least twice per year, assessment of the patient’s overall condition and his neurological, cardiac, and nephrological status. Magnetic Resonance Imaging (MRI) of the brain and the heart was done annually.
Ethics Statement
The study protocol was reviewed by the ethical committee of the medical faculty of the Justus Liebig University Giessen. To use routine clinical data the ethical board recommend obtaining patient’s written informed consent from each participant if possible. In 13 patients a written consent was obtained, one patient could not be contacted any longer. The ethical board approved the conduction of the analysis.
Cardiac Assessment
cTNI, was measured by the ADVIA Centaur® TnI-Ultra immunoassay (Bayer HealthCare, Tarrytown USA). A cTNI cut-off level of ≥0.01 ng/ml was considered as relevant elevation. Furthermore, brain natriuretic peptide (BNP) was determined in all patients. Values >37 pg/ml were considered as abnormal.
In all patients, a routine electrocardiogram (ECG), a Holter-ECG and a cardiac ultrasound were performed. Arrhythmia was diagnosed if one of the following symptoms was detected in Holter-ECG: persistent or intermittent atrial fibrillation or flatter, sustained tachycardia (heart rate ≥100/minute for more than 30 seconds), non-sustained tachycardia (heart rate ≥100/minute for less than 30 seconds in at least 3 subsequent heart cycles), incomplete bundle branch block (QRS-duration: 100–119 ms) or complete bundle branch block (QRS-duration ≥120 ms).
Cardiac MRI findings were analyzed by an experienced radiologist blinded from any clinical data. The following parameters were systematically assessed: left ventricular ejection fraction (LVEF), left ventricular (LV) hypertrophy, right ventricular (RV) hypertrophy, left ventricular posterior wall thickness measured during diastole (LVPWd), interventricular septum thickness during diastole (IVSd), LV mass index, and late gadolinium enhancement (LGE). The LGE technique (8-mm slice thickness, breath hold, short heart axis) was applied to detect changes in tissue integrity in the left ventricle. Short-axis views at the basal, mid, and apical segments were used for the semiquantitative assessment of the appearance of gadolinium enhancement in every left ventricular segment indicating the occurrence of intramyocardial fibrosis.
Nephrological Assessment
Glomerular filtration rate (eGFR) was calculated according to the simplified MDRD equation: eGFR (ml/min/1,73 m2 body surface) = 186×(serum creatinine)−1.154×(age) −0.203×0.742 (if female)×1.212 (if black) [12]. In addition, protein and albumin excretion was determined in a 24 h urine sample. The reference range for proteinuria was <159 mg/24 h, and that for albuminuria was <30 mg/24 h.
Neurological Assessment
A thorough neurological examination was performed in all patients at least twice per year. Small fibre dysfunction was assessed by quantitative sensory testing. Affection status of the brain supplying arteries was determined by measuring intima-media thickness in the common carotid artery by means of transcranial Doppler sonography. In addition, all patients underwent a brain MRI for assessment of white matter lesions.
Statistical Analysis
Data are presented as median values and range. cTNI levels were related to the nephrological data and various cardiac and cardiac MRI parameters obtained at last follow-up examination. Cardiac MRI and other parameters were compared between patients with cTNI values ≥0.01 ng/ml and those with normal values (<0.01 ng/ml).
Results
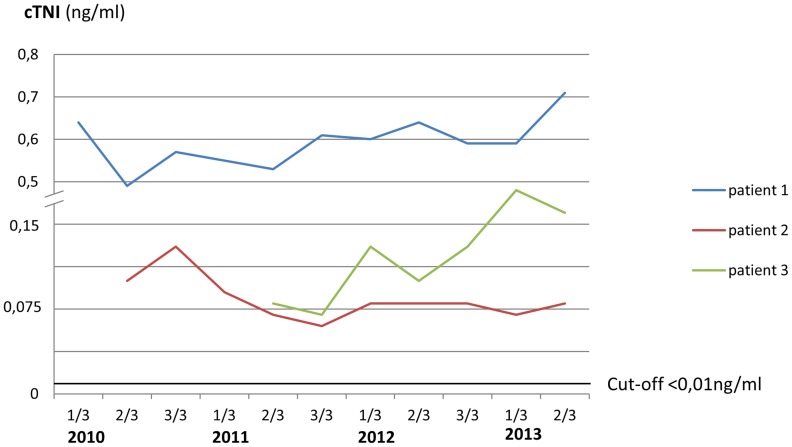
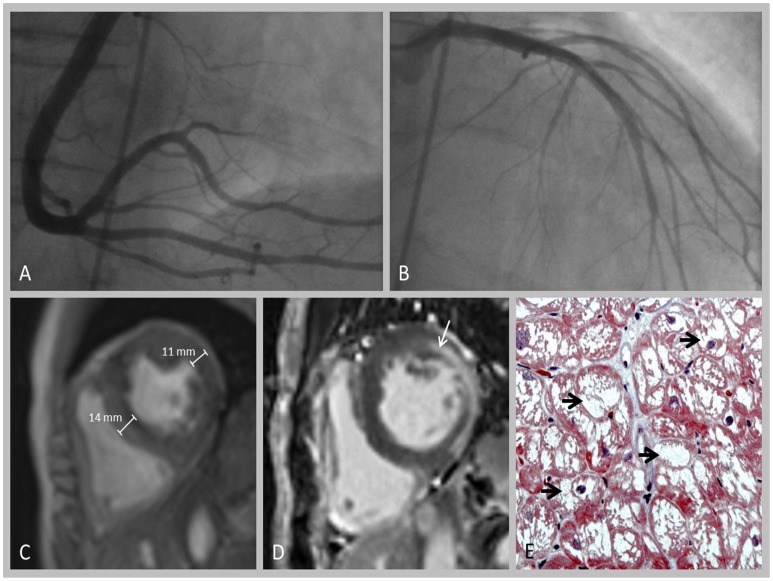
The underlying mutations in all 14 patients are summarized in table 1. In 3 out of 14 patients (2 females and one male) (21%), repetitive cTNI assessments (3–5 times per year) demonstrated continuously elevated values, reflecting persistent cTNI-elevation (Figure 1). Anthropometrical and clinical data, medical history, and laboratory and cardiac findings of these three patients in relation to subjects without cTNI-elevation and to normal values are summarized in table 2. All three individuals with elevated cTNI levels were treated by ERT since 5, 3, and 6 years, respectively; while cTNI-measurements were started 5, 3 and 4 years after initiation of ERT. cTNI in subjects with increased values ranged from 0.06 to 0.71 ng/ml. None of them had vascular risk factors other than FD, including hypertension, diabetes and hyper-cholesterolemia. One patient temporarily complained of symptoms compatible with angina pectoris, whereas none of them had an acute coronary syndrome or stroke prior to or after making the diagnosis of FD. A coronary angiography performed in 2 of them did not reveal coronary artery stenoses. Patient 2 underwent a myocardial biopsy, demonstrating a relevant accumulation of Gb3 in cardiomyocytes (Figure 2).
Table 1. Synopsis of alpha-galactosidase gene mutations in 14 patients included in the study.
| Mutation | Patients |
| c.424>C [C142R] | Patient 1 |
| c.514T>G [C172G] | Patient 2 |
| c.994dupA [p.Arg332Lysfs*7] | Patient 3 |
| c.755G>C [R252T] | 1 female, 2 males |
| c.376A>G [S126G] | 2 females, 2 males |
| c.1025G>A [R342Q] | 1 female, 1 male |
| c.416A>G [N139S] | 1 female |
| c.376A>G [s126G] | 1 female |
Figure 1. cTNI-values in 3 patients with Fabry disease.
Table 2. Baseline data, medical history, biomarkers and cardiac work up in patients with FD in relation to cardiac troponin I elevation and normal values.
| Patient 1 | Patient 2 | Patient 3 | FD-patients without cTNIelevation (n = 11) | |
| cTNI (ng/ml); median (range) | 0.59 (0.49–0.71) | 0.08 (0.06–0.13) | 0.073 (0.07–0.18) | <0.01 |
| Baseline data | ||||
| Age | 61 | 59 | 35 | 50 (21–80) |
| Male | No | No | Yes | 5 |
| BMI (kg/m2) | 19.8 | 19.3 | 22.4 | 23.6 (20.4–30.8) |
| Medical history | ||||
| Hypertension | No | No | No | 0 |
| Diabetes mellitus | No | No | No | 0 |
| Hypercholesterolemia (n = 9) | No | No | No | 0 |
| Current Smoker | No | No | No | 0 |
| Peripheral artery occlusive disease | No | No | No | 0 |
| Angina pectoris | Yes | No | No | 0 |
| Intima media thickness | No | No | No | 0 |
| Acute coronary syndrome | No | No | No | 0 |
| Previous stroke | No | No | No | 3 |
| Angioceratoma | No | Yes | Yes | 3 |
| Cornea verticillata | Yes | Yes | Yes | 1 |
| Gastrointestinal symptoms | Yes | Yes | Yes | 2 |
| Small fibre neuropathy* | Yes | Yes | Yes | 3 |
| White matter lesions (cerebral MRI) | Yes | n.d. | No | 5 |
| Clinical chemistry | ||||
| α-GAL-A activity (nmol MU/mg protein); (reference range >33); (n = 13) | 5 | 2.6 | 0.02 | 49 (26–63) |
| Serum-Lyso-Gb3 (ng/ml) (reference range <0.5), (n = 13) | 15.2 | 21.7 | 67.1 | 0.1 (LLQ§-0.73) |
| Brain natriuretic peptide (pg/ml); (reference range <37); (n = 12) | 161 | 288 | 15 | 16 (2–90) |
| Serum-Creatinin (mg/ml) | 1.1 | 0.7 | 1.2 | 0.8 (0.7–1) |
| eGFR(ml/min/1.73 m2) | 53.4 | 88.7 | 73.2 | 84.4 (64.9–151.3) |
| Proteinuria (mg/24 h); (reference range <159 mg/24 h) | 1141 | 1062 | 318 | 81.8 (52–160.2) |
| Albuminuria (mg//24 h); (reference range <30 mg/24 h) | 657 | 739 | 204 | 21 (17–30.9) |
| Cardiac MRI (n = 13) | ||||
| LVEF (%); (reference range ≤60%) | 77 | 65 | 64 | 62 (46–70) |
| LV-hypertrophy/concentric | Yes | Yes | Yes | 0 |
| LV-hypertrophy/eccentric | No | No | No | 0 |
| RV-hypertrophy | No | No | No | 0 |
| Ventricular wall thickness (mm); (reference range ≤11 mm)† | 14 | 11 | 10 | 8 (6–11) |
| Ventricular Septum (mm); (reference range ≤11 mm) | 14 | 14 | 8 | 7 (5–10) |
| LV-mass index (g/m2) (reference range ≤115 g/m2 inmales and ≤95 g/m2 in females) | 133.4 | 92 | 141.9 | 101 (65.2–139.5) |
| LGE | 1 | 1 | 1 | 0 |
| Electrocardiogram | ||||
| Sinus rhythm | Yes | Yes | Yes | 11 |
| PQ-duration (ms); (reference range ≤200 ms) | 216 | 116 | 134 | 138 (116–164) |
| QRS-duration (ms); (reference range ≤100 ms) | 90 | 110 | 94 | 88 (76–112) |
| QT-duration (ms); (reference range <460 ms) | 411 | 415 | 384 | 397 (370–432) |
| Holter | ||||
| Arrhythmia$ | 1 | 1 | 0 | 1 |
| Pacemaker | No | No | No | 1 |
*a small fibre dysfunction was proved by quantitative sensory testing or by skin biopsy.
measurement end-diastolic in the posterior wall of the left ventricle.
Arrhythmia was considered if one of the following conditions was detected: persistent or intermittent atrial fibrillation of flatter, sustained tachycardia (heart rate ≥100/minute for more than 30 seconds), non-sustained tachycardia (heart rate ≥100/minute for less than 30 seconds in at least 3 subsequent hear cycles), incomplete bundle branch block (QRS-duration: 100–119 ms) or complete bundle branch block (QRS-duration ≥120 ms).
lower level of quantification.
Figure 2. Cardiac work up in patient 2.
A+B: Coronary angiography (A: right coronary artery; B: left coronary artery) demonstrating no relevant pathology. C+D: Cardiac MRI showing increase in myocardial wall thickness (C) and pathological late gadolinium enhancement (D, arrow). E: Myocardial biopsy revealing strong accumulation of Gb3, as indicated by numerous vacuoles within the cardiomyocytes (arrow).
All FD patients revealed values for the LVEF within normal ranges. One patient showed left ventricular hypertrophy and 5 had elevated BNP-values. Ventricular tachycardias were detected by Holter-ECG in 3 patients, necessitating pacemaker implantation in one. Comparison of cardiac MRI parameters between patients with and without cTNI elevation revealed higher LVPWd thickness in those with increased cTNI levels, whereas IVSd and EF did not differ between the two groups. Importantly, all three patients with elevated cTNI values showed LGE, whereas this was not the case in any of the other subjects. The three patients with increased cTNI-levels had concentric left ventricular hypertrophy, two showed distinctly increased IVSd thickness (both 14 mm). All three subjects with cTNI elevation had proteinuria and albuminuria, while eGFRs were not relevantly reduced (53.4–88.7 ml/min/1.73 m2).
During follow-up, in the patients with continuously increased cTNI-values, serial echocardiographic examinations revealed no significant changes of cardiac wall thickness (data not shown).
Discussion
The main findings of this study were that approximately 20% of patients in this FD cohort had continuously increased cTNI values, and that cTNI elevation was highly correlated with pathologic LGE in the cardiac MRI.
In FD it is assumed that the cardiac pathology is mainly related to progressive cardiac hypertrophy [13]. Storage of Gb3 has been demonstrated in various structures of the heart including cardiomyocytes, cells of the conduction system, fibroblasts and endothelium cells within all types of vessels [14]. The proposed pathophysiological mechanism is an absolute and relative ischemia determined by cardiac hypertrophy on one hand, and angiopathy of small arteriols and capillaries on the other [14], [15].
In two of our patients with elevated cTNI values, coronary macroangiopathy could be ruled out by angiography, performed to clarify cTNI elevation. Persistent cTNI elevation secondary to small vessel stenosis also seems unlikely, because no clinical signs and no electrocardiographic abnormalities characteristic of myocardial ischemia were detected. Furthermore, distribution of LGE within the myocardial wall does not support the hypothesis of an ischemic mechanism in these subjects. Therefore, a direct damage of cardiomyocytes by abnormal Gb3 storage appears to be the most likely cause of persistent cTNI elevation. This assumption is also supported by the marked accumulation of Gb3 in cardiomyocytes as observed in the myocardial biopsy of patient two (Figure 2).
Since cTNI elevation is usually considered highly specific for myocardial damage, this finding often prompts further examinations such as coronary angiography. Therefore, the observation of increased cTNI-values in FD patients without coronary artery disease is of clinical relevance, since it may help to avoid nonessential invasive procedures [9], [16].
However, when interpreting our results, potential causes for cTNI elevation other than cardiac pathology needs to be taken into account. Elevated cTNI values not related to myocardial damage have been described in patients with critical illness, sepsis, stroke, and seizures [9], [17]–[24]. The latter two causes are typical central nervous system symptoms complicating FD. But since none of our three patients with continuously increased cTNI levels suffered from stroke or epilepsy, these mechanisms can be excluded. Measuring cTNI by immunoassays as done in our study has been identified as a potential source of false positive results [22]. However, as cTNI determinations were performed in all our patients with the same test and device, and since only subjects with LGE in cardiac MRI had elevated values, this makes a systematic analytical error in our cohort unlikely.
Further, it has been shown that renal insufficiency can result in elevated cTNI values [22], [25]–[29]. Indeed, all three subjects with cTNI-elevation in our study group also showed signs of renal involvement such as reduced GFR, proteinuria, albuminuria, or mildly increased creatinine values. Brunet and colleagues investigated cTNI alterations in patients with end-stage renal disease [25]. Comparing their data with those obtained in our subjects with relatively well preserved renal function, shows similar cTNI levels in two of our individuals, while the third one had even distinctly higher values than patients with end-stage renal disease. But collectively, it can not be excluded definitely that renal impairment contributed additionally to continuous cTNI-elevations in our patients. Conversely, our findings highlight that FD is a differential diagnosis in patients with otherwise unexplained cTNI elevations.
Late gadolinium enhancement in cMRI is a valid and sensitive method for visualizing myocardial interstitial abnormalities in FD [30], [31]. In this study all subjects with cTNI elevation also had LGE, whereas this was not the case in any of the other patients, thus pointing to considerable convergence between cTNI-values and cardiac pathology in FD.
Recently, a correlation between genotype, phenotype and clinical severity in FD has been emphasized [32]. Lyso-Gb3, is a biomarker considered to be closely related to disease severity [32]. Our patients with continuously increased cTNI values also had elevated lyso-Gb3 levels, but due to the small patient sample and since Gb3 values were not determined systematically in all our patients, we were not able to relate reliably cTNI levels to results of mutational analysis and lyso-Gb3 values.
Notably, all 3 FD subjects with cTNI elevations received ERT. Since these patients started ERT before first cTNI determination, it is not clear whether their cTNI values increased during or remained elevated despite ERT. However, the impact of a continuous cTNI release from cardiomyocytes under ERT deserves particular attention, since it can be speculated that ERT in its current form is not sufficient to avoid ongoing cardiac damage in such patients [33]. In addition, longitudinal cTNI determination or assessment of the so-called high sensitive troponin could serve to assess the therapeutic effect of ERT on the heart [34].
In summary, continuous cTNI release seems not to be infrequent in FD, and occurred in this study cohort exclusively in subjects with evidence of cardiac involvement. In FD, a persistent cTNI elevation appears to be primarily related to direct cardiac muscular injury by Gb3 deposition in myocytes. Further studies with larger numbers of patients are required to determine whether cTNI is a valid surrogate marker of cardiac involvement in FD.
Funding Statement
The authors have no support or funding to report.
References
- 1. Mehta A, Ricci R, Widmer U, Dehout F, Garcia LA, et al. (2004) Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 34: 236–242. [DOI] [PubMed] [Google Scholar]
- 2. Weidemann F, Strotmann JM, Breunig F, Niemann M, Maag R, et al. (2008) Misleading terms in Anderson-Fabry disease. Eur J Clin Invest 38: 191–196. [DOI] [PubMed] [Google Scholar]
- 3. Chimenti C, Morgante E, Tanzilli G, Mangieri E, Critelli G, et al. (2008) Angina in fabry disease reflects coronary small vessel disease. Circ Heart Fail 1: 161–169. [DOI] [PubMed] [Google Scholar]
- 4. O’Mahony C, Elliott P (2010) Anderson-Fabry disease and the heart. Prog Cardiovasc Dis 52: 326–335. [DOI] [PubMed] [Google Scholar]
- 5. Takenaka T, Teraguchi H, Yoshida A, Taguchi S, Ninomiya K, et al. (2008) Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol 51: 50–59. [DOI] [PubMed] [Google Scholar]
- 6. Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, et al. (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fauler G, Rechberger GN, Devrnja D, Erwa W, Plecko B, et al. (2005) Rapid determination of urinary globotriaosylceramide isoform profiles by electrospray ionization mass spectrometry using stearoyl-d35-globotriaosylceramide as internal standard. Rapid Commun Mass Spectrom 19: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 8. Feriozzi S, Germain DP, Di Vito R, Legrand A, Ricci R, et al. (2007) Cystatin C as a marker of early changes of renal function in Fabry nephropathy. J Nephrol 20: 437–443. [PubMed] [Google Scholar]
- 9. Adams JE, Bodor GS, vila-Roman VG, Delmez JA, Apple FS, et al. (1993) Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation 88: 101–106. [DOI] [PubMed] [Google Scholar]
- 10. Tanislav C, Feustel A, Franzen W, Wusten O, Schneider C, et al. (2011) Persistent increase in cardiac troponin I in Fabry disease: a case report. BMC Cardiovasc Disord 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rolfs A, Fazekas F, Grittner U, Dichgans M, Martus P, et al. (2013) Acute cerebrovascular disease in the young: the Stroke in Young Fabry Patients study. Stroke 44: 340–349. [DOI] [PubMed] [Google Scholar]
- 12. Tutarel O, Denecke A, Bode-Boger SM, Martens-Lobenhoffer J (2011) Schieffer B, et al (2011) Symmetrical dimethylarginine outperforms CKD-EPI and MDRD-derived eGFR for the assessment of renal function in patients with adult congenital heart disease. Kidney Blood Press Res 34: 41–45. [DOI] [PubMed] [Google Scholar]
- 13. Linhart A, Palecek T, Bultas J, Ferguson JJ, Hrudova J, et al. (2000) New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J 139: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 14. Hulkova H, Ledvinova J, Poupetova H, Bultas J, Zeman J, et al. (1999) [Postmortem diagnosis of Fabry disease in a female heterozygote leading to the detection of undiagnosed manifest disease in the family]. Cas Lek Cesk 138: 660–664. [PubMed] [Google Scholar]
- 15. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, et al. (2003) Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 349: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 16. Norris JW, Hachinski VC, Myers MG, Callow J, Wong T, et al. (1979) Serum cardiac enzymes in stroke. Stroke 10: 548–553. [DOI] [PubMed] [Google Scholar]
- 17. Sieweke N, Allendorfer J, Franzen W, Feustel A, Reichenberger F, et al. (2012) Cardiac Troponin I elevation after epileptic seizure. BMC Neurol 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, et al. (2006) Neuroanatomic correlates of stroke-related myocardial injury. Neurology 66: 1325–1329. [DOI] [PubMed] [Google Scholar]
- 19. Brobbey A, Ravakhah K (2004) Elevated serum cardiac troponin I level in a patient after a grand mal seizure and with no evidence of cardiac disease. Am J Med Sci 328: 189–191. [DOI] [PubMed] [Google Scholar]
- 20. Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G (2004) Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-alpha. Cerebrovasc Dis 18: 194–199. [DOI] [PubMed] [Google Scholar]
- 21. Dixit S, Castle M, Velu RP, Swisher L, Hodge C, et al. (2000) Cardiac involvement in patients with acute neurologic disease: confirmation with cardiac troponin I. Arch Intern Med. 160: 3153–3158. [DOI] [PubMed] [Google Scholar]
- 22. Inbar R, Shoenfeld Y (2009) Elevated cardiac troponins: the ultimate marker for myocardial necrosis, but not without a differential diagnosis. Isr Med Assoc J 11: 50–53. [PubMed] [Google Scholar]
- 23. Stollberger C, Finsterer J (2004) Cardiac troponin levels following monitored epileptic seizures. Neurology 62: 1453. [DOI] [PubMed] [Google Scholar]
- 24. Woodruff BK, Britton JW, Tigaran S, Cascino GD, Burritt MF, et al. (2003) Cardiac troponin levels following monitored epileptic seizures. Neurology 60: 1690–1692. [DOI] [PubMed] [Google Scholar]
- 25. Brunet P, Oddoze C, Paganelli F, Indreies M, Faure V, et al. (2008) Cardiac troponins I and T in hemodialysis patients without acute coronary syndrome. Int J Cardiol 129: 205–209. [DOI] [PubMed] [Google Scholar]
- 26. Colivicchi F, Bassi A, Santini M, Caltagirone C (2004) Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke 35: 2094–2098. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Higgins JA (2003) Elevation of cardiac troponin I indicates more than myocardial ischemia. Clin Invest Med 26: 133–147. [PubMed] [Google Scholar]
- 28. Korff S, Katus HA, Giannitsis E (2006) Differential diagnosis of elevated troponins. Heart 92: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lim W, Qushmaq I, Devereaux PJ, Heels-Ansdell D, Lauzier F, et al. (2006) Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med 166: 2446–2454. [DOI] [PubMed] [Google Scholar]
- 30.Strotmann J, Breunig F, Wanner C, Weidemann F (2007) Progression of Fabry cardiomyopathy. Clin Ther 29 Suppl A: S13–4. [DOI] [PubMed]
- 31.Weidemann F, Strotmann J (2008) Early detection of Fabry disease: cardiac cases. Clin Ther 30 Suppl B: S46. [DOI] [PubMed]
- 32.Niemann M, Rolfs A, Stork S, Bijnens B, Breunig F et al.. (2014) Gene Mutations Versus Clinically Relevant Phenotypes-Lyso-Gb3 Defines Fabry Disease. Circ Cardiovasc Genet [Epub ahead of print]. [DOI] [PubMed]
- 33. Weidemann F, Niemann M, Stork S, Breunig F, Beer M, et al. (2013) Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 274: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gamble JH, Carlton EW, Orr WP, Greaves K (2013) High-sensitivity cardiac troponins-no more ‘negatives’. Expert Rev Cardiovasc Ther 11: 1129–1139. [DOI] [PubMed] [Google Scholar]