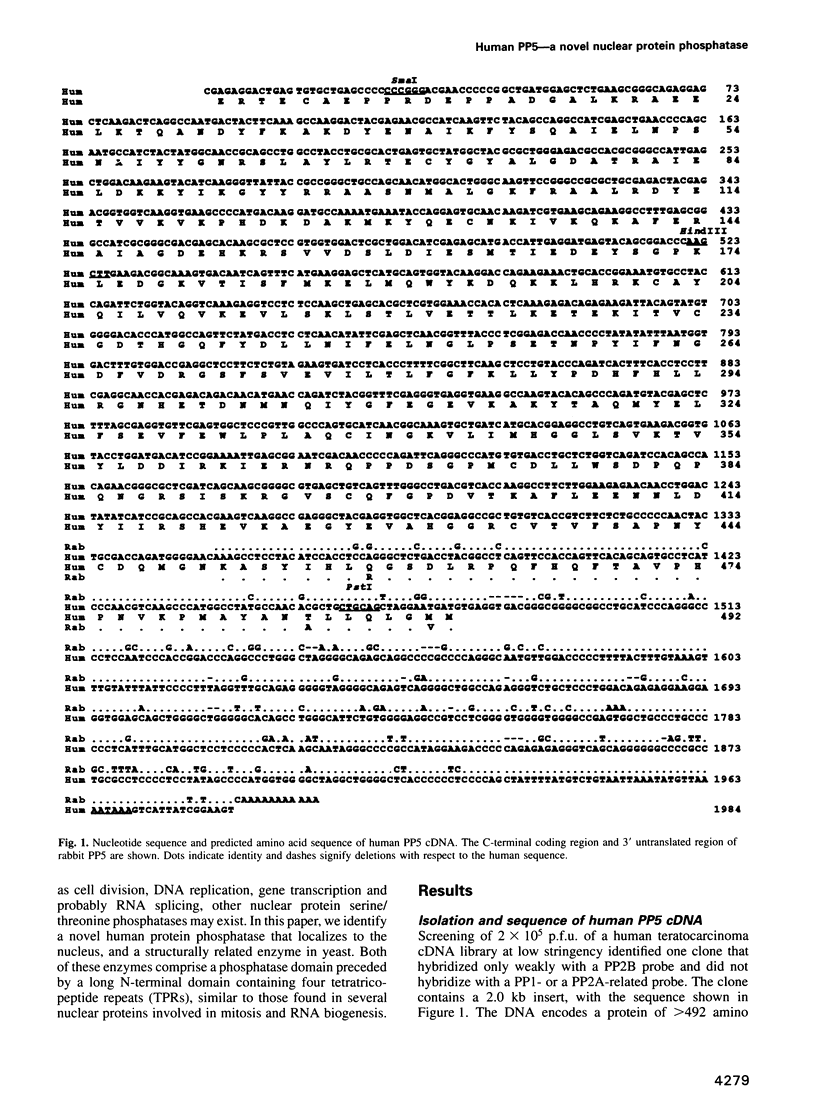
Abstract
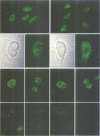
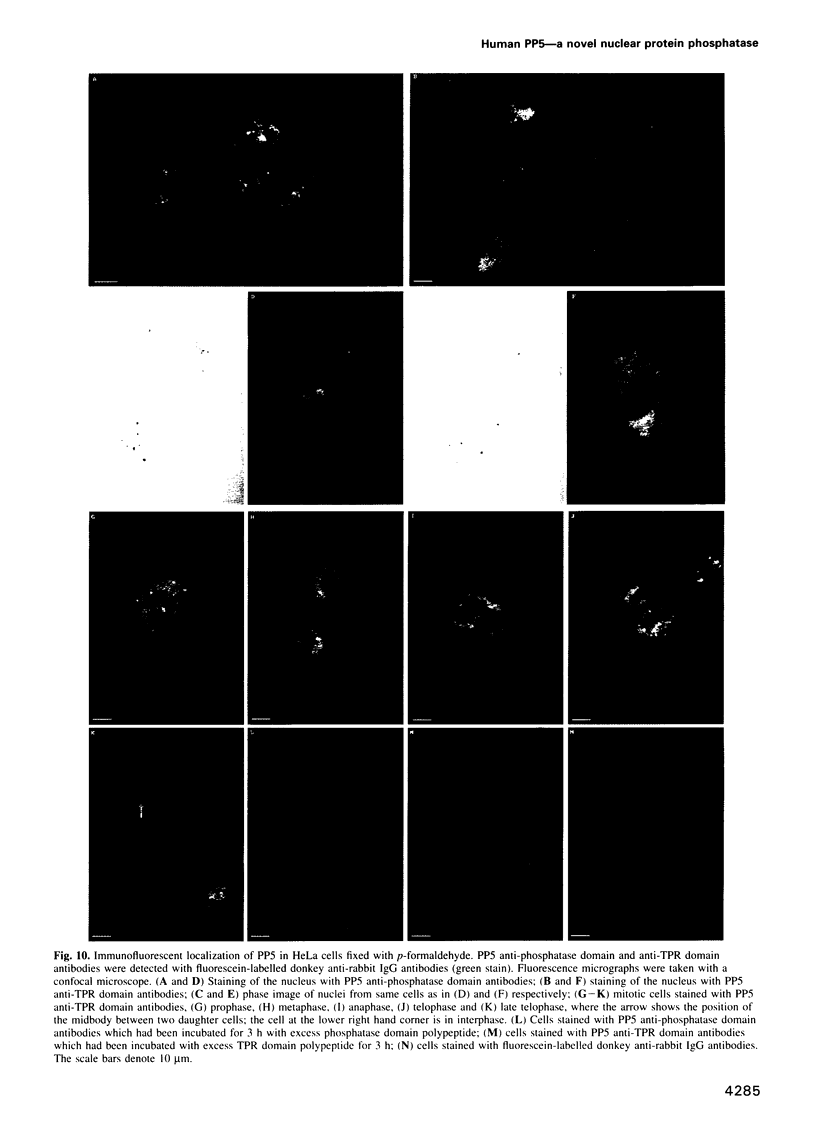
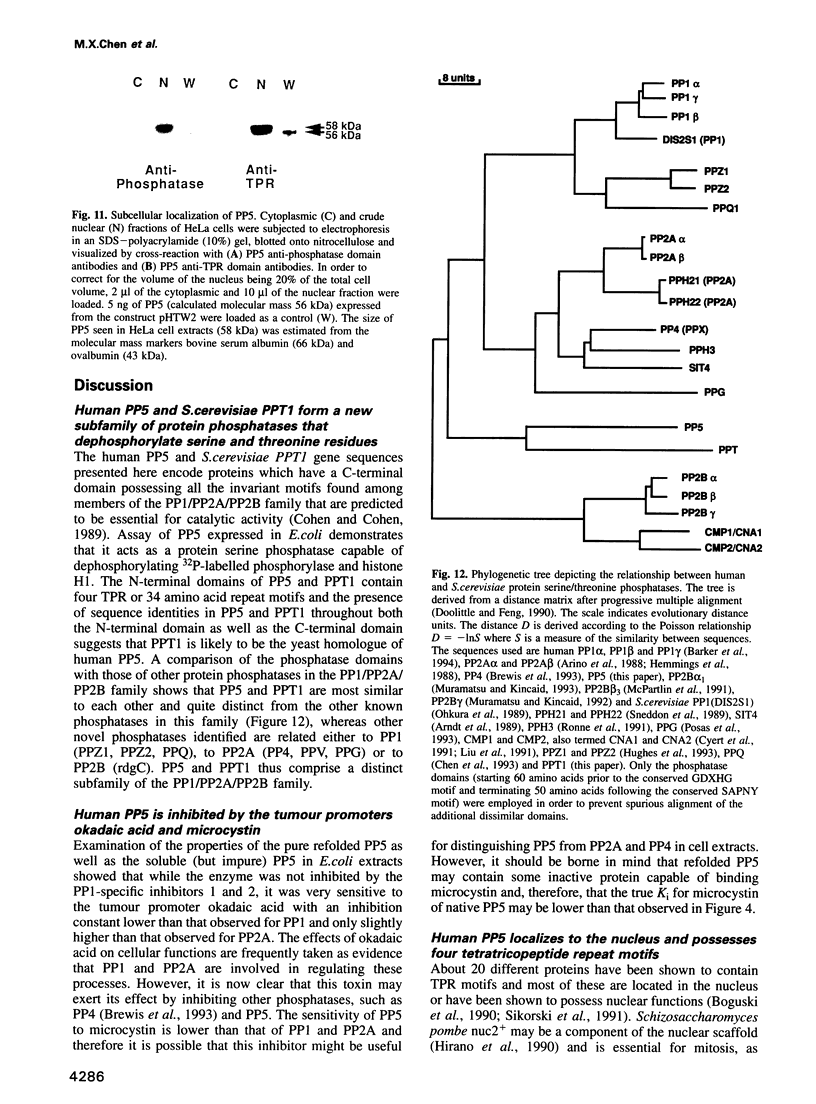
A novel human protein serine/threonine phosphatase, PP5, and a structurally related phosphatase in Saccharomyces cerevisiae, PPT1, have been identified from their cDNA and gene respectively. Their predicted molecular mass is 58 kDa and they comprise a C-terminal phosphatase catalytic domain and an N-terminal domain, which has four repeats of 34 amino acids, three of which are tandemly arranged. The phosphatase domain possesses all the invariant motifs of the PP1/PP2A/PP2B gene family, but is not closely related to any other known member (< or = 40% identity). Thus PP5 and PPT1 comprise a new subfamily. The repeats in the N-terminal domain are similar to the tetratricopeptide repeat (TPR) motifs which have been found in several proteins that are required for mitosis, transcription and RNA splicing. Bacterially expressed PP5 is able to dephosphorylate serine residues in proteins and is more sensitive than PP1 to the tumour promoter okadaic acid. A 2.3 kb mRNA encoding PP5 is present in all human tissues examined. Investigation of the intracellular distribution of PP5 by immunofluorescence, using two different antibodies raised against the TPR and phosphatase domains, localizes PP5 predominantly to the nucleus. This suggests that, like other nuclear TPR-containing proteins, it may play a role in the regulation of RNA biogenesis and/or mitosis.
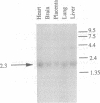
Full text
PDF
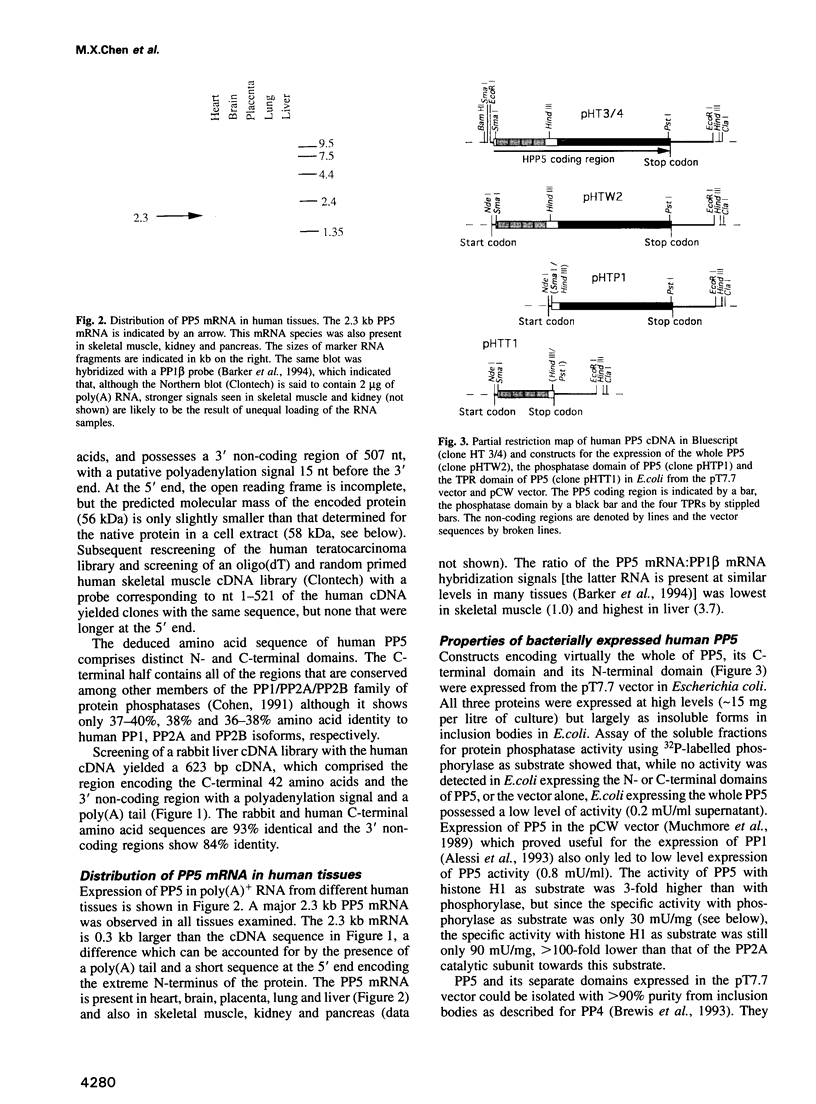




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Street A. J., Cohen P., Cohen P. T. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur J Biochem. 1993 May 1;213(3):1055–1066. doi: 10.1111/j.1432-1033.1993.tb17853.x. [DOI] [PubMed] [Google Scholar]
- Arino J., Woon C. W., Brautigan D. L., Miller T. B., Jr, Johnson G. L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt K. T., Styles C. A., Fink G. R. A suppressor of a HIS4 transcriptional defect encodes a protein with homology to the catalytic subunit of protein phosphatases. Cell. 1989 Feb 24;56(4):527–537. doi: 10.1016/0092-8674(89)90576-x. [DOI] [PubMed] [Google Scholar]
- Barker H. M., Brewis N. D., Street A. J., Spurr N. K., Cohen P. T. Three genes for protein phosphatase 1 map to different human chromosomes: sequence, expression and gene localisation of protein serine/threonine phosphatase 1 beta (PPP1CB). Biochim Biophys Acta. 1994 Jan 13;1220(2):212–218. doi: 10.1016/0167-4889(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Berndt N., Campbell D. G., Caudwell F. B., Cohen P., da Cruz e Silva E. F., da Cruz e Silva O. B., Cohen P. T. Isolation and sequence analysis of a cDNA clone encoding a type-1 protein phosphatase catalytic subunit: homology with protein phosphatase 2A. FEBS Lett. 1987 Nov 2;223(2):340–346. doi: 10.1016/0014-5793(87)80316-2. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Sikorski R. S., Hieter P., Goebl M. Expanding family. Nature. 1990 Jul 12;346(6280):114–114. doi: 10.1038/346114a0. [DOI] [PubMed] [Google Scholar]
- Brewis N. D., Street A. J., Prescott A. R., Cohen P. T. PPX, a novel protein serine/threonine phosphatase localized to centrosomes. EMBO J. 1993 Mar;12(3):987–996. doi: 10.1002/j.1460-2075.1993.tb05739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. X., Chen Y. H., Cohen P. T. PPQ, a novel protein phosphatase containing a Ser + Asn-rich amino-terminal domain, is involved in the regulation of protein synthesis. Eur J Biochem. 1993 Dec 1;218(2):689–699. doi: 10.1111/j.1432-1033.1993.tb18423.x. [DOI] [PubMed] [Google Scholar]
- Chen M. X., Chen Y. H., Cohen P. T. Polymerase chain reactions using Saccharomyces, Drosophila and human DNA predict a large family of protein serine/threonine phosphatases. FEBS Lett. 1992 Jul 13;306(1):54–58. doi: 10.1016/0014-5793(92)80836-6. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Berndt N. Reactivation of protein phosphatase 1 expressed at high levels from recombinant baculovirus. Methods Enzymol. 1991;201:408–414. doi: 10.1016/0076-6879(91)01037-3. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P. T. Cloning of protein-serine/threonine phosphatases. Methods Enzymol. 1991;201:398–408. doi: 10.1016/0076-6879(91)01036-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Cohen P. Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem J. 1989 Jun 15;260(3):931–934. doi: 10.1042/bj2600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
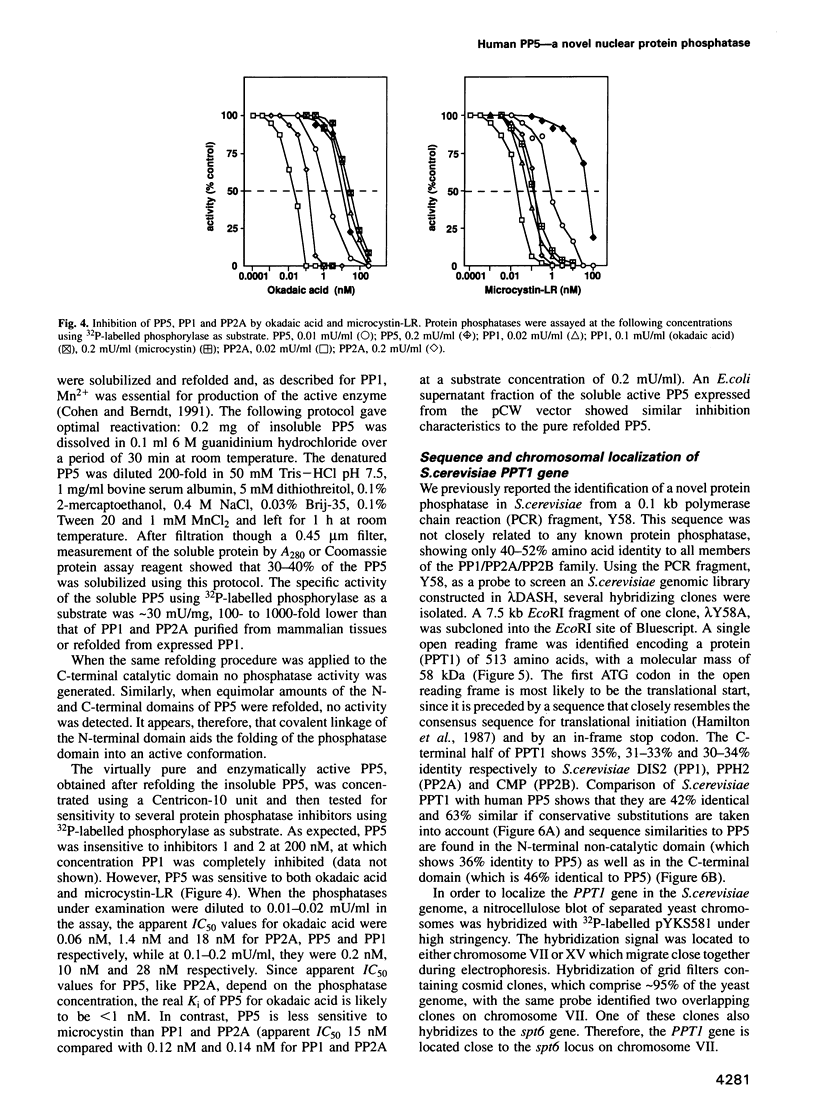
- Cohen P. T. Important roles for novel protein phosphatases dephosphorylating serine and threonine residues. Biochem Soc Trans. 1993 Nov;21(4):884–888. doi: 10.1042/bst0210884. [DOI] [PubMed] [Google Scholar]
- Cohen P., Foulkes J. G., Holmes C. F., Nimmo G. A., Tonks N. K. Protein phosphatase inhibitor-1 and inhibitor-2 from rabbit skeletal muscle. Methods Enzymol. 1988;159:427–437. doi: 10.1016/0076-6879(88)59042-0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D., Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrádi V., Axton J. M., Glover D. M., Cohen P. T. Molecular cloning and chromosomal localization of a novel Drosophila protein phosphatase. FEBS Lett. 1989 Apr 24;247(2):391–395. doi: 10.1016/0014-5793(89)81377-8. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Nearest neighbor procedure for relating progressively aligned amino acid sequences. Methods Enzymol. 1990;183:659–669. doi: 10.1016/0076-6879(90)83043-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ferrigno P., Langan T. A., Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993 Jul;4(7):669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. H., Krebs E. G. Commentary on 'The Phosphorylase b to a Converting Enzyme of Rabbit Skeletal Muscle". Biochim Biophys Acta. 1989;1000:297–301. doi: 10.1016/s0006-3002(89)80024-1. [DOI] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
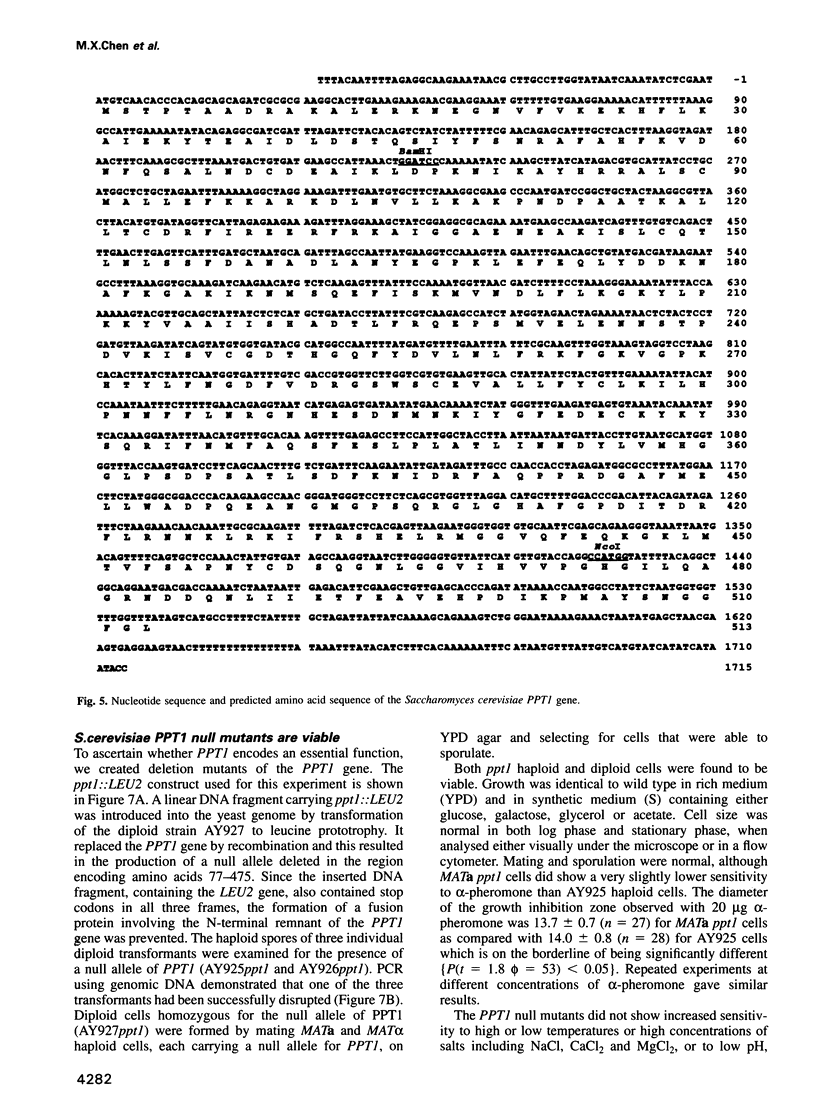
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T., Riezman H., Suda K., Schatz G. Import of proteins into mitochondria: nucleotide sequence of the gene for a 70-kd protein of the yeast mitochondrial outer membrane. EMBO J. 1983;2(12):2169–2172. doi: 10.1002/j.1460-2075.1983.tb01718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Wernet W., Mayer R., Maurer F., Hofsteenge J., Stone S. R. The nucleotide sequence of the cDNA encoding the human lung protein phosphatase 2A beta catalytic subunit. Nucleic Acids Res. 1988 Dec 9;16(23):11366–11366. doi: 10.1093/nar/16.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Kinoshita N., Morikawa K., Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+. Cell. 1990 Jan 26;60(2):319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard M. J., Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993 May;18(5):172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Hughes V., Müller A., Stark M. J., Cohen P. T. Both isoforms of protein phosphatase Z are essential for the maintenance of cell size and integrity in Saccharomyces cerevisiae in response to osmotic stress. Eur J Biochem. 1993 Aug 15;216(1):269–279. doi: 10.1111/j.1432-1033.1993.tb18142.x. [DOI] [PubMed] [Google Scholar]
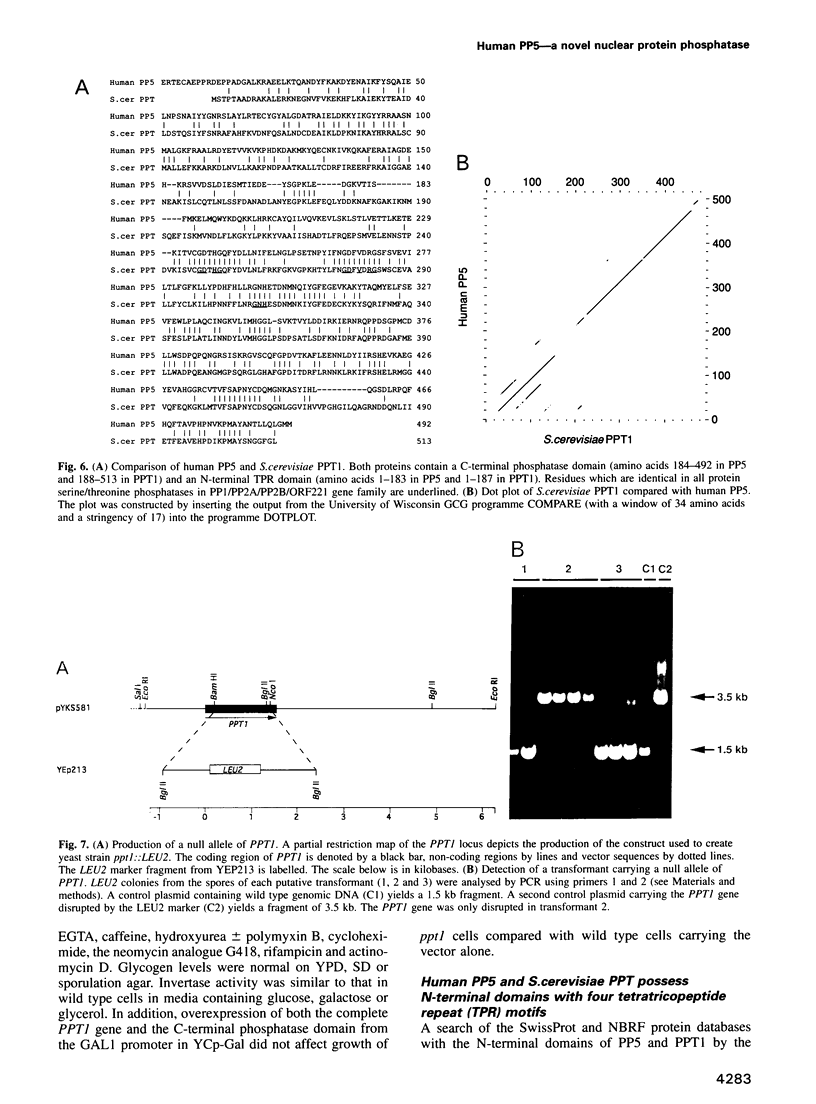
- Icho T., Wickner R. B. Metal-binding, nucleic acid-binding finger sequences in the CDC16 gene of Saccharomyces cerevisiae. Nucleic Acids Res. 1987 Oct 26;15(20):8439–8450. doi: 10.1093/nar/15.20.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes S., Mellgren R. L., Schlender K. K. Isolation and characterization of an inhibitor-sensitive and a polycation-stimulated protein phosphatase from rat liver nuclei. Biochim Biophys Acta. 1986 Aug 29;888(1):135–142. doi: 10.1016/0167-4889(86)90079-0. [DOI] [PubMed] [Google Scholar]
- Kuret J., Bell H., Cohen P. Identification of high levels of protein phosphatase-1 in rat liver nuclei. FEBS Lett. 1986 Jul 28;203(2):197–202. doi: 10.1016/0014-5793(86)80741-4. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Hines L. K., Levin D. E. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol Cell Biol. 1993 Sep;13(9):5843–5853. doi: 10.1128/mcb.13.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. G., Tang N., Thompson S., Miller J., Katze M. G. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol. 1994 Apr;14(4):2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain P., Choulika A. The molecular characterization of PRP6 and PRP9 yeast genes reveals a new cysteine/histidine motif common to several splicing factors. EMBO J. 1990 Sep;9(9):2775–2781. doi: 10.1002/j.1460-2075.1990.tb07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Fields F. O., Kunisawa R., Bishop J. M., Thorner J. A candidate protein kinase C gene, PKC1, is required for the S. cerevisiae cell cycle. Cell. 1990 Jul 27;62(2):213–224. doi: 10.1016/0092-8674(90)90360-q. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ishii S., Tokai M., Tsutsumi H., Ohki O., Akada R., Tanaka K., Tsuchiya E., Fukui S., Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991 May;227(1):52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Mann D. J., Dombrádi V., Cohen P. T. Drosophila protein phosphatase V functionally complements a SIT4 mutant in Saccharomyces cerevisiae and its amino-terminal region can confer this complementation to a heterologous phosphatase catalytic domain. EMBO J. 1993 Dec;12(12):4833–4842. doi: 10.1002/j.1460-2075.1993.tb06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marck C., Lefebvre O., Carles C., Riva M., Chaussivert N., Ruet A., Sentenac A. The TFIIIB-assembling subunit of yeast transcription factor TFIIIC has both tetratricopeptide repeats and basic helix-loop-helix motifs. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4027–4031. doi: 10.1073/pnas.90.9.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum D., Monosov E., Subramani S. The pas8 mutant of Pichia pastoris exhibits the peroxisomal protein import deficiencies of Zellweger syndrome cells--the PAS8 protein binds to the COOH-terminal tripeptide peroxisomal targeting signal, and is a member of the TPR protein family. J Cell Biol. 1993 May;121(4):761–774. doi: 10.1083/jcb.121.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartlin A. E., Barker H. M., Cohen P. T. Identification of a third alternatively spliced cDNA encoding the catalytic subunit of protein phosphatase 2B beta. Biochim Biophys Acta. 1991 Feb 16;1088(2):308–310. doi: 10.1016/0167-4781(91)90069-x. [DOI] [PubMed] [Google Scholar]
- Mirabito P. M., Morris N. R. BIMA, a TPR-containing protein required for mitosis, localizes to the spindle pole body in Aspergillus nidulans. J Cell Biol. 1993 Feb;120(4):959–968. doi: 10.1083/jcb.120.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore D. C., McIntosh L. P., Russell C. B., Anderson D. E., Dahlquist F. W. Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol. 1989;177:44–73. doi: 10.1016/0076-6879(89)77005-1. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Kincaid R. L. Molecular cloning and chromosomal mapping of the human gene for the testis-specific catalytic subunit of calmodulin-dependent protein phosphatase (calcineurin A). Biochem Biophys Res Commun. 1992 Oct 15;188(1):265–271. doi: 10.1016/0006-291x(92)92379-c. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Kincaid R. L. Molecular cloning of a full-length cDNA encoding the catalytic subunit of human calmodulin-dependent protein phosphatase (calcineurin A alpha). Biochim Biophys Acta. 1993 Jul 28;1178(1):117–120. doi: 10.1016/0167-4889(93)90117-8. [DOI] [PubMed] [Google Scholar]
- Nicolet C. M., Craig E. A. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989 Sep;9(9):3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Posas F., Clotet J., Muns M. T., Corominas J., Casamayor A., Ariño J. The gene PPG encodes a novel yeast protein phosphatase involved in glycogen accumulation. J Biol Chem. 1993 Jan 15;268(2):1349–1354. [PubMed] [Google Scholar]
- Rameau G., Puglia K., Crowe A., Sethy I., Willis I. A mutation in the second largest subunit of TFIIIC increases a rate-limiting step in transcription by RNA polymerase III. Mol Cell Biol. 1994 Jan;14(1):822–830. doi: 10.1128/mcb.14.1.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T., Carrello A., Mark P. J., Warner B. J., Simpson R. J., Moritz R. L., House A. K. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J Biol Chem. 1993 Jun 25;268(18):13187–13192. [PubMed] [Google Scholar]
- Rhee S. K., Icho T., Wickner R. B. Structure and nuclear localization signal of the SKI3 antiviral protein of Saccharomyces cerevisiae. Yeast. 1989 May-Jun;5(3):149–158. doi: 10.1002/yea.320050304. [DOI] [PubMed] [Google Scholar]
- Ronne H., Carlberg M., Hu G. Z., Nehlin J. O. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991 Oct;11(10):4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Samejima I., Matsumoto T., Nakaseko Y., Beach D., Yanagida M. Identification of seven new cut genes involved in Schizosaccharomyces pombe mitosis. J Cell Sci. 1993 May;105(Pt 1):135–143. doi: 10.1242/jcs.105.1.135. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Schultz J., Marshall-Carlson L., Carlson M. The N-terminal TPR region is the functional domain of SSN6, a nuclear phosphoprotein of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4744–4756. doi: 10.1128/mcb.10.9.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki M., Kinoshita N., Ohkura H., Yoshida T., Toda T., Yanagida M. Isolation and characterization of the fission yeast protein phosphatase gene ppe1+ involved in cell shape control and mitosis. Mol Biol Cell. 1993 Mar;4(3):303–313. doi: 10.1091/mbc.4.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Michaud W. A., Hieter P. p62cdc23 of Saccharomyces cerevisiae: a nuclear tetratricopeptide repeat protein with two mutable domains. Mol Cell Biol. 1993 Feb;13(2):1212–1221. doi: 10.1128/mcb.13.2.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Michaud W. A., Wootton J. C., Boguski M. S., Connelly C., Hieter P. TPR proteins as essential components of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1991;56:663–673. doi: 10.1101/sqb.1991.056.01.075. [DOI] [PubMed] [Google Scholar]
- Simon A. J., Saville S. P., Jamieson L., Pocklington M. J., Donnelly S. F., Ron D., Milner Y., Mochly-Rosent D., Orr-Sternlicht E. Characterization of PKC2, a gene encoding a second protein kinase C isotype of Saccharomyces cerevisiae. Curr Biol. 1993 Dec 1;3(12):813–821. doi: 10.1016/0960-9822(93)90215-a. [DOI] [PubMed] [Google Scholar]
- Steele F. R., Washburn T., Rieger R., O'Tousa J. E. Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell. 1992 May 15;69(4):669–676. doi: 10.1016/0092-8674(92)90230-a. [DOI] [PubMed] [Google Scholar]
- Steger H. F., Söllner T., Kiebler M., Dietmeier K. A., Pfaller R., Trülzsch K. S., Tropschug M., Neupert W., Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990 Dec;111(6 Pt 1):2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E. M., Yamano H., Kinoshita N., Yanagida M. Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Curr Biol. 1993 Jan;3(1):13–26. doi: 10.1016/0960-9822(93)90140-j. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Van der Leij I., Franse M. M., Elgersma Y., Distel B., Tabak H. F. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11782–11786. doi: 10.1073/pnas.90.24.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Smouse D., Perrimon N. The crooked neck gene of Drosophila contains a motif found in a family of yeast cell cycle genes. Genes Dev. 1991 Jun;5(6):1080–1091. doi: 10.1101/gad.5.6.1080. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva E. F., Cohen P. T. Isolation of a cDNA likely to encode a novel Ca2+-dependent/calmodulin-stimulated protein phosphatase. Biochim Biophys Acta. 1989 Dec 22;1009(3):293–296. doi: 10.1016/0167-4781(89)90118-8. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva O. B., da Cruz e Silva E. F., Cohen P. T. Identification of a novel protein phosphatase catalytic subunit by cDNA cloning. FEBS Lett. 1988 Dec 19;242(1):106–110. doi: 10.1016/0014-5793(88)80995-5. [DOI] [PubMed] [Google Scholar]