Abstract
Rat liver parenchyma harbors equal numbers of epidermal growth factor (EGF) and insulin receptors. Following administration of a saturating dose of EGF (10 micrograms/100 g body weight), there was a rapid (t1/2 approximately 1.1 min) internalization of receptor coincident with its tyrosine phosphorylation at residue 1173 and receptor recruitment of the adaptor protein SHC, its tyrosine phosphorylation and its association with GRB2 and the Ras guanine nucleotide exchange factor, mSOS, largely in endosomes. This led to a cytosolic pool of a complex of tyrosine-phosphorylated SHC, GRB2 and mSOS. It was demonstrated that these constituents were linked to Ras activation by the characteristic decrease in Raf-1 mobility on SDS-PAGE, which was maintained for 60 min after a single bolus of administered EGF. While insulin administration (15 micrograms/100 g body weight) led to insulin receptor beta-subunit tyrosine phosphorylation and internalization, there was little detectable tyrosine phosphorylation of SHC, recruitment of GRB2, association of a complex with mSOS or any detectable change in the mobility of Raf-1. Therefore, in normal physiological target cells in vivo, distinct signaling pathways are realized after EGF or insulin receptor activation, with regulation of this specificity most probably occurring at the locus of the endosome.
Full text
PDF
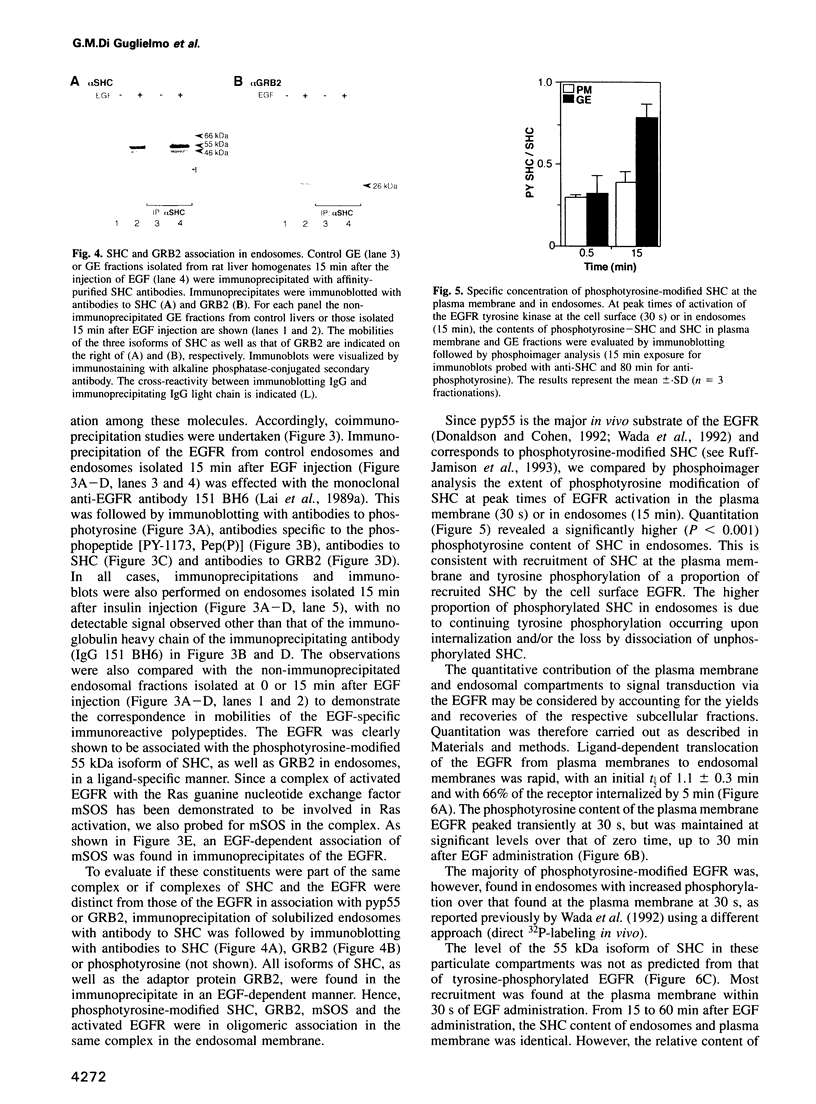
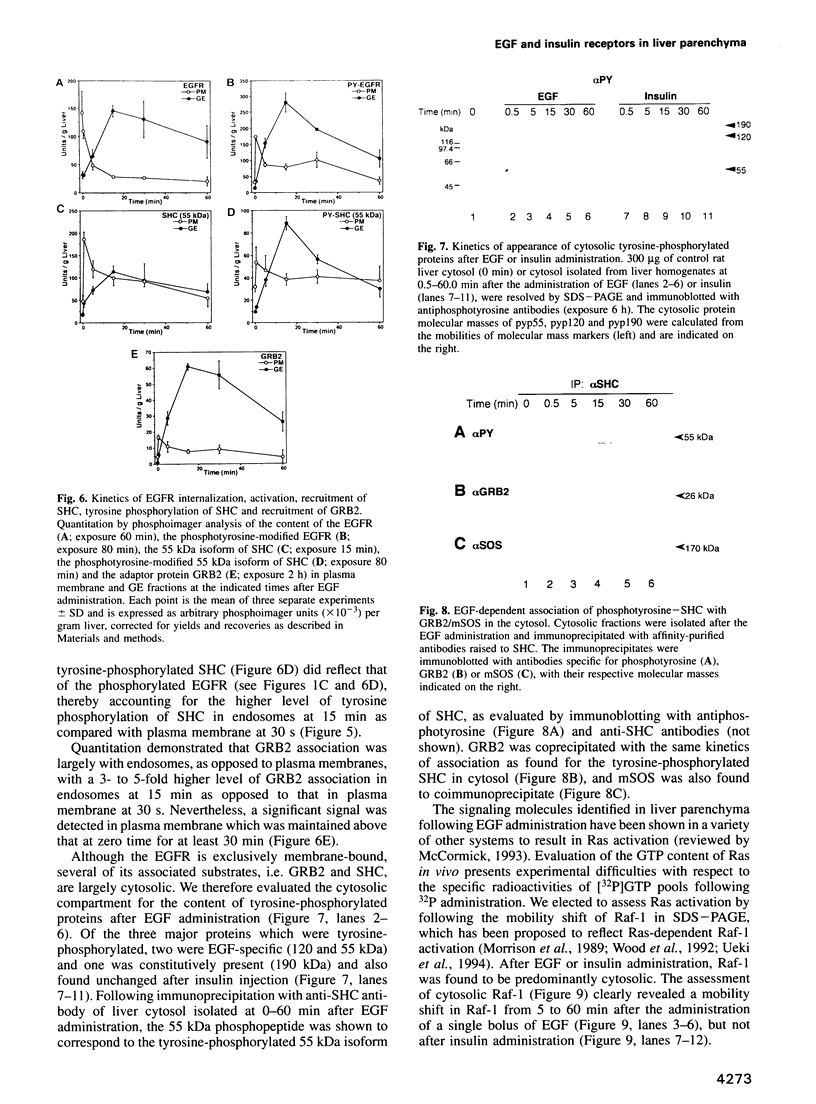
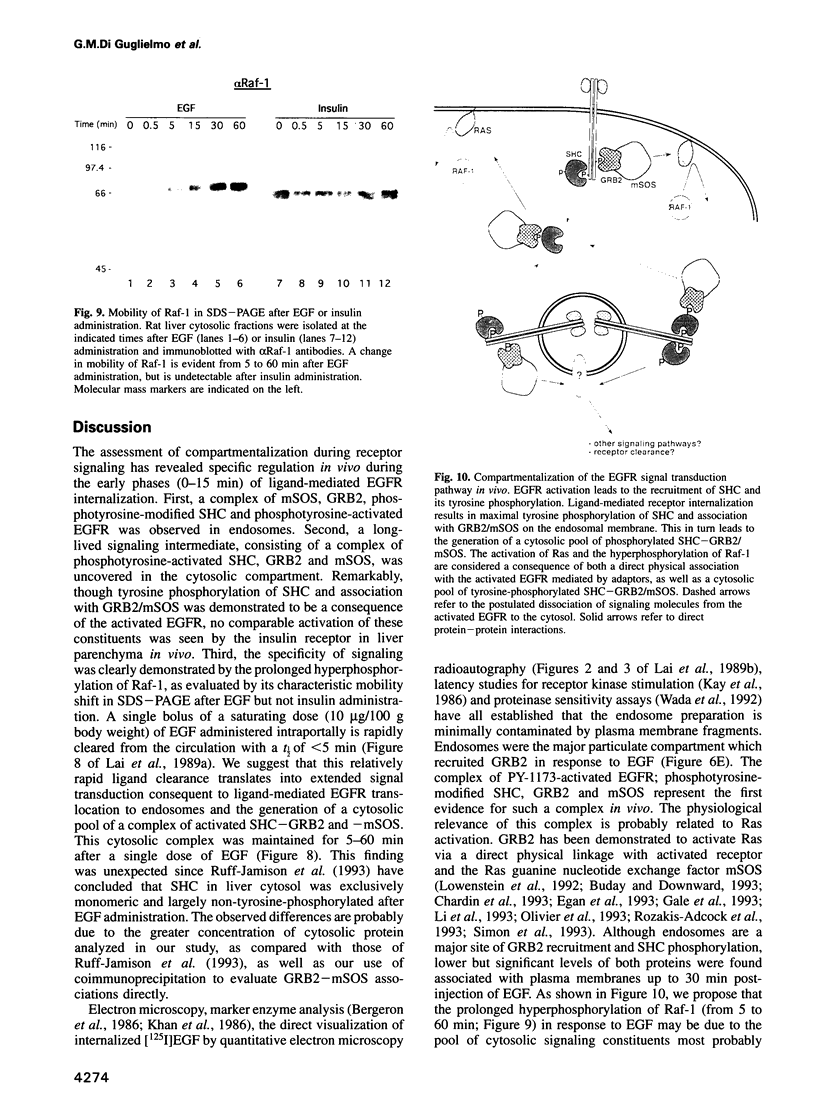



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Authier F., Rachubinski R. A., Posner B. I., Bergeron J. J. Endosomal proteolysis of insulin by an acidic thiol metalloprotease unrelated to insulin degrading enzyme. J Biol Chem. 1994 Jan 28;269(4):3010–3016. [PubMed] [Google Scholar]
- Backer J. M., Kahn C. R., White M. F. The dissociation and degradation of internalized insulin occur in the endosomes of rat hepatoma cells. J Biol Chem. 1990 Sep 5;265(25):14828–14835. [PubMed] [Google Scholar]
- Baltensperger K., Kozma L. M., Cherniack A. D., Klarlund J. K., Chawla A., Banerjee U., Czech M. P. Binding of the Ras activator son of sevenless to insulin receptor substrate-1 signaling complexes. Science. 1993 Jun 25;260(5116):1950–1952. doi: 10.1126/science.8391166. [DOI] [PubMed] [Google Scholar]
- Bangalore L., Tanner A. J., Laudano A. P., Stern D. F. Antiserum raised against a synthetic phosphotyrosine-containing peptide selectively recognizes p185neu/erbB-2 and the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11637–11641. doi: 10.1073/pnas.89.23.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer A. G., Rotin D., Ureña J. M., Skolnik E. Y., Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol. 1994 Aug;14(8):5192–5201. doi: 10.1128/mcb.14.8.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Evrard P., Berthet J. Electron microscopic examination of subcellular fractions. I. The preparation of representative samples from suspensions of particles. J Cell Biol. 1967 Jan;32(1):181–191. doi: 10.1083/jcb.32.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Rachubinski R., Searle N., Borts D., Sikstrom R., Posner B. I. Polypeptide hormone receptors in vivo: demonstration of insulin binding to adrenal gland and gastrointestinal epithelium by quantitative radioautography. J Histochem Cytochem. 1980 Aug;28(8):824–835. doi: 10.1177/28.8.6255031. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Searle N., Khan M. N., Posner B. I. Differential and analytical subfractionation of rat liver components internalizing insulin and prolactin. Biochemistry. 1986 Apr 8;25(7):1756–1764. doi: 10.1021/bi00355a046. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burgess J. W., Wada I., Ling N., Khan M. N., Bergeron J. J., Posner B. I. Decrease in beta-subunit phosphotyrosine correlates with internalization and activation of the endosomal insulin receptor kinase. J Biol Chem. 1992 May 15;267(14):10077–10086. [PubMed] [Google Scholar]
- Chang C. P., Lazar C. S., Walsh B. J., Komuro M., Collawn J. F., Kuhn L. A., Tainer J. A., Trowbridge I. S., Farquhar M. G., Rosenfeld M. G. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993 Sep 15;268(26):19312–19320. [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Cohen S., Fava R. A., Sawyer S. T. Purification and characterization of epidermal growth factor receptor/protein kinase from normal mouse liver. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6237–6241. doi: 10.1073/pnas.79.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J. J., 2nd, Kay D. G., Lai W. H., Posner B. I., Bergeron J. J. Selective degradation of insulin within rat liver endosomes. J Cell Biol. 1990 Jan;110(1):35–42. doi: 10.1083/jcb.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. W., Cohen S. Epidermal growth factor stimulates tyrosine phosphorylation in the neonatal mouse: association of a M(r) 55,000 substrate with the receptor. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8477–8481. doi: 10.1073/pnas.89.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Faure R., Baquiran G., Bergeron J. J., Posner B. I. The dephosphorylation of insulin and epidermal growth factor receptors. Role of endosome-associated phosphotyrosine phosphatase(s). J Biol Chem. 1992 Jun 5;267(16):11215–11221. [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Hsuan J. J., Totty N., Waterfield M. D. Identification of a novel autophosphorylation site (P4) on the epidermal growth factor receptor. Biochem J. 1989 Sep 1;262(2):659–663. doi: 10.1042/bj2620659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C., Stahle C., Harkins R. N., Fausto N., Smith G. H., Merlino G. T. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990 Jun 15;61(6):1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Karnovsky M. J. The ultrastructural basis of capillary permeability studied with peroxidase as a tracer. J Cell Biol. 1967 Oct;35(1):213–236. doi: 10.1083/jcb.35.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. G., Lai W. H., Uchihashi M., Khan M. N., Posner B. I., Bergeron J. J. Epidermal growth factor receptor kinase translocation and activation in vivo. J Biol Chem. 1986 Jun 25;261(18):8473–8480. [PubMed] [Google Scholar]
- Kelly K. L., Ruderman N. B. Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185-kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low density membrane vesicle. J Biol Chem. 1993 Feb 25;268(6):4391–4398. [PubMed] [Google Scholar]
- Khan M. N., Baquiran G., Brule C., Burgess J., Foster B., Bergeron J. J., Posner B. I. Internalization and activation of the rat liver insulin receptor kinase in vivo. J Biol Chem. 1989 Aug 5;264(22):12931–12940. [PubMed] [Google Scholar]
- Khan M. N., Savoie S., Bergeron J. J., Posner B. I. Characterization of rat liver endosomal fractions. In vivo activation of insulin-stimulable receptor kinase in these structures. J Biol Chem. 1986 Jun 25;261(18):8462–8472. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai W. H., Cameron P. H., Doherty J. J., 2nd, Posner B. I., Bergeron J. J. Ligand-mediated autophosphorylation activity of the epidermal growth factor receptor during internalization. J Cell Biol. 1989 Dec;109(6 Pt 1):2751–2760. doi: 10.1083/jcb.109.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W. H., Cameron P. H., Wada I., Doherty J. J., 2nd, Kay D. G., Posner B. I., Bergeron J. J. Ligand-mediated internalization, recycling, and downregulation of the epidermal growth factor receptor in vivo. J Cell Biol. 1989 Dec;109(6 Pt 1):2741–2749. doi: 10.1083/jcb.109.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Margolis B. L., Lax I., Kris R., Dombalagian M., Honegger A. M., Howk R., Givol D., Ullrich A., Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem. 1989 Jun 25;264(18):10667–10671. [PubMed] [Google Scholar]
- Marti U., Burwen S. J., Jones A. L. Biological effects of epidermal growth factor, with emphasis on the gastrointestinal tract and liver: an update. Hepatology. 1989 Jan;9(1):126–138. doi: 10.1002/hep.1840090122. [DOI] [PubMed] [Google Scholar]
- McCormick F. Signal transduction. How receptors turn Ras on. Nature. 1993 May 6;363(6424):15–16. doi: 10.1038/363015a0. [DOI] [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., King E., Jr, Mondon C. E., Glinsmann W. H. Effects of insulin on net carbohydrate alterations in perfused rat liver. Am J Physiol. 1967 Jan;212(1):179–183. doi: 10.1152/ajplegacy.1967.212.1.179. [DOI] [PubMed] [Google Scholar]
- O'Keefe E., Hollenberg M. D., Cuatrecasas P. Epidermal growth factor. Characteristics of specific binding in membranes from liver, placenta, and other target tissues. Arch Biochem Biophys. 1974 Oct;164(2):518–526. doi: 10.1016/0003-9861(74)90062-9. [DOI] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pronk G. J., McGlade J., Pelicci G., Pawson T., Bos J. L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993 Mar 15;268(8):5748–5753. [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., McGlade J., Pawson T., Chen K., Cohen S. Epidermal growth factor stimulates the tyrosine phosphorylation of SHC in the mouse. J Biol Chem. 1993 Apr 15;268(11):7610–7612. [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. Galloylglucoses of low molecular weight as mordant in electron microscopy. I. Procedure, and evidence for mordanting effect. J Cell Biol. 1976 Sep;70(3):608–621. doi: 10.1083/jcb.70.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. A., Dodson G. S., Rubin G. M. An SH3-SH2-SH3 protein is required for p21Ras1 activation and binds to sevenless and Sos proteins in vitro. Cell. 1993 Apr 9;73(1):169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993 Jun 25;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., Carpenter G. Interaction of activated EGF receptors with coated pit adaptins. Science. 1993 Jul 30;261(5121):612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- Ueki K., Matsuda S., Tobe K., Gotoh Y., Tamemoto H., Yachi M., Akanuma Y., Yazaki Y., Nishida E., Kadowaki T. Feedback regulation of mitogen-activated protein kinase kinase kinase activity of c-Raf-1 by insulin and phorbol ester stimulation. J Biol Chem. 1994 Jun 3;269(22):15756–15761. [PubMed] [Google Scholar]
- Wada I., Lai W. H., Posner B. I., Bergeron J. J. Association of the tyrosine phosphorylated epidermal growth factor receptor with a 55-kD tyrosine phosphorylated protein at the cell surface and in endosomes. J Cell Biol. 1992 Jan;116(2):321–330. doi: 10.1083/jcb.116.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Chen W. S., Rosenfeld M. G., Gill G. N. Analysis of deletions of the carboxyl terminus of the epidermal growth factor receptor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J Biol Chem. 1990 Jan 25;265(3):1750–1754. [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]