Abstract
In some diseases, a very important role is played by the ability of bacteria to form multi-dimensional complex structure known as biofilm. The most common disease of the oral cavity, known as dental caries, is a top leader. Streptococcus mutans, one of the many etiological factors of dental caries, is a microorganism which is able to acquire new properties allowing for the expression of pathogenicity determinants determining its virulence in specific environmental conditions. Through the mechanism of adhesion to a solid surface, S. mutans is capable of colonizing the oral cavity and also of forming bacterial biofilm. Additional properties enabling S. mutans to colonize the oral cavity include the ability to survive in an acidic environment and specific interaction with other microorganisms colonizing this ecosystem. This review is an attempt to establish which characteristics associated with biofilm formation—virulence determinants of S. mutans—are responsible for the development of dental caries. In order to extend the knowledge of the nature of Streptococcus infections, an attempt to face the following problems will be made: Biofilm formation as a complex process of protein–bacterium interaction. To what extent do microorganisms of the cariogenic flora exemplified by S. mutans differ in virulence determinants “expression” from microorganisms of physiological flora? How does the environment of the oral cavity and its microorganisms affect the biofilm formation of dominant species? How do selected inhibitors affect the biofilm formation of cariogenic microorganisms?
Introduction
In the 18th century, it was demonstrated that microorganisms live not only in a single-cell form, but are also capable of forming clusters suspended in a mucilaginous extracellular substance. The pathogenicity of certain microbial species such as Streptococcus mutans, Staphylococcus epidermidis, Legionella pneumophila or Pseudomonas aeruginosa is inseparably associated with their ability to form biofilms on solid surfaces, e.g., tissues, catheters or implants [1–4]. This feature allows microorganisms to form three-dimensional structures in which cells become more resistant to antibiotics and changing environmental conditions, among others, through changes occurring as a result of interbacterial interactions and the presence of an exopolysaccharide matrix protecting the entire structure [5, 6].
Microorganism pathogenicity
Interactions observed between pathogen and host have been the subject of research and discussion for many years. The historical approach to the problem of microorganism pathogenicity postulated by, amongst others, Koch, puts the pathogen or host in the main position by this featured affiliation to one of them. The term ‘pathogenicity determinants’ is related to the features which determine a microorganism’s ability to cause disease, but which themselves are not required for its survival [7]. Henderson et al. defined pathogenicity determinants as pathogen components which cause damage in a host organism; this may include factors vitally important for the microorganisms [8]. These definitions do not, however, take into account the role of host susceptibility to infection, indicating only that pathogen properties are responsible for disease development. According to these definitions, only those microorganisms which cause diseases in healthy people are pathogens and not opportunistic or commensal microorganisms which are only able to infect the hosts with immune system disorders.
Casadevall and Pirofski [9] proposed a new definition for pathogenicity and pathogenicity determinants of the microorganisms taking into account the state of host immunological defense. A given microorganism pathogenicity is expressed as a range of damage which is caused by the microorganism itself and by the immune system as a response to a pathogen. The state of a host’s immunological defense is the main determinant for bacterial pathogens and it determines the infection course and cure [9].
The determination of microorganism pathogenicity determinants according to the classification proposed by Casadevall and Pirofski (six classes) poses some interpretation problems in class I, since it seems that the key role in these infections is played by a host’s condition. The infected human constitutes a complex ecosystem in which homeostasis in the field of the bacteria-immune system is observed in the physiological state. The development of bacteremia occurs in cases of disturbances in this system, usually as a result of immunity deficiency or, more rarely, as a result of an overexpression of the features determining bacteria pathogenicity. There are, however, some bacteria, e.g., S. salivarius or most bacteria colonizing the oral cavity, which live in the human environment but are rarely described as pathogens, even in those patients with an impaired immune system. This suggests that even pathogens of a low virulence have to possess a minimum set of features determining their pathogenicity, which would allow them to penetrate and proliferate in a host organism.
In the study by Kreikemeyer et al. [10] concerning biofilm structure, the determinants of Streptococcus pathogenicity are related to the discovery of long filamentous structures similar to the pilus observed on bacteria surfaces. These structures exhibit adhesive properties and may play a key role in adhering to host cells and tissues, as well as in biofilm formation by pathogens of the Streptococcus species. The study demonstrated that the described pilus in S. pyogenes are responsible for bacteria adhesion and the formation of microcolonies on host cell surfaces, and also for aggregation itself, especially when influenced by human saliva. Also, in the case of S. agalactiae, these structures are engaged in pathogen interaction with host cells, e.g., adherence and bypassing the epithelium barrier. They are also responsible for bacteria clustering in a biofilm-like structure. In the pathogenesis of the diseases caused by S. pneumoniae, as in the case of other Streptococcus species, these structures are engaged in adhesion and colonization processes; however, compared to other Streptococcus species, the frequency of pilus occurrence is lower (less than 30 %) [11].
There are studies suggesting that S. mutans isolates have a greater ability to form biofilm than the isolates of other Streptococcus species, which colonize the human oral cavity environment [12, 13]. The studies focused on S. mutans cells, which form biofilm and proved that they exhibit a different expression of some proteins in comparison to planktonic cultures, e.g., an increase in exopolyphosphatase expression and a decrease of lactate dehydrogenase or pyruvate kinase expression [14]. Increased virulence of cells forming biofilm can also be associated with a higher tolerance to low pH, as compared to planktonic cultures.
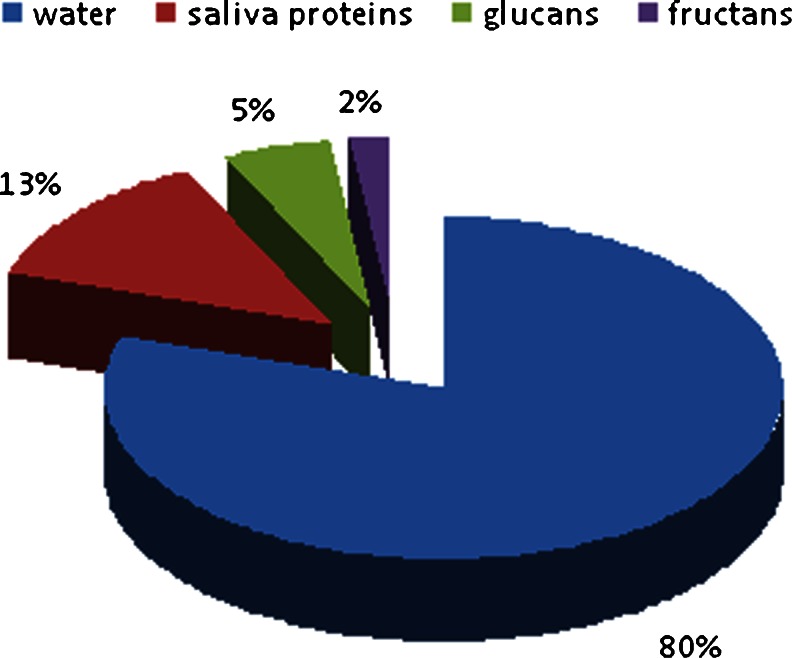
The main components of the biofilm formed on the surface of teeth include: glucan (10–20 % of dry weight), fructan (1–2 % of dry weight) and proteins (40 % dry matter). Moreover, it differs from the surrounding saliva in terms of the levels of lipids, calcium, magnesium, fluorine, and phosphorus. In situ, in 80 % it consists of water [6]. Thus, an amorphous membrane formed in such conditions provides ideal conditions for bacterial survival and determines the virulence of the biofilm structure. Physical and biochemical matrix features allow for the adhesion of microorganisms, promote cohesion (the aggregation of cells), and act as a source of energy reservoirs. Moreover, limited diffusion to and from the biofilm helps to focus the ions and other nutrients in the microenvironment, such as biofilm; however, it hinders the penetration of substances from outside, including antibiotics [6]. It can be observed that bacteria in the biofilm resemble other organisms for which the clustering was also an evolutionary adaptation for survival.
The ability of bacteria of the S. mutans species to form biofilms is significant from a clinical point of view, mainly in the context of carries etiology; however, there are also single casuistic cases of infective endocarditis (IE) with the involvement of this bacteria [15, 16]. The development of IE is observed when endocardial damage occurs followed by the formation of a very small blood clot, in which platelets play a crucial role. If, at the same time, microorganisms enter the bloodstream, they may use these favorable growth conditions for deposition and biofilm formation.
Such microclots are formed at the interface of the mitral and tricuspid valves from the side of atria and from the side of chambers at the aortic and pulmonary valves [17]. The survival of S. mutans in the bloodstream, where it is very rarely observed after dental surgery, is associated with the presence of several virulence factors on the surface of bacteria, and these have been described in different cases of IE caused by this microorganism. First are those factors responsible for the increased resistance of proteins to phagocytosis, namely, the fibronectin-binding protein (so-called autolysin A, AtlA) [18] and serotype-specific polysaccharide [19]. Next, C antigens increased platelet aggregation, initiating the coagulation cascade process and, consequently, often leading to hypercoagulable states [20]. An equally important role is attributed to the collagen-binding protein (Cnm) [21], so that the S. mutans bacterium can adhere to the heart tissue and penetrate the endothelium of the coronary arteries, consequently leading to endocarditis [22].
Biofilm formation as a complex process of protein–bacterium interaction
The most common oral cavity infectious disease in which an important causative role is played by biofilms formed by microorganisms on the teeth and gums surface is dental caries. One of the main etiological factors of the above-mentioned disease is S. mutans [23–25].
Caries is the most common childhood illness. It is estimated to occur five times more often than the second most common childhood illness, asthma [26]. The Decayed, Missing and Filled Teeth (DMFT) index rate was observed for Bolivia, Guatemala, Bosnia and Herzegovina, and Philippines. Directly behind them in contrast, the lowest DMFT index rate was observed for Russia, Europe’s Eastern Bloc including Poland, and most South American countries [27, 28].
Comparing the DMFT index rate for European countries, one can observe that the epidemiological situation in Poland is not ideal. In 2012, the DMFT index rate for 12-year-olds was estimated at 4.4, whereas the lowest observed in Holland was estimated at 0.9 and the highest in Latvia was estimated at 7.7. In terms of 6-year-olds, the situation deteriorates because, among the countries under investigation, the highest DMFT index rate equal to 5.1 was recorded in Poland. It is estimated that, in Poland, dental caries is observed in 35–50 % of children aged 2–3 years and in 50–60 % of children aged 3–4 years [27, 29]. According to alarming reports from the World Health Organization (WHO) and the National Institute of Public Health (PZH), dental caries is observed in almost 100 % of children aged 6–7 years. Caries in permanent teeth starts just after the dentition of the first molar teeth and has been reported in approximately 90 % of Polish 12-year-old children [30, 31].
In the view of the above data, it is extremely important to understand more fully the mechanisms of cariogenic strain activity on the basis of the biofilm formed by them, which may be applied in the prevention and early diagnostics of dental caries in children.
Biofilms present in the oral cavity are three-dimensional structures, consisting of bacterial strains anchored to solid surfaces such as tooth enamel, tooth roots or dental implants. They are embedded in an exopolysaccharide matrix [32]. Over 700 different bacterial species incorporating into biofilms have been identified so far [33, 34]. The structure and composition of the exopolysaccharide matrix is determined by the conditions existing in the oral cavity and change over time. The extracellular polysaccharides (EPS) also affect the physical and biochemical properties of the biofilm [6, 35].
The primary sources of EPS are glucosyltransferase (GTF) and fructosyltransferase (Ftf), products of interaction with sucrose and starch hydrolysates. Exopolymers contained in the polysaccharide matrix form its stability and provide possibilities of binding bacterial cells [6, 35, 36]. Research on the composition of the polysaccharide matrix revealed the presence of components as shown in Fig. 1.
Fig. 1.
The percentage composition of polysaccharide matrix (the figure was prepared based on the data published by Bowen and Koo [6])
The process of biofilm formation begins with the coating of the tooth surface through the salivary pellicle [6, 32]. This pellicle is formed by salivary components (such as proline-rich proteins, amylase, lysozyme, histatin, peroxidase, mucin, and bacterial components, e.g., Ftf, Gtf, lipoteichoic acid) specifically adsorbed to the acquired enamel pellicle (AEP) [6, 37]. AEP is the basis for microorganism-induced biofilm formation colonizing the oral cavity [37]. Single S. mutans cells or their aggregates fuse with pellicles via two independent mechanisms: sucrose-dependent and sucrose-independent [23, 25, 32].
The sucrose-dependent mechanism
Glucosyltransferases (GtfS)
The sucrose-dependent mechanism of plaque formation is based on glucosyltransferases (GTFB, -C, and-D) produced by S. mutans in combination with glucan-binding proteins (GBPs) [6, 25, 38]. Glucosyltransferases play critical roles in virulent dental plaque development and are responsible for glucans formation from sucrose. The synthesized glucans provide the possibility of both bacterial adhesion to the tooth enamel and microorganisms to each other. Thanks to this process, microcolonies are formed which favor the formation of biofilm. Each of the three types of Gtf plays a different, though similar role in biofilm formation and, therefore, the loss or mutation of one of them impairs the whole process [6, 38].
In vivo, GtfS very rapidly (approximately 1 min) adsorbs into the hydroxyapatite surface (sHA) of the enamel-coated salivary pellicle. The highest affinity for the sHA is reported for GTFC (formally known as GtfSI), which is a hydrophilic compound and produces a mixture of soluble (with mostly α-1,6-linkages) and insoluble glucans. However, it has a hydrophobic domain which is related to its affinity for dental plaque. This enables the interaction with saliva proteins in the pellicle, such as lysozyme or α-amylase. GtfB (known as GtfI), a glucosyltransferase, is primarily responsible for the interaction with other S. mutans bacteria which mainly synthesize insoluble glucan rich in α-1,3-linkages. It is responsible for the formation of highly differentiated microcolonies forming the structure of biofilm [39]. Its activity significantly increases when there is a glucose in the environment, which is not a typical situation and is observed extremely rarely [6, 40].
GtfD (GtfS) predominantly forms soluble, quickly metabolizable polysaccharides and acts as a primer for GtfB.
Glucosyltransferases also interact with the components of saliva: amylase, in consequence blocking the activity and adsorption to hydroxyapatite; lysozyme, which decreases the activity of GtfB, but does not affect the characteristics of glucans formed by the enzyme; peroxidase, which inhibits the activity of all three glycosyltransferases.
GtfS also have the ability to bind to other bacterial cells, even if these are not bacteria synthesizing their own glycosyltransferases. Therefore, cooperation between Gtfs and microorganisms allows for the building of a plate strongly attached to the teeth surface with stable bonds between bacterial cells [6, 35].
Genes encoding GtfB and GtfC lie close to each other, have a very similar amino acid composition (95 % homology), and are subject to the same regulatory processes. These genes are expressed in response to acidification of the environment or in situations of glucose or sucrose excess in the environment. Many factors additionally influence the physiological expression of these genes. One of them is catabolism and factors like RegM, an inhibitory regulator of S. mutans [6]. The protein regulating the catabolism in Streptococcus and Staphylococcus bacteria, RegM, is also known as catabolite control protein A (CCPA) [41]. Its regulatory function is based on the inhibition of genes involved in the utilization of alternative carbon sources and the activation of the expression of genes whose products are involved in the elimination of excess carbon from the cell [42–44]. Inactivation of the RegM of S. mutans results in a very strong decrease in the promoter expression of gtfBC [6]. Other factors are the products of genes: luxS (AI-2 autoinducer-coding synthesis), which affects the expression of gtfB and gtfC [6, 25, 45], and also ropA (encoding for the trigger factor) regulating the production of GtfB and GtfD [6, 23]. Also, the VicRK signal transduction system affects the physiological expression of Gtfs. In a mutant strain of S. mutans which does not have this system, a significant decrease in gtfD gene expression, as well as increased expression of the gtfB gene, was observed.
The gtfD gene lies distant from other genes, is characterized by lesser homology (50 %) and is subject to different regulatory mechanisms. Its expression is specifically induced by copper ions [6]. Apart from Gtfs, the synthesis and structure of glucans is also affected by enzymes such as mutanase and α-1,6-glucosidase. Therefore, the structure of glucans in the biofilm matrix is changeable, and in mature dental plaque, water-insoluble polysaccharides dominate [6, 46, 47].
Glucans binding of proteins (Gbps)
Another component of the sucrose-dependent mechanism is Gbps mediating the binding of bacteria to glucans. Four types of this protein are known: GbpA, -B, -C, and -D [6, 25, 38]. The GBPC protein (and probably GbpB) is associated with the bacterial cell wall and, therefore, acts as a specific receptor for glucan. All four types of proteins play a role in microorganism adhesion and biofilm formation; however, the GbpD protein seems to play a key role [6]. Research on the utility of GS5 (deletion of the gbpB gene) and UACA2 (a strain of the gbpB gene expression encoding antisense RNA) of S. mutans strains showed that the absence or mutation of the gene encoding GbpB results in a change of cell shape and a slowing down of its growth [38]. This disables the appropriate development of biofilm, which, instead of a diverse, dense formation, becomes a product of non-regular cell clusters surrounded by a matrix of unusual structure.
The expression patterns of GbpB which have been studied by Fujita et al. [40] may have some relationships with S. mutans virulence. Attention was paid to the relationship of the profiles and biological activity of the above-mentioned proteins with the virulence of isolated proteins. GbpB expression analysis isolates revealed the existence of several expression patterns. Strains that showed single and multiple bonding were classified as S and M strains, while strains without explicit GbpB expression were classified as N type. The GbpB expression pattern distribution was found to be different for the Japanese and Finnish clinical strains simultaneously.
For the Japanese strains, the highest frequency was reported for S (81 %), followed by the M (15 %) and N types (4 %), while in the Finnish population, the frequencies were estimated at 42 % for the M type, 37 % for the S type, and 21 % for the N type without the explicit expression of GbpB. Studies show a clear differentiation of each type depending on the origin of clinical isolates. Geographic variation seems to be crucial in determining the virulence of the strains, which, depending on the environment, do or do not acquire specific virulence factors, constituting a kind of adaptation which allows them to colonize a new ecological niche. An explanation of the distribution and specificity of GbpB expression patterns may be useful for the evaluation of S. mutans virulence in individual patients [40].
The sucrose-independent mechanism
The sucrose-independent mechanism is not relevant in the virulence of S. mutans. In the second mechanism of adhesion (sucrose-independent), an interaction is observed between the adhesive particles of S. mutans and the AEP. Agglutinins found in saliva are involved in the process of adhesion and aggregation of S. mutans thanks to interaction with the I/II antigen, which is a multifunctional PI adhesin (also known as AgB, SpaP, or Pac1 adhesin) anchored in the bacterial cell wall, and encoded by the spaP gene [23–25, 48].
The protein family of Ag I/II, represented by SpaP, SspA, or SspB, is identified not only on the surface of S. mutans, but also on other microorganisms, such as Streptococcus pyogenes, Streptococcus agalactiae, or Streptococcus suis [48]. Genetic sequences encoding Ag I/II comprise six distinct regions. The most important of these are the A region rich in alanine and the P region rich in proline. Region V, located between them, is composed in the majority of different sequences found in individual strains. The A and V regions encode adhesive epitopes appearing on the surface of bacterial cells (so-called adhesive types) responsible for affinity to the salivary glycoproteins [49]. The contribution of the A, P, and V regions to the adhesion has been confirmed by studies using mutant strains [48]. None of them had the ability to adhere to solid surfaces coated with the salivary pellicle. The expression and biological activity of the P1 protein in S. mutans is also dependent on multiple gene products, i.e., luxS, ropA, and srtA genes (encoding the enzyme responsible for the attachment of P1 adhesin to the cell wall) [22, 50].
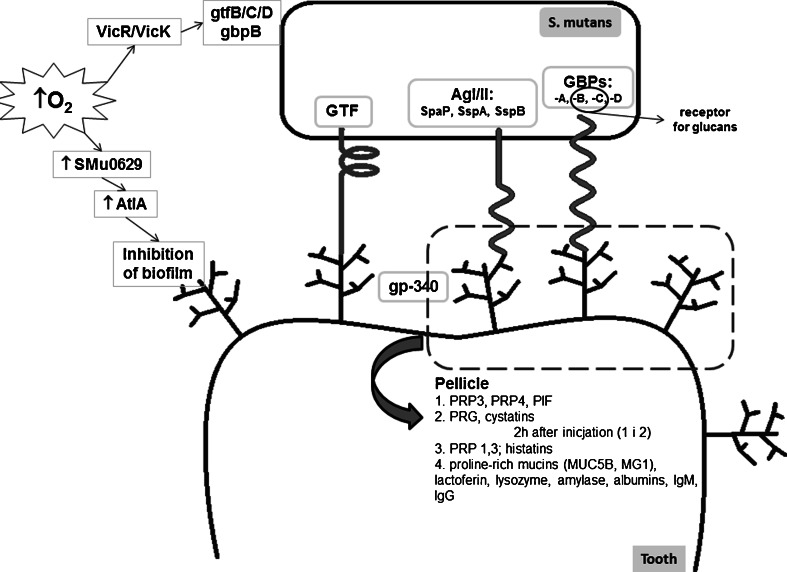
The SpaP protein and other proteins of the Ag I/II family specifically interact with glycoprotein-340 (gp-340) found in saliva. It is interesting that the gp-340 dissolved in the liquid phase of saliva plays a role in the aggregation of bacterial cells and, thus, purifies the oral cavity. However, if gp-340 is adsorbed on the surface of the teeth or gums, it acts as a receptor for surface bacterial adhesins initiating the adhesion process [22, 48] (Fig. 2). The Ag I/II protein family is also involved in the interaction between microorganisms, e.g., Streptococcus gordonii and Porphyromonas gingivalis, and in the aggregation of cells in the absence of gp-340 [48].
Fig. 2.
The function of Streptococcus mutans in the process of the formation of biofilms on the surface of teeth. Glycosyltransferases (GTF) are an indispensable element for the proper functioning of S. mutans. In the early phase of biofilm development, S. mutans are bound to the teeth surface. It is thought that this binding is the first step in the formation of plaque. Surface adhesins of S. mutans (so-called antigen I/II) interact with α-galactosides from saliva, forming the structure of pellicula. Other groups of compounds belonging to S. mutans and located on the surface of teeth which participate in the formation of pellicula are glucan-binding proteins (GBPs) or GTF. Salivary proteins with which can interact the surface adhesins of S. mutans can be divided into four groups depending on the time of pellicula formation. Proline-rich proteins (PRP) participate in the first stage of the formation of pellicula. Two hours later, enamel is formed by cystatins, peptides with high affinity to hydroxyapatite. Then, peptides with low molecular weight and with bacteriological properties against S. mutans start playing a role in this model. In the last stage, mucin-rich proteins, lactoferrin, lysozyme, amylase, albumin, or IgM and IgG antibodies are involved in the process. S. mutans are aggregated on the surface of teeth in the presence of saccharose. Additionally, GTF synthetizes extracellular glucans, which is another key step in the development of plaque. GBP is a S. mutans receptor that differs from GTF and specifically binds glucans. GTF contain a glucan-binding domain and, therefore, they act as receptors for glucans. Therefore, S. mutans binds initially developed glucan through GBP and GTF, which gives a basis for the aggregation of S. mutans
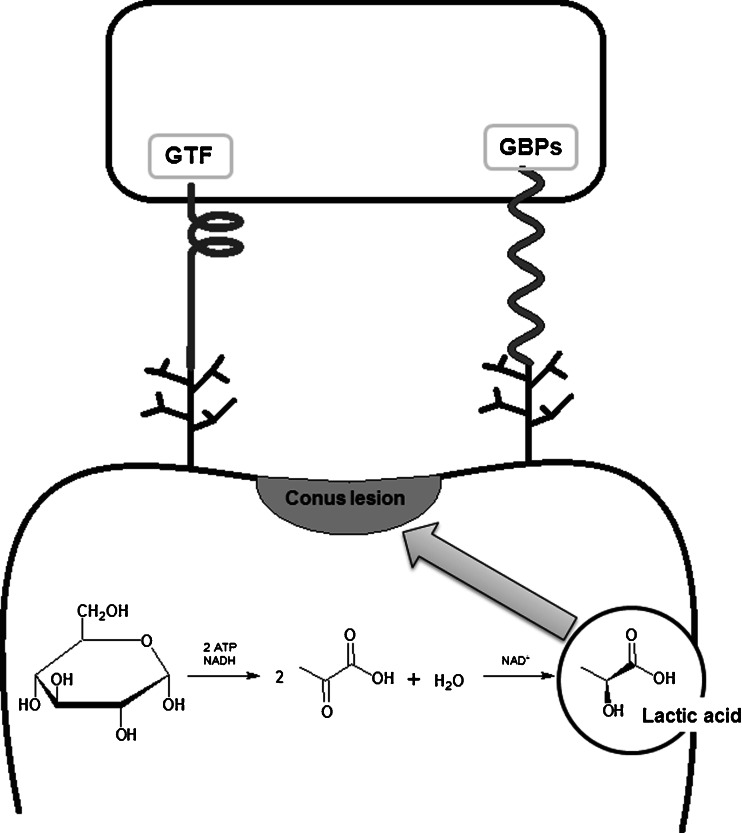
A key role in the interactions between S. mutans and saliva agglutinins is played by the actual structure and location of P1 protein, as has been proven through the use of mutant strains (spaP and srtA) of this microorganism. Additionally, it has been shown that the lack of expression of the gene encoding sortase A enzyme (SrtA) results not only in an abnormal location of the P1 adhesin, but also of other surface proteins, which is not negligible in the context of bacterial aggregation ability [23]. The survival of S. mutans inside biofilms, at a very low pH inside microcolonies of other bacterial species (as observed in the oral cavity), should be considered as three mechanism-dependent phenomena which include: the upregulation system of F1F0-ATPase, biosynthesis of membrane fatty acids (for instance, FabM), and branched-chain amino acid (BCAA) (for instance, IIvC). This process may be the main driving force in the survival of S. mutans inside a mixed biofilm (Fig. 3); thus, innovative therapies aimed at inhibiting biofilm formation should be oriented along these three mechanisms and should be directed at all three simultaneously and not just in one of them [51].
Fig. 3.
Production of lactic acid by Streptococcus mutans. Metabolism of various carbohydrates (including glucose and fructose) by bacterial biofilm. Production and secretion of a significant amount of lactic acid, which can cause demineralization of teeth structure that can finally result in the development of decay
Salivary agglutinins
In the process of biofilm formation, a role equally important as that of surface bacterial adhesins is played by salivary agglutinins. This has been confirmed in vitro [23, 24]. Researchers have also used mutant bacterial strains of the major genes encoding the P1 protein to study the differences and interactions during biofilm formation. The results clearly indicate a less satisfactory process in the absence of salivary agglutinins, as well as significantly less sufficiency in the mutant strains compared to the parental strain [22, 23]. It has also been proven that the initial stage of adhesion and biofilm formation of S. mutans may be stimulated by salivary agglutinins and other salivary proteins (e.g., mucin of high molecular mass or acidic proline-rich proteins) (Fig. 2) [23, 52].
Under specific conditions, the expression of the virulence factors of S. mutans and biofilm formation in the oral cavity may be modulated in two ways: through the environment in which bacterial growth was reported as well as by the presence of other microorganisms, and the interactions between them [25].
Culturing S. mutans strains under aerobic conditions induces an 80 % reduction of bacterial ability to form biofilms [23]. Oxygen availability is the causative agent of variations in bacterial cell surface composition and modification of the production of autolysins and, specifically, in the signal transduction system of VicRK (Fig. 2). The production of AtlA autolysin is conditioned by the SMu0629 gene expression of oxidoreductase activity. Under aerobic conditions, increased expression of this gene is being observed and, thus, the overproduction of AtlA autolysin, which inhibits the formation of biofilm. Vick kinase transducer is a system regulating the expression and activity of AtlA autolysin. In order to confirm oxygen-induced alterations in autolysin, studies on mutant strains for SMu0629 and vicK genes were conducted. Strains from which the above-mentioned genes were removed adapted better to aerobic conditions and showed a greater ability to form biofilm as compared to the UA159 parental strain [52–54].
Diet habits
Diet is another factor influencing the process of biofilm formation. The occurrence of dental caries is strongly correlated to diet. Meal cariogenicity is affected not only by the content of carbohydrates but also by the frequency of their consumption. Particular carbohydrates differ in their ability to cause caries. Saccharose is definitely the most cariogenic carbohydrate [55]. It constitutes the main center of S. mutans metabolism. These bacteria exhibit not only the ability to decompose this carbohydrate, but also produce glucans which are significant in interactions between tooth enamel and cariogenic bacteria [6].
The presence of high levels of carbohydrates in children’s diets, especially in the drinks given to children immediately before sleep or during the night, constitutes a significant factor in ECC development [56]. Falling asleep with a sweetened nipple or bottle in the mouth stimulates the formation of carious lesions. Saliva excretion decreases during sleep, which accelerates the cariogenic strength of the activity of acids derived from carbohydrate metabolism. The opinions concerning the effect of feeding with milk on dental caries development still remain controversial. According to researchers, cow milk does not contain high amounts of lactose and, moreover, no significant relationship with carious lesion formation has been demonstrated. Human milk, in turn, despite having higher amounts of lactose, also contains case in which adsorbs to the tooth surface and retards caries development. Moreover, it contains considerable amounts of calcium and phosphates, which additionally stimulate enamel remineralization [57]. Also, the significant influence of sparkling drinks and fruit juice on dental caries development in children and teenagers has been demonstrated. Acids common in sparkling drinks, such as citric or phosphoric acids, considerably lower the oral cavity pH and stimulate an exchange of calcium ions present in the enamel on hydrons. It has also been demonstrated that drinking through a straw elongates and increases liquid contact with the tooth surface, which unprofitably affects their structure [58].
It is not the level of carbohydrates in a meal but the frequency of consumption of meals with high carbohydrate levels that is a significant factor [59]. Also, time plays a substantial role in tooth mineralization. It has been demonstrated that alternate processes of enamel demineralization and remineralization occur during the day. The loss of hydroxyapatite mineral components is related to the period when bacteria fermenting carbohydrates may freely develop and synthesize acids into carbohydrates metabolism byproducts. Time acts in favor of cariogenic pathogens which adapt to low pH values, which favor the progress of the cariogenic process. The length of change in the period on the enamel surface is especially significant in cases of early childhood caries characterized by an aggressive course. Noticing the first changes on the upper incisor surface is extremely important, since carious lesions quickly spread on molar teeth [60]. Moreover, the changes in the daily rhythm of saliva excretion play a significant role in caries exposure. Saliva secretion is decreased during the night hours, which limits its ability to protect against enamel demineralization [58].
Currently, studies on the development of products to prevent dental caries development are being conducted. Some are aimed at inhibiting the development of biofilm through the influence on Gtfs. There are a number of synthetic and natural glycosyltransferase inhibitors, i.e., hop components, green tea, medicinal plant extracts, plant polyphenols of high molecular mass (e.g., curcumin), cardiolipin, putrescine, cadaverine, or hypochlorite compounds or metal ions (Fe2+, Zn2+, Cu2+). In order to increase their effectiveness, there is a tendency to develop formulations for oral hygiene which best use the potential of the above-mentioned substances [6, 61, 62].
Biofilm structure
The formation of biofilm is a multistep and very complicated process. A number of relevant factors and conditions are required in the oral cavity for the process to run correctly.
Interactions occurring between agglutinins of saliva and bacteria, and simultaneously between microorganisms, might cause the formation of fur composed of cells beginning the colonization: Actinomyces species, Streptococcus species, Lactobacillus species, Candida species. They transform into different types of biofilm in the first layer of subgingival plaque. Biofilm maturation is followed by the aggregation of subsequent bacteria and their growth. After 7 days, the number of Streptococcus bacteria decreases, but the number of Fusobacterium nucleatum increases. After 3 weeks, intact subgingival plaque begins to resemble morphologically supragingival plaque [32].
In the architecture of supragingival biofilm, four layers can be distinguished. The first layer of biofilm consists of cells displaying little fluorescence relative to cells in the top layer. This indicates the presence of Actinomyces sp. In this layer, physiologically inactive or dead cells can also be found. In the intermediate layer, many spindle-shaped cells are found, whose fluorescence indicates Fusobacterium nucleatum and Tannerella sp., mostly T. forsythia. These bacteria can benefit from the proximal location of dead cells (e.g., they acquire sugar building their cell wall). The third layer of biofilm and the intermediate layer mainly consist of a bacterial cluster termed the Cytophaga–Flavobacterium–Bacteroides (CFB), which consists mainly of Gram-negative bacteria Tannerella sp., Prevotella sp., and Bacteroidetes sp.
Apart from this, long cigar-like Synergistetes bacteria from the A group forming palisade-like stroma are also found in this layer. These bacteria are in direct contact with the cells of the host immune system resembling polynuclear leukocytes, which, thereby, suggests their important role in the interactions occurring between host and biofilm. Observations of the top biofilm layer have shown that Spirochaetes bacteria are the most abundant. Among them, bacterial aggregates called rough and fine brushes are found [32].
More heterogeneous than subgingival biofilm is supragingival biofilm. It consists of two layers: basal and top, described in Table 1. All the above-mentioned factors and conditions provide an adequate oral microbial habitat for various kinds of microorganisms. The varied morphology of biofilm expands and targets attacks on potential anticaries compounds. At the same time, however, it is a complex structure, the variability of which makes it difficult to determine the destination points that underlie effective prevention related to the treatment of periodontal disease.
Table 1.
Composition of supragingival biofilm (the table was prepared based on data published by Zijnge et al. [32])
| Basal layer—attached to the surface of teeth and consists of four types of biofilm |
The first type: - rod-shaped cells of Actinomyces located vertically towards the surface of the teeth |
|
The second type: - Actinomyces sp. - cocci chains, not identified as Streptococcus, located vertically towards the surface of the teeth | |
|
The third type: - filamentous bacteria - Streptococcus creating distinct colonies around yeast cells (Candida sp.) | |
|
The fourth type: - mainly Streptococcus developing in the vicinity of Lactobacillus sp. located vertically to the surface of the teeth | |
| Top layer—covers all types of biofilm-forming basal layer |
- Streptococcus sp. in a form of heterogeneous distributed cells, or as a flat, thin layer on the top - heterogeneous distributed bacteria creating CFB cluster - external layer: Lactobacillus sp. surrounded with cells with various morphology |
Interbacterial interactions and the process of biofilm formation
Interactions between microorganisms colonizing the oral cavity are consecutive major factors affecting the development of biofilm [25, 63, 64]. Interactions occurring between microorganisms can result in both the acceleration and inhibition of this process. Thus, the virulence of S. mutans depends not only on the environmental conditions of the oral cavity, but also on the composition of the bacterial flora.
The analysis of interactions between S. mutans (as one of the etiological factors of dental caries) and the components of physiological flora, e.g., S. oralis, S. sanguinis, or Lactobacillus casei, was made possible by the use of dual cultures [25]. In studies on this topic, single bacterial cultures acting as the control group were compared to dual cultures. The results of the experiments are summarized in Table 2.
Table 2.
The results obtained in a study with the use of dual cultures (the table was prepared on the basis of data published by Wen et al. [25])
| Dual culture | Biofilm formation | Expression of pathogenic factors of S. mutans | |||
|---|---|---|---|---|---|
| spaP | gtfB | gbpB | luxS | ||
| S. mutans + S. oralis | Not a relevant change | 30-fold decrease | No decrease | 30-fold decrease | 15-fold decrease |
| S. mutans + S. sanguinis | Significantly important decrease | Small decrease | Small decrease | Small decrease | Change not statistically significant |
| S. mutans + L. casei | Small increase(around 2-fold) | 40-fold decrease | 40-fold decrease | 40-fold decrease | 7-fold decrease |
The study of Zijnge at al. [32] suggests that S. mutans and S. sanguinis are microorganisms which possess an inhibitory effect on biofilm formation ability. Thus, taking into account this observation, researchers have decided to isolate and analyze proteins produced by S. salivarius [63]. Based on these studies, they found that substances responsible for biofilm formation inhibition are: fructosyltransferase (Ftf) and exo-β-D-fructosidase (FruA).
Ftf is an enzyme involved in the metabolism of sucrose to fructose characterized as an extracellular homopolymer, levan (fructan). Next, the enzyme FruA performs a hydrolysis reaction of the formed fructan to fructose. Fructosidase is encoded by the FruA gene, which is identified in many different species of streptococci and in fungi. The mechanism of action of both enzymes allows them to effectively inhibit the sucrose-dependent adhesion of the S. mutans pathway [63]. Authors have confirmed the inhibitory effect of FruA on the process of biofilm formation by S. mutans. To confirm this, a fructanase mixture (the mixture used was derived from a culture of Aspergillus niger and showed significant FruA activity) containing exo- and endo-inulinase was added for the cultivation of this bacterium. It was shown that these enzymes did not affect bacterial growth inhibition; however, they strongly inhibited their ability to form biofilm [63]. In addition, the ability of FruA fructosidase to use sucrose from the oral environment causes a lack of availability of this carbohydrate for S. mutans. This phenomenon is observed because FruA digests sucrose before S. mutans glucosyltransferases manage to produce the glucans necessary for biofilm formation [63].
Another microorganism strongly affecting S. mutans-induced biofilm in the oral cavity is Lactobacillus spp. (LB). LB bacteria are commensal microorganisms colonizing, among others, the human oral cavity. LB is strongly associated with the development of dental caries in the dentine, because it ferments sugars into acidic products, which decrease the pH in the oral cavity, and promote the development of biofilm (Fig. 3) [65]. On the other hand, low pH and antibacterial agents, such as hydrogen peroxide or bacteriocins produced by LB microorganisms, favor the purification of the oral cavity from microorganisms which are non-adaptive to such environmental conditions, for instance, Porphyromonas gingivalis [65]. Thanks to such properties, LB bacteria can be widely used as probiotics [34, 65–68]. LB microorganisms, by interacting with other microorganisms and the production of antimicrobial proteins, contribute to maintaining the balance in the natural flora of many ecosystems, e.g., the oral cavity [66].
Research conducted on various Lactobacillus microorganisms has confirmed the inhibitory effect of many of them on cariogenic microorganisms colonizing the oral cavity, among others S. mutans. Among the most active microorganisms are: L. paracasei, L. plantarum, L. salivarius, and L. rhamnosus [66]. It is also known that the above-mentioned probiotics do not affect the adhesive ability of S. mutans; however, they are able to inhibit the proliferation of the pathogen, and, thus, retard biofilm formation and development [68, 69]. Unfortunately, the mechanism of interaction between Lactobacillus and S. mutans has not yet been sufficiently investigated. Detailed in vitro studies focused on Lactobacillus reuteri [34, 65] have shown that different strains of this species interact with S. mutans with different forces. These interactions are dependent on the pH of the environment and the ability of probiotics to produce hydrogen peroxide and reuterin, an antibacterial protein formed from glycerol, resistant to proteolytic and lipolytic enzymes [24, 68]. The strains also differed in terms of their ability to adhere to surfaces coated with saliva. This feature is very important because bacteria unconjugated to the solid phase are quickly removed by swallowing [63]. Similar differences in activity against S. mutans have been observed among different strains of Lactobacillus salivarius [66].
The role of Lactobacillus bacteria to sustain oral purity is also a result of their specific coaggregation with S. mutans cells. This relationship applies to L. paracasei and L. rhamnosus [67]. The fusion of microorganisms in common aggregates prevents the adhesion of S. mutans to the surface of teeth and gums. This allows the removal of the pathogen from the oral cavity with saliva before the first stage of biofilm formation begins, that is, adhesion [67].
Tahmourespour et al. showed that a biosurfactant derivative produced by probiotic bacterium, Lactobacillus fermentum, inhibits biofilm formation. In the presence of S. mutans, the above-mentioned ability to form a derivative was inhibited, probably due to the inhibition of two key enzymes, GtfB and GtfC. A decrease in the gene expression encoding the mentioned glucosyltransferases in the presence of a biosurfactant derivative produced by L. fermentum [70, 71] has been reported. Ogawa et al. noted that S. salivarius can inhibit biofilm formation. Exo-beta-D-fructosidase synthesized by S. salivarius blocks the synthesis of polysaccharides, which reduces the amount of extracellular matrix produced by S. mutans [63].
Interactions occurring between S. mutans and other microorganisms in the oral cavity may contribute to both the development as well as the inhibition of biofilm formation. Through ongoing research, we have obtained new data on these interactions which enables the search for and use of novel, more specific and effective measures for the prevention of and fight against dental caries. In this fight, it is important to utilize, apart from synthetic products, the potential of natural products, such as probiotics and specific antimicrobial proteins. These substances exhibit a significantly lower number of side effects than artificially made specimens and are suitable for use under specific environmental conditions, which allows a high level of activity of the specimens to be maintained over a long period of time.
Inhibition of biofilm formation—treatment perspectives in dental caries
First, second, and third-generation medicines (phenolic compounds, chlorhexidine, delmopinol)
The growing problem of strain resistance to antibiotics compels us to seek other methods which deal with pathogens that occur in the oral cavity. Nowadays, we know of a number of substances with effective antimicrobial activity inhibiting biofilm development, for instance, chlorhexidine [72], delmopinol [73], or phenolic compounds [74]. Unfortunately, most of these substances cause side effects such as vomiting, diarrhea, addiction, or teeth discoloration. Therefore, alternative substances of antibacterial activity are being sought which would be safe for the users [33, 75].
“Liquid enamel”—calcium phosphate
For many years, the primary role in the fight against dental caries has been played by calcium phosphate, which possesses remineralization properties [76–78]. A special calcium phosphate resin, which was designed for the gradual release of large amounts of these elements in places that require reconstruction, was established very quickly. Later, this compound gained the form of a nanoparticle of amorphous calcium phosphate composite (NACP), which was also as widely used in dentistry as its precursor. The advantages of the nanocomposite are:
Improved mechanical features for its use in dentistry as compared to the classic combination of elements;
Increased release of ions in acidic environments favoring the formation of voids and dental caries;
Fast neutralization and raising of low pH to a safe level (about 6).
Nanoparticles of quaternary ammonium salts (QAS)
In the search for a substance which, apart from remineralization properties, would possess antibacterial ability, NACP was enriched with quaternary ammonium salts (QAS). A specific application was found for a quaternary ammonium called dimethacrylate (QADM). This compound has two active antibacterial domains at its ends, which increase the desired properties, and, additionally, mixes with other dental media easily [77]. Thanks to this change, a new composite of strong antibacterial activity was found. Its application results in shortening microorganism viability both as a planktonic form and in biofilms among others (Streptococcus sp. and Lactobacillus sp.), decreasing acid production by bacteria and a reduction of microorganism metabolic activity [76, 77]. This is possible thanks to the amphiphilicity of QAS compounds, which allow for these substances to react with the lipid part of the cell membrane and interfere with its function. As a result, it indirectly affects the activity of enzymes involved in the transport of substances through the membrane lipid, and, thus, alters the metabolic activity of bacterial cells [79].
Antibacterial nanoemulsions
Lethal QAS properties against a broad spectrum of microorganisms have also been used in antibacterial nanoemulsions. In this context, cetylpyridinium chloride (CPC) was used [80]. Antibacterial nanoemulsion is characterized as a dispersing substance, namely, water and a lipid substance composed of surfactant, which forms nanoemulsion droplets. It is not toxic to humans or animals; however, it exhibits antibacterial, antifungal, and antiviral activity. Antibacterial properties of the emulsion result from the activity of nanodrops on bacterial cell membranes destabilizing the integration of its lipids [80].
Cetylpyridinium chloride (CPC)
CPC has the ability to inhibit Ftfs enzymes (fructosyltransferases), which play an important role in microorganism-induced biofilm formation in the oral cavity. Thanks to this property, CPC plays the role of an antibacterial substance (affecting the cell membrane of a microorganism) and a substance inhibiting the development of biofilm. Increasing the efficiency of nanoemulsions through CPC enrichment has led to their wider application in products for oral hygiene, such as toothpastes and mouthwashes, and in dental materials, e.g., varnishes and dental fillings [80].
12-methacryloyloxydodecylpyridinium bromide (MDPB)
Among QAS, 12-methacryloyloxydodecylpyridinium bromide (MDPB) has also found an application in the fight against dental caries [81, 82]. The properties of MDPB and the possibility of its application have attracted the attention of research groups [82]. In experiments, the authors have investigated the effect of MDPB on the bacterial flora of the oral cavity, interactions with dental materials, and the possibility of a synergistic effect of this compound with nanoparticles of silver (NAg).
MDPB is a monomer characterized by antibacterial activity against aerobic and anaerobic bacteria isolated from translucent zones (i.e., Actinomyces, S. mutans) and antifungal activity (e.g., Candida albicans) [82]. Stiffened by polymerization of the filling bonding layer, it remains active against pathogens and, at the same time, does not negatively affect either human cells or the binding capacity of the filling material [82].
Silver nanoparticles
Silver compounds became the research objective for Cheng et al. [81], who drew attention to their application in conjunction with QADM. Silver is known for its lethal activity against a wide spectrum of bacteria, viruses, and fungi. Its antibacterial activity is due to the ability of cell membrane disintegration, internal penetration, and destruction of intracellular organelles. An additional mechanism allowing for the inactivation of bacterial enzymes by inhibiting bacterial DNA replication capability enhances the efficacy of silver compounds. Low toxicity and long-term antibacterial activity due to the gradual release and lower resistance of bacteria against these compounds in relation to antibiotics are other advantages in the fight against microorganisms [81–83]. The sheer number of mechanisms and properties describing the activity of substances containing silver particles lead to the inability for microorganisms to sustain full protection against their effects. An additional difficulty is the role of silver compounds as catalysts, rather than substances participating in chemical reactions [84].
Silver nanoparticles have a huge active surface, and, therefore, their level in dental composites is estimated at only 0.05 to 0.1 % by weight. This proportion provides both effective antibacterial activity as well as a lack of effect on the mechanical filling properties [81].
NAg with MDPB and NAg with QADM
Research conducted by both groups showed that the combinations of NAg with MDPB and NAg with QADM give much better results than the use of these substances alone. Based on Table 3, it can be observed that the combination of silver nanoparticles with MDPB and QADM does not affect the mechanical properties of the dental material and is not toxic to human cells. A major advantage of such combinations is the increase in the ability to inhibit microorganism growth, as well as the significant reduction in their metabolic activity and vitality.
Table 3.
The effect of MDPB + NAg and QADM + NAg on chosen traits (the table was prepared based on data published by Cheng et al. [81] and Zhang et al. [82])
| Trait | Control | MDPB + NAg | QADM + NAg |
|---|---|---|---|
| Growth inhibition zone | 1 mm | 10-fold higher | 8-fold higher |
| The strength of binding of dental material | 30–32 MPa | No changes | No changes |
| Metabolic activity of bacteria measured by absorbance in the enzymatic assay (reduction of MTTa at 540 nm wavelength). | 0.5 A540/cm2 | 0.05 A540/cm2 | No changes |
| Viability of bacteria described as a ratio of the number of live cells to dead cells based on the image from a fluorescence microscope | Long lifetime | Significantly lowered in comparison to control | Significantly lowered in comparison to control |
| CFU (colony forming unit, number of microorganisms in the study material) after application of antibacterial agents |
23 × 106 for control MDPB 2.5 × 106 for control QADM |
0.5 × 106 | 0.9 × 104 |
aMTT – tetrazolium dye
Variations of silver nanoparticles with MDPB, QADM, and with silver diamine fluoride (SDF)
The combination of silver with fluoride led to the formation of silver diamine fluoride (SDF), characterized by antibacterial properties, for which the metal component is responsible, and remineralization properties due to the presence of fluoride [85]. It has been shown that SDF can be used as an element of dental caries prevention [86], as well as a substance inhibiting the development of disease by reducing the amount of cariogenic microorganisms in the oral cavity and that it has a positive influence in zones of tooth enamel demineralization [85]. During studies on the described compound, the sensitivity of S. mutans and Actinomyces naeslundii to its function was also demonstrated [87].
However, studies examining the effects of SDF on dual-species biofilm formed by S. mutans and Lactobacillus acidophilus illustrate much better the effectiveness of this compound in the natural oral environment, in which biofilm is characterized as a structure of multi- and not single species. Mei et al. [72] showed that, in dual-species biofilm, the antibacterial activity of silver ions is much lower than in the biofilm of single species; however, it does not disappear completely. This is confirmed by the ratio of dead and live cells, which, for the control, was equal to 0.02, whereas for the samples with SDF addition, it was 6.74. These studies, apart from confirming SDF antibacterial activity against S. mutans and L. acidophilus, also proved that this substance slows the process of enamel demineralization and protects collagen from damage. This dual action of SDF may contribute to a wider use in oral hygiene products and to clinical success in the fight against dental caries.
Natural inhibitors: chitosan
Antibacterial substances which could be included in the formulations of oral hygiene products and would not exert side effects are still being researched. One such substance is chitosan, a polysaccharide formed by the N-acetylation reaction of chitin [88]. Both compounds are present in the plant, fungi, and animal worlds, and have natural antibacterial and antifungal properties. A limitation in the use of chitosan for oral hygiene products, however, was proven by its insolubility in water, because the compound is dissolved only in acids. Attempts have been made to modify this property, and as a result of the Maillard reaction or sugar modification, water-soluble chitosan of unchanged antibacterial properties was obtained. It has been shown [88] that such modified chitosan exhibits the highest antibacterial activity against microorganisms isolated from the oral cavity in an environment with a pH range between 5 (for Klebsiella pneumoniae, Streptococcus mutans, Staphylococcus aureus) and 8 (for Enterobacter gergoviae, Lactobacillus brevis, Staphylococcus saprophyticus).
The optimum temperature for chitosan activity, in which the antibacterial activity remained at the level of 50–96 %, was estimated at 37 °C in relation to the above-mentioned bacterial species. For K. pneumoniae, L. brevis, and S. saprophyticus, the minimum bactericidal concentration of chitosan (MBC) was 500 μg/ml, whereas for other species, it was 400 μg/ml. It was also shown that 5 s is sufficient for chitosan to exhibit antibacterial activity in relation to the above-mentioned microorganisms species at the level of 99.6 %, and 20s was required to reach the level of 99.9 %.
Thus, it was proven that water-soluble chitosan has a comparable efficacy in the fight against dental caries in relation to commonly used antibacterial mouthwashes for oral hygiene. A very strong argument for the use of chitosan in such formulations is also its much lower toxicity than the conventionally used alcohols, chlorhexidines, or cetylpyridinium chloride [88], which are commonly used in products for oral cavity disinfection in diseases of the upper respiratory tract, e.g., Tantum Verde.
Natural inhibitors—antimicrobial peptides (AMPs): chrisofsin-1, D-Nal-Pac-525
Another safe and potential solution to the problem of dental caries in humans are antimicrobial peptides (AMPs). They exhibit a lethal effect against many microorganisms but do not cause side effects for humans [33].
AMPs represent a family of short polypeptides, typically associated with congenital human and other organism immune systems. An example of such a protein is chrisofsin-1 amphipathic, an α-helical protein of broad activity spectrum against both Gram-negative and Gram-positive bacteria. Chrisofsin-1 possesses a hydrophilic domain at the C-terminal end of the polypeptide chain.
Via electrostatic interactions with negatively-charged phospholipids of bacterial cell membranes, it covers the membrane tightly, and then the hydrophilic domain penetrates into the structure of the membrane, forming a number of pores inside. Wang et al. observed the first changes in cell shape during their studies [33] using chrisofsin-1 at concentrations of 2–4 mg/ml (depending on the bacteria species). Thus, chrisofsin-1 exhibits potent destructive action against lipid components of microorganism cell membranes, such as S. mutans, S. sanguinis, S. sobrinus, S. gordonii, Actinomyces sp., and Lactobacillus sp. and, thus, it is able to induce disintegration and bacterial lysis.
This contributes to reduced viability of pathogens, both as planktonic forms as well as those grouped in biofilm. Compared to S. mutans, chrisofsin-1 exhibits lethal activity after 30 min with the use of an 8 times higher concentration than the minimum inhibitory concentration (MIC). For comparison, E. faecalis was destroyed after 5 min and L. fermentis after 60 min with the use of 12 times the MIC. Among the strains under investigation, only A. viscosus was observed to be more resistant than S. mutans. Its eradication required the application of 8 times the MIC for 60 min. This shows the different effects of chrisofsin-1 in relation to particular bacterial species [32].
Microorganisms remaining in the biofilm structure are 10–1,000 times more resistant than those in the planktonic phase. For this reason, AMPs could be used most effectively in the early stages of biofilm formation. It would prevent its growth and the colonization of the oral cavity by other pathogens. In the phase of biofilm maturation, AMPs would only delay the cariogenic process [33].
The D-Nal-Pac-525 peptide appeared to be another example of an AMP with high antibacterial activity. The high anticaries activity of D-Nal-Pac-525 peptide is associated with the tryptophan conversion in the tryptophan-rich (Trp-rich) Pac-525 peptide fragment to the D-β-naphthylalanines fragment [62]. Studies with the use of scanning and transmission electron microscopes have shown that the D-Nal-Pac-525 peptide causes morphological changes and cell membrane damages of S. mutans with a MIC of 4 ug/ml, while the inhibition of biofilm formation was observed under an MIC of 2 ug/ml. The effective anti-biofilm protection of the D-Nal-Pac-525 peptide was highlighted in the first stage of its formation (planktonic and colonization phase of bacteria) (Fig. 2), with no effect on pre-existing bacterial biofilms. The above-mentioned results are comparable to beta-defensin (6 mM) action in human saliva. Data suggest the use of AMPs exemplified by D-Nal-Pac-525 as new therapeutic agents which combat the problems caused by the occurrence of bacterial strains resistant to antibiotics [62].
The problem of infectious diseases is inherently associated with the growing number of microorganisms becoming resistant to a broader spectrum of antibiotics (multidrug resistance). Therefore, the attention of scientists has increasingly been drawn to antibacterial, antifungal, and antiviral substances of natural origin. Many metabolites of plant origin, such as polyphenols, alkaloids, tannin, terpenoids, steroids, or flavonoids, are known for such properties [61, 89, 90]. Because of their proven antibacterial activity, these compounds act as supplements in oral hygiene products.
Most of the above-mentioned substances have found an application in dentistry. They are included in composites and dental restorative resins, the properties of which allow for the reduction of biofilm formation and reduce the probability of dental caries episodes at the same place. They have been introduced into treatment because commonly used methods of treating developing dental caries usually do not allow for the removal of bacteria from the mineralized tooth tissues. In such situations, it is suggested that antibacterial agents should be applied on already developed dentin [91]. These are usually fillings which release ions [92] in the form of dicalcium phosphate anhydrous nanoparticles (DPCA) or tetracalcium phosphate (TTCP) often enriched with fluorine [92], or modifications of such fillings, e.g., with the addition of QAS or silver nanoparticles [76, 77, 81, 82].
Iodine compounds
Studies on substances allowing for the reduction of dental caries incidence also include iodine compounds such as potassium iodide or povidone–iodine (PVP-I). PVP-I is a complex of iodide and polyvinylpyrrolidone, which is a specific “reservoir” of free iodine ions forming an active domain of the whole structure. Iodine slowly released from the complex is able to penetrate the bacterial membrane and enter into the cytosol, where it deactivates the key proteins, fatty acids, and nucleotides in the metabolism of the cell [93]. Additionally, the slow release of iodine reduces its toxic effects for human cells [94] and its short-term use does not irritate even damaged oral mucosa. No side effects, such as discoloration of teeth, tongue, or taste change, have been reported [95].
Hosaka et al. [95] decided to test the antibacterial activity of PVP-I in relation to the two microorganisms associated with oral cavity diseases, Porphyromonas gingivalis and Fusobacterium nucleatum. They showed that PVP-I, at a concentration of 7 %, is able to reach the activity allowing 100 % of P. gingivalis species to die after 3 min, and an identical effectiveness against F. nucleatum with the use of 5 % of PVP-I was observed after 30 s. The results obtained for dual-species biofilm formed by the microorganisms under investigation indicated that this biofilm is 200 times more resistant to the activity of the tested substance than mono-species cultures, although the activity of 5 % PVP-I allows bacteria which form this biofilm to be killed.
Unfortunately, it has also been shown that the concentrations of PVP-I (0.23–0.47 %) used in common mouthwashes are not sufficient for a significant reduction of P. gingivalis and F. nucleatum biofilm formation ability during a time of 30 s to 1 min, which was estimated as the average time of mouthwashing by the public [95]. Based on these reports, one may conclude that other bacterial species will also exhibit a different sensitivity to PVP-I activity, and a multi-species biofilm will be much more resistant to both planktonic cells as well as structures formed by a lower number of microorganisms.
The antibacterial activity of PVP-I has also been tested for S. mutans strains isolated from children with early childhood caries [96, 97]. It has been proven that the application of 10 % PVP-I on healthy teeth and on teeth with dental caries every 3 months for 1 year significantly reduces the number of these microorganisms in the oral cavity as compared to baseline levels [96, 98]. This reduced number of cariogenic bacteria is, therefore, likely to reduce the probability of childhood dental caries. Similar conclusions can be drawn from studies conducted on a group of 172 children, which showed that combination therapy of dental caries through the application of 10 % PVP-I and dental varnish containing 5 % sodium fluoride reduces the incidence of new carious lesions by 31 % in relation to the application of the varnish alone [86].
Ursolic acid
Composite resins containing ursolic acid which inhibited the biofilm formation of S. mutans at a concentration of 0.1–0.5 wt.% [99] have been proposed as another solution to the problem of dental caries. This compound has similar properties to those tested in clinical trials: triterpenoids of recorded anticancer, antiwrinkle, and antibacterial activity.
Aromatic amino acid pathways
There is little information concerning the effect of amino acids on the ability of S. mutans to form biofilms. Kolodkin-Gal et al. conducted a study on biofilm structure decomposition on the example of Bacillus subtilis. This microorganism produced a factor which inhibited biofilm formation and next induced structure disassembly. Analysis of this factor’s composition demonstrated that it is a mixture of four D-amino acids: leucine, methionine, tyrosine, and tryptophan [100]. A similar inhibitory effect of the D-amino acid mixture was observed in the case of biofilm formation by Staphylococcus aureus [101] and Pseudomonas aeruginosa [102]. Recent studies have indicated that increased levels of L-tryptophan itself may also give the signal for biofilm decomposition; a suitable experiment was conducted on the example of biofilm formed by E. coli [103]. Our study indicates that another aromatic L-amino acid, i.e., tyrosine, has similar properties and may inhibit biofilm formation by S. mutans (paper submitted to the editorial board). It needs to be verified as to whether other aromatic amino acids may exhibit anti-biofilm formation activity for S. mutans species, and also whether inhibition caused by these compounds is limited to ex vivo models, as well as whether and to what degree they may be applied in clinical situations (proposed animal model).
The increasingly broader range of substances protecting us from the occurrence of carious lesions while not causing side effects creates hope for the design of oral hygiene products allowing for the effective fight against this disease and for the complete protection of children from the development of early caries lesions in primary teeth.
Summary
Studies on biofilm formed by S. mutans clearly show that the virulence of S. mutans strains is dependent on environmental conditions, and, thus, on in vivo model host-dependent characteristics. On the other hand, to form a biofilm structure, it is not sufficient to ensure appropriate environmental conditions. Microorganisms alone must possess characteristics which, in terms of the above-mentioned favorable conditions, will allow the adhesion and formation of microcolonies [6, 35, 36].
Cariogenic strains of S. mutans exhibit large variation due to the occupation and expression of characteristics considered as virulence determinants: (A) most S. mutans strains exhibit the ability to survive and reproduce in acidic pH, without eliminating the possibility to survive in alkaline medium levels (described in case studies isolated cases of bacteremia), (B) most S. mutans strains possess the activity of specific proteins (enzymes) associated with biofilm formation, depending on a number of biochemical properties of the host (EPS production, the condition of the host immune system).
Biofilm formation in the oral cavity is a complex process, dependent not only on EPS produced by S. mutans and the host, but also on other species colonizing the oral cavity. The occurrence of potentially non-pathogenic species in the oral cavity environment seems to be the key point.
This study is an introduction to the research which aims to confirm or exclude the virulence of selected Streptococcus strains in clinical material, as well as shedding new light on the development of potential compounds blocking the metabolism of Streptococcus mutans through influence on specific proteins involved in the formation of the complex structure which is a biofilm.
Acknowledgments
Conflict of interest
All authors confirm that there is no conflict of interest.
References
- 1.Huang L, Xu QA, Liu C, Fan MW, Li YH. Anti-caries DNA vaccine-induced secretory immunoglobulin A antibodies inhibit formation of Streptococcus mutans biofilms in vitro. Acta Pharmacol Sin. 2013;34(2):239–246. doi: 10.1038/aps.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura H, Isshiki Y, Sakuda K, Sakuma K, Kondo S. Effects of oakmoss and its components on biofilm formation of Legionella pneumophila. Biol Pharm Bull. 2013;36(5):833–837. doi: 10.1248/bpb.b12-01047. [DOI] [PubMed] [Google Scholar]
- 3.Wojtyczka RD, Kępa M, Idzik D, Kubina R, Kabała-Dzik A, Dziedzic A, Wąsik TJ. In vitro antimicrobial activity of ethanolic extract of Polish propolis against biofilm forming Staphylococcus epidermidis strains. Evid Based Complement Alternat Med. 2013;2013:590703. doi: 10.1155/2013/590703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao K, Tseng BS, Beckerman B, Jin F, Gibiansky ML, Harrison JJ, Luijten E, Parsek MR, Wong GC. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature. 2013;497(7449):388–391. doi: 10.1038/nature12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welin-Neilands J, Svensäter G. Acid tolerance of biofilm cells of Streptococcus mutans. Appl Environ Microbiol. 2007;73(17):5633–5638. doi: 10.1128/AEM.01049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45(1):69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. MBio. 2010;1(3):pii: e00178-10. doi: 10.1128/mBio.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60(2):316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall A, Pirofski LA. Host–pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67(8):3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreikemeyer B, Gámez G, Margarit I, Giard JC, Hammerschmidt S, Hartke A, Podbielski A. Genomic organization, structure, regulation and pathogenic role of pilus constituents in major pathogenic Streptococci and Enterococci. Int J Med Microbiol. 2011;301(3):240–251. doi: 10.1016/j.ijmm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Bagnoli F, Moschioni M, Donati C, Dimitrovska V, Ferlenghi I, Facciotti C, Muzzi A, Giusti F, Emolo C, Sinisi A, Hilleringmann M, Pansegrau W, Censini S, Rappuoli R, Covacci A, Masignani V, Barocchi MA. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J Bacteriol. 2008;190:5480–5492. doi: 10.1128/JB.00384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaykus LA, Wang HH, Schlesinge LS. Food-borne microbes: shaping the host ecosystem. Washington: ASM Press; 2009. p. 124. [Google Scholar]
- 13.Tamura S, Yonezawa H, Motegi M, Nakao R, Yoneda S, Watanabe H, Yamazaki T, Senpuku H. Inhibiting effects of Streptococcus salivarius on competence-stimulating peptide-dependent biofilm formation by Streptococcus mutans. Oral Microbiol Immunol. 2009;24(2):152–161. doi: 10.1111/j.1399-302X.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 14.Svensäter G, Welin J, Wilkins JC, Beighton D, Hamilton IR. Protein expression by planktonic and biofilm cells of Streptococcus mutans. FEMS Microbiol Lett. 2001;205(1):139–146. doi: 10.1016/S0378-1097(01)00459-1. [DOI] [PubMed] [Google Scholar]
- 15.Jung CJ, Yeh CY, Shun CT, Hsu RB, Cheng HW, Lin CS, Chia JS. Platelets enhance biofilm formation and resistance of endocarditis-inducing streptococci on the injured heart valve. J Infect Dis. 2012;205(7):1066–1075. doi: 10.1093/infdis/jis021. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Urano-Tashiro Y, Konishi K. Adhesins of oral streptococci. Nihon Saikingaku Zasshi. 2013;68(2):283–293. doi: 10.3412/jsb.68.283. [DOI] [PubMed] [Google Scholar]
- 17.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Moreillon P, de Jesus Antunes M, Thilen U, Lekakis J, Lengyel M, Müller L, Naber CK, Nihoyannopoulos P, Moritz A, Zamorano JL, ESC Committee for Practice Guidelines Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30(19):2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 18.Jung CJ, Zheng QH, Shieh YH, Lin CS, Chia JS. Streptococcus mutans autolysin AtlA is a fibronectin-binding protein and contributes to bacterial survival in the bloodstream and virulence for infective endocarditis. Mol Microbiol. 2009;74(4):888–902. doi: 10.1111/j.1365-2958.2009.06903.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakano K, Ooshima T. Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 2009;4:891–902. doi: 10.2217/fmb.09.64. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto-Nakano M, Tsuji M, Inagaki S, Fujita K, Nagayama K, Nomura R, Ooshima T. Contribution of cell surface protein antigen c of Streptococcus mutans to platelet aggregation. Oral Microbiol Immunol. 2009;24:427–430. doi: 10.1111/j.1399-302X.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 21.Abranches J, Miller JH, Martinez AR, Simpson-Haidaris PJ, Burne RA, Lemos JA. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun. 2011;79:2277–2284. doi: 10.1128/IAI.00767-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abranches J, Zeng L, Bélanger M, Rodrigues PH, Simpson-Haidaris PJ, Akin D, Dunn WA, Jr, Progulske-Fox A, Burne RA. Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol. 2009;24:141–145. doi: 10.1111/j.1399-302X.2008.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn SJ, Ahn SJ, Wen ZT, Brady LJ, Burne RA. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect Immun. 2008;76(9):4259–4268. doi: 10.1128/IAI.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AU, Islam B, Khan SN, Akram M. A proteomic approach for exploring biofilm in Streptococcus mutans. Bioinformation. 2011;5(10):440–445. doi: 10.6026/97320630005440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson SN, Snesrud E, Liu J, Ong AC, Kilian M, Schork NJ, Bretz W. The dental plaque microbiome in health and disease. PLoS One. 2013;8(3):e58487. doi: 10.1371/journal.pone.0058487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawka B, Dreher P, Herda J, Szwiec I, Krasicka M. Próchnica zębów u dzieci problemem społecznym. Probl Hig Epidemiol. 2010;91(1):5–7. [Google Scholar]
- 28.Krzyściak W, Pluskwa KK, Jurczak A, Kościelniak D (2013) The pathogenicity of the Streptococcus genus. Eur J Clin Microbiol Infect Dis (in press) [DOI] [PMC free article] [PubMed]
- 29.Składnik-Jankowska J, Kaczmarek U. Istotny Wskaźnik Próchnicy u 12-letnich dzieci z różnych środowisk województwa dolnośląskiego. Czas Stomatol. 2010;63(3):166–173. [Google Scholar]
- 30.World Health Organization (WHO) (2011) Poland: health system review. Health systems in transition, Vol. 13, No. 8 [PubMed]
- 31.PZH Raports (2007–2012) Informacje o zachorowaniach na choroby zakaźne i zatruciach w Polsce oraz szczepieniach ochronnych w latach 2007–2012 dostępne na stronie internetowej. Available online at: http://www.pzh.gov.pl/oldpage/epimeld/index_p.html#01
- 32.Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJ. Oral biofilm architecture on natural teeth. PLoS One. 2010;5(2):e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Tao R, Tong Z, Ding Y, Kuang R, Zhai S, Liu J, Ni L. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides. 2012;33(2):212–219. doi: 10.1016/j.peptides.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Kang MS, Oh JS, Lee HC, Lim HS, Lee SW, Yang KH, Choi NK, Kim SM. Inhibitory effect of Lactobacillus reuteri on periodontopathic and cariogenic bacteria. J Microbiol. 2011;49(2):193–199. doi: 10.1007/s12275-011-0252-9. [DOI] [PubMed] [Google Scholar]
- 35.Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR, 3rd, Heydorn A, Koo H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8(4):e1002623. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab C, Walter J, Tannock GW, Vogel RF, Gänzle MG. Sucrose utilization and impact of sucrose on glycosyltransferase expression in Lactobacillus reuteri. Syst Appl Microbiol. 2007;30(6):433–443. doi: 10.1016/j.syapm.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Siqueira WL, Bakkal M, Xiao Y, Sutton JN, Mendes FM. Quantitative proteomic analysis of the effect of fluoride on the acquired enamel pellicle. PLoS One. 2012;7(8):e42204. doi: 10.1371/journal.pone.0042204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duque C, Stipp RN, Wang B, Smith DJ, Höfling JF, Kuramitsu HK, Duncan MJ, Mattos-Graner RO. Downregulation of GbpB, a component of the VicRK regulon, affects biofilm formation and cell surface characteristics of Streptococcus mutans. Infect Immun. 2011;79(2):786–796. doi: 10.1128/IAI.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita K, Matsumoto-Nakano M, Inagaki S, Ooshima T. Biological functions of glucan-binding protein B of Streptococcus mutans. Oral Microbiol Immunol. 2007;22(5):289–292. doi: 10.1111/j.1399-302X.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujita K, Takashima Y, Inagaki S, Nagayama K, Nomura R, Ardin AC, Grönroos L, Alaluusua S, Ooshima T, Matsumoto-Nakano M. Correlation of biological properties with glucan-binding protein B expression profile in Streptococcus mutans clinical isolates. Arch Oral Biol. 2011;56(3):258–263. doi: 10.1016/j.archoralbio.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Zeng L, Choi SC, Danko CG, Siepel A, Stanhope MJ, Burne RA. Gene regulation by CcpA and catabolite repression explored by RNA-Seq in Streptococcus mutans. PLoS One. 2013;8(3):e60465. doi: 10.1371/journal.pone.0060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giammarinaro P, Paton JC. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect Immun. 2002;70(10):5454–5461. doi: 10.1128/IAI.70.10.5454-5461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng L, Burne RA. Comprehensive mutational analysis of sucrose-metabolizing pathways in Streptococcus mutans reveals novel roles for the sucrose phosphotransferase system permease. J Bacteriol. 2013;195(4):833–843. doi: 10.1128/JB.02042-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai J, Tong H, Qi F, Dong X. CcpA-dependent carbohydrate catabolite repression regulates galactose metabolism in Streptococcus oligofermentans. J Bacteriol. 2012;194(15):3824–3832. doi: 10.1128/JB.00156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsang P, Merritt J, Shi W, Qi F. IrvA-dependent and IrvA-independent pathways for mutacin gene regulation in Streptococcus mutans. FEMS Microbiol Lett. 2006;261(2):231–234. doi: 10.1111/j.1574-6968.2006.00351.x. [DOI] [PubMed] [Google Scholar]
- 46.Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol. 2010;192(12):3024–3032. doi: 10.1128/JB.01649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein MI, Xiao J, Lu B, Delahunty CM, Yates JR, 3rd, Koo H. Streptococcus mutans protein synthesis during mixed-species biofilm development by high-throughput quantitative proteomics. PLoS One. 2012;7(9):e45795. doi: 10.1371/journal.pone.0045795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brady LJ, Maddocks SE, Larson MR, Forsgren N, Persson K, Deivanayagam CC, Jenkinson HF. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol Microbiol. 2010;77(2):276–286. doi: 10.1111/j.1365-2958.2010.07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edberg SC. Does the possession of virulence factor genes mean that those genes will be active? J Water Health. 2009;7(Suppl 1):S19–S28. doi: 10.2166/wh.2009.066. [DOI] [PubMed] [Google Scholar]
- 50.Crowley PJ, Seifert TB, Isoda R, van Tilburg M, Oli MW, Robinette RA, McArthur WP, Bleiweis AS, Brady LJ. Requirements for surface expression and function of adhesin P1 from Streptococcus mutans. Infect Immun. 2008;76(6):2456–2468. doi: 10.1128/IAI.01315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLean JS, Fansler SJ, Majors PD, McAteer K, Allen LZ, Shirtliff ME, Lux R, Shi W. Identifying low pH active and lactate-utilizing taxa within oral microbiome communities from healthy children using stable isotope probing techniques. PLoS One. 2012;7(3):e32219. doi: 10.1371/journal.pone.0032219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn SJ, Burne RA. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J Bacteriol. 2007;189(17):6293–6302. doi: 10.1128/JB.00546-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibbons RJ, Hay DI. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989;68:1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- 54.Yamada A, Tamura H, Kato H. Identification and characterization of an autolysin gene, atlg, from Streptococcus sobrinus. FEMS Microbiol Lett. 2009;291(1):17–23. doi: 10.1111/j.1574-6968.2008.01426.x. [DOI] [PubMed] [Google Scholar]
- 55.Aires CP, Del Bel Cury AA, Tenuta LM, Klein MI, Koo H, Duarte S, Cury JA. Effect of starch and sucrose on dental biofilm formation and on root dentine demineralization. Caries Res. 2008;42(5):380–386. doi: 10.1159/000154783. [DOI] [PubMed] [Google Scholar]
- 56.Bhalla S, Tandon S, Satyamoorthy K. Salivary proteins and early childhood caries: a gel electrophoretic analysis. Contemp Clin Dent. 2010;1(1):17–22. doi: 10.4103/0976-237X.62515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaczmarek U. Aspekt bakteryjny próchnicy zębów mlecznych. Dent Med Probl. 2004;41(3):509–514. [Google Scholar]
- 58.Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55(4):358–367. doi: 10.1111/j.1834-7819.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- 59.Moynihan PJ. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull World Health Organ. 2005;83(9):694–699. [PMC free article] [PubMed] [Google Scholar]
- 60.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 61.Hu P, Huang P, Chen MW. Curcumin reduces Streptococcus mutans biofilm formation by inhibiting sortase A activity. Arch Oral Biol. 2013;58(10):1343–1348. doi: 10.1016/j.archoralbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Cheng JW, Yu HY, Xin Y, Tang L, Ma Y. Effect of the antimicrobial peptide D-Nal-Pac-525 on the growth of Streptococcus mutans and its biofilm formation. J Microbiol Biotechnol. 2013;3(8):1070–1075. doi: 10.4014/jmb.1212.12035. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa A, Furukawa S, Fujita S, Mitobe J, Kawarai T, Narisawa N, Sekizuka T, Kuroda M, Ochiai K, Ogihara H, Kosono S, Yoneda S, Watanabe H, Morinaga Y, Uematsu H, Senpuku H. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl Environ Microbiol. 2011;77(5):1572–1580. doi: 10.1128/AEM.02066-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redanz S, Standar K, Podbielski A, Kreikemeyer B. A five-species transcriptome array for oral mixed-biofilm studies. PLoS One. 2011;6(12):e27827. doi: 10.1371/journal.pone.0027827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jalasvuori H, Haukioja A, Tenovuo J. Probiotic Lactobacillus reuteri strains ATCC PTA 5289 and ATCC 55730 differ in their cariogenic properties in vitro. Arch Oral Biol. 2012;57(12):1633–1638. doi: 10.1016/j.archoralbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Teanpaisan R, Piwat S, Dahlén G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett Appl Microbiol. 2011;53(4):452–459. doi: 10.1111/j.1472-765X.2011.03132.x. [DOI] [PubMed] [Google Scholar]
- 67.Lang C, Böttner M, Holz C, Veen M, Ryser M, Reindl A, Pompejus M, Tanzer JM. Specific Lactobacillus/Mutans Streptococcus co-aggregation. J Dent Res. 2010;89(2):175–179. doi: 10.1177/0022034509356246. [DOI] [PubMed] [Google Scholar]
- 68.Söderling EM, Marttinen AM, Haukioja AL. Probiotic lactobacilli interfere with Streptococcus mutans biofilm formation in vitro. Curr Microbiol. 2011;62(2):618–622. doi: 10.1007/s00284-010-9752-9. [DOI] [PubMed] [Google Scholar]
- 69.Samot J, Lebreton J, Badet C. Adherence capacities of oral lactobacilli for potential probiotic purposes. Anaerobe. 2011;17(2):69–72. doi: 10.1016/j.anaerobe.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Tahmourespour A, Salehi R, Kermanshahi RK, Eslami G. The anti-biofouling effect of Lactobacillus fermentum-derived biosurfactant against Streptococcus mutans. Biofouling. 2011;27(4):385–392. doi: 10.1080/08927014.2011.575458. [DOI] [PubMed] [Google Scholar]
- 71.Kim do K, Kim KH, Cho EJ, Joo SJ, Chung JM, Son BY, Yum JH, Kim YM, Kwon HJ, Kim BW, Kim TH, Lee EW. Gene cloning and characterization of MdeA, a novel multidrug efflux pump in Streptococcus mutans. J Microbiol Biotechnol. 2013;23(3):430–435. doi: 10.4014/jmb.1301.01028. [DOI] [PubMed] [Google Scholar]
- 72.Mei ML, Chu CH, Low KH, Che CM, Lo EC (2013) Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal (in press) [DOI] [PMC free article] [PubMed]
- 73.García-Godoy F, Hicks MJ. Maintaining the integrity of the enamel surface: the role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J Am Dent Assoc. 2008;139(Suppl):25S–34S. doi: 10.14219/jada.archive.2008.0352. [DOI] [PubMed] [Google Scholar]
- 74.Goyal D, Sharma S, Mahmood A. Inhibition of dextransucrase activity in Streptococcus mutans by plant phenolics. Indian J Biochem Biophys. 2013;50(1):48–53. [PubMed] [Google Scholar]
- 75.Melo MA, Guedes SF, Xu HH, Rodrigues LK. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31(8):459–467. doi: 10.1016/j.tibtech.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou X, Xu HHK. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. J Dent Res. 2012;91:460–466. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng L, Weir MD, Zhang K, Wu EJ, Xu SM, Zhou X, Xu HH. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent Mater. 2012;28(8):853–862. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Waal SV, van der Sluis LW. Potential of calcium to scaffold an endodontic biofilm, thus protecting the micro-organisms from disinfection. Med Hypotheses. 2012;79(1):1–4. doi: 10.1016/j.mehy.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 79.Obłak E, Gamian A. The biological activity of quaternary ammonium salts (QASs) Postepy Hig Med Dosw (Online) 2010;64:201–211. [PubMed] [Google Scholar]
- 80.Lee VA, Karthikeyan R, Rawls HR, Amaechi BT. Anti-cariogenic effect of a cetylpyridinium chloride-containing nanoemulsion. J Dent. 2010;38(9):742–749. doi: 10.1016/j.jdent.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HH. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater. 2013;29(4):462–472. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, Bai Y, Xu HH. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J Dent. 2013;1(5):464–474. doi: 10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim YJ, Lee DY, Lee JY, Lim YK. The effect of silver ion-releasing elastomers on mutans streptococci in dental plaque. Korean J Orthod. 2012;42(2):87–93. doi: 10.4041/kjod.2012.42.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.You C, Han C, Wang X, Zheng Y, Li Q, Hu X, Sun H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–9201. doi: 10.1007/s11033-012-1792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mei ML, Chu CH, Lo EC, Samaranayake LP. Preventing root caries development under oral biofilm challenge in an artificial mouth. Med Oral Patol Oral Cir Bucal. 2013;18(4):e557–e563. doi: 10.4317/medoral.18768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milgrom PM, Tut OK, Mancl LA. Topical iodine and fluoride varnish effectiveness in the primary dentition: a quasi-experimental study. J Dent Child (Chic) 2011;78(3):143–147. [PubMed] [Google Scholar]
- 87.Chu CH, Mei L, Seneviratne CJ, Lo EC. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22:2–10. doi: 10.1111/j.1365-263X.2011.01149.x. [DOI] [PubMed] [Google Scholar]
- 88.Chen CY, Chung YC. Antibacterial effect of water-soluble chitosan on representative dental pathogens Streptococcus mutans and Lactobacilli brevis. J Appl Oral Sci. 2012;20(6):620–627. doi: 10.1590/S1678-77572012000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafiei Z, Shuhairi NN, Md Fazly Shah Yap N, Harry Sibungkil CA, Latip J. Antibacterial activity of myristica fragrans against oral pathogens. Evid Based Complement Alternat Med. 2012;2012:825362. doi: 10.1155/2012/825362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noumedem JA, Mihasan M, Lacmata ST, Stefan M, Kuiate JR, Kuete V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against gram-negative multidrug-resistant bacteria. BMC Complement Alternat Med. 2013;13(1):26. doi: 10.1186/1472-6882-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Łukomska-Szymańska M, Olbert-Sieroszewska V, Żurawska-Olszewska J, Szczerba I, Krzemiński Z, Sokołowski J. Antibacterial properties of 6th generation bonding systems. Dent Med Probl. 2010;47(3):304–308. [Google Scholar]
- 92.Chen MH. Update on dental nanocomposites. J Dent Res. 2010;89(6):549–560. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 93.Durani P, Leaper D. Povidone–iodine: use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J. 2008;5:376–387. doi: 10.1111/j.1742-481X.2007.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson MJ, Horn ME, Lin YC, Parks PJ, Peterson ML. Efficacy of concurrent application of chlorhexidine gluconate and povidone iodine against six nosocomial pathogens. Am J Infect Control. 2010;38(10):826–831. doi: 10.1016/j.ajic.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 95.Hosaka Y, Saito A, Maeda R, Fukaya C, Morikawa S, Makino A, Ishihara K, Nakagawa T. Antibacterial activity of povidone–iodine against an artificial biofilm of Porphyromonas gingivalis and Fusobacterium nucleatum. Arch Oral Biol. 2012;57(4):364–368. doi: 10.1016/j.archoralbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 96.Simratvir M, Singh N, Chopra S, Thomas AM. Efficacy of 10% povidone iodine in children affected with early childhood caries: an in vivo study. J Clin Pediatr Dent. 2010;34(3):233–238. doi: 10.17796/jcpd.34.3.l552816527xtv122. [DOI] [PubMed] [Google Scholar]
- 97.Berkowitz RJ, Koo H, McDermott MP, Whelehan MT, Ragusa P, Kopycka-Kedzierawski DT, Karp JM, Billings R. Adjunctive chemotherapeutic suppression of mutans streptococci in the setting of severe early childhood caries: an exploratory study. J Public Health Dent. 2009;69(3):163–167. doi: 10.1111/j.1752-7325.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berkowitz RJ, Amante A, Kopycka-Kedzierawski DT, Billings RJ, Feng C. Dental caries recurrence following clinical treatment for severe early childhood caries. Pediatr Dent. 2011;33(7):510–514. [PubMed] [Google Scholar]
- 99.Kim S, Song M, Roh BD, Park SH, Park JW. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor Dent Endod. 2013;38(2):65–72. doi: 10.5395/rde.2013.38.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328(5978):627–629. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol. 2011;193(20):5616–5622. doi: 10.1128/JB.05534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol. 2012;10:39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 103.Shimazaki J, Furukawa S, Ogihara H, Morinaga Y. L-Tryptophan prevents Escherichia coli biofilm formation and triggers biofilm degradation. Biochem Biophys Res Commun. 2012;419(4):715–718. doi: 10.1016/j.bbrc.2012.02.085. [DOI] [PubMed] [Google Scholar]