Abstract
Synapsins are nerve-terminal proteins that are linked to synaptic transmission and key factors in several forms of synaptic plasticity. While synapsins are generally assumed to be ubiquitous in synaptic terminals, whether they are excluded from certain types of terminals is of interest. In the visual pathway, synapsins are lacking in photoreceptor and bipolar cell terminals as well as in retinogeniculate synapses. These are the terminals of the first three feedforward synapses in the visual pathway, implying that lack of synapsins may be a common property of terminals that provide the primary driver activity onto their postsynaptic neurons. To further investigate this idea, we studied the fourth driver synapse, thalamocortical synapses in visual cortex, using glutamatergic terminal antibody markers anti-VGluT1 and VGluT2, anti-Synapsin I and II, and confocal microscopy to analyze co-localization of these proteins in terminals. We also used pre-embedding immunocytochemical labeling followed by electron microscopy to investigate morphological similarities or differences between terminals containing synapsins or VGluT2. In visual cortex, synapsin coincided extensively with non-TC-neuron marker, VGluT1, while thalamocortical terminal marker VGluT2 and synapsin overlap was sparse. Morphologically, synapsin-stained terminals were smaller than non-stained, while VGluT2 positive thalamocortical terminals constituted the largest terminals in cortex. The size discrepancy between synapsin- and VGluT2-positive terminals, together with the complementary staining patterns, indicate that thalamocortical synapses are devoid of synapsins, and support the hypothesis that afferent sensory information is consistently transmitted without involvement of synapsins. Furthermore, VGluT2 and synapsins were colocalized in other brain structures, suggesting that lack of synapsins is not a property of VGluT2 containing terminals, but a property of primary driver terminals in the visual system.
Keywords: synaptic transmission, synapsin, synaptic terminals, thalamus, LGN, visual cortex
INTRODUCTION
Synapsins are a family of nerve specific phosphoproteins encoded by three genes in vertebrates, Synapsin I, II and III (Südhof et al., 1989; Hosaka and Südhof, 1998; Kao et al., 1998; Ferreira et al., 2000). They are located in nerve terminals, involved in trafficking of transmitter vesicles, in particular in regulation of the reserve pool of vesicles (De Camilli et al., 1990; Walaas and Greengard, 1991; Greengard et al., 1993). The reserve pool regulation depends on activity across the synapse (De Camilli et al., 1990; Walaas and Greengard, 1991; Greengard et al., 1993). Thereby Synapsin I (SynI) and Synapsin II (SynII) play an important role in synaptic plasticity, particularly in short-term plasticity (cf. Cesca et al., 2010). Synapsin III seems to be mainly involved in early development of the CNS, and in the adult brain there is little Synapsin III (Ferreira et al., 2000; Pieribone et al., 2002). Synapsins have a widespread distribution in the CNS, suggesting general involvement in synaptic terminals (Südhof et al., 1989). A remarkable exception is found in retina where the ribbon-containing terminals of photoreceptors and bipolar cells lack synapsin, while terminals of other types of retinal cells that form conventional synapses, contain synapsin (Mandell et al., 1992).
In sensory systems, information is relayed stepwise through ascending levels of principal neurons, or drivers (cf. e.g. Sherman and Guillery, 1998), which bear the main information of the sensory pathway, and determine the receptive field properties of the postsynaptic neuron (Sherman and Guillery, 1998; Viaene et al., 2011a). At each level the effect of the driver input is adjusted or modified through input from modulator neurons. In retina the synapsin-lacking neurons, photoreceptors and bipolar cells, are drivers, whereas the synapsin-expressing neurons, horizontal and amacrine cells, are modulators. It was suggested that the lack of synapsins in photoreceptors and bipolar cells could be related to their driver function rather than to the particular morphology of their ribbon synapses, and the presence of synapsins in modulator terminals could be related to specific functions of these neurons such as low release probability or low vesicle reserve. In support of this hypothesis, Kielland et al. (2006) showed that the terminals of retinal ganglion cells, which are drivers that form conventional synapses on the thalamocortical (TC) relay neurons in the dorsal lateral geniculate nucleus (dLGN), do not contain SynI or SynII (cf. also Wei et al., 2011), whereas terminals from modulators to TC neurons, i.e. thalamic inhibitory neurons and excitatory corticogeniculate feedback neurons, contain synapsin. Moreover, it is known that mRNA expression for synapsins is notably low in dLGN (Zurmöhle et al., 1996), implying that a major neuron population, possibly relay cells, may not synthesize synapsins.
In this study we investigated whether synapsins are lacking also in the driver input to the next level of the visual pathway, i.e. the TC terminals in Layer 4 of primary visual cortex (e.g. Sherman and Guillery, 1998; Viaene et al. 2011a). Light- and electron-microscopy were combined with antibody staining for the established TC terminal marker in Layer 4, VGluT2 (Nahmani and Erisir, 2005; Coleman et al., 2010), the non-TC neuron marker VGluT1, and the two synapsin isoforms, SynI and SynII. Moreover, we investigated expression of synapsins in TC terminals in Layer 6, which also receives a substantial input from dLGN.
EXPERIMENTAL PROCEDURES
The experimental procedures were approved by the Norwegian Animal Research Authority according to the Norwegian Animal Welfare Act and the European Communities Council Directive 86/609/EEC, or by the Institutional Animal Care and Use Committee at the University of Virginia in accordance with the National Institutes of Health guidelines for humane handling of animals.
Tissue preparation
A total of three strains of mice were used; wild type C57BL/6, and SynI or SynII knockouts based on the same strain (described by Chin et al., 1995; Ferreira et al., 1998; Etholm and Heggelund, 2009). A total of 12 mice were used: Six mice for light-microscopic analyses of VGluT and synapsin in V1, one each of SynI and SynII knockouts, and four mice for electron microscopy analysis. The ages of mice ranged between P38 and P90. Mice were given a lethal dose of pentobarbital (in excess of 100 mg/kg) and perfused transcardially with Tyrodes buffer (containing in mM: NaCl 137, dextrose 5.5, MgCl2 1.2, KCl 2, NaH2PO4 0.4, CaCl2 0.9) for 1 min., followed either by 4% paraformaldehyde (in 0.1 M phosphate buffer; pH 7.4), or a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde (for SynII LM experiments), or 4% paraformaldehyde and 0.5% glutaraldehyde (for EM experiments) for fixation. Brains were post-fixed overnight in the perfusion solution, dissected out the following day, and cut into blocks and sliced into 60 μm slices using a vibratome slicer. Free floating sections were either incubated for staining immediately, or treated with 1% sodium borohydride and stored at 4° C in 0.1% sodium azide, 0.01 M phosphate buffered saline (PBS).
Immunohistochemistry
Sections were incubated in 1% bovine serum albumin (BSA) blocking solution (with the addition of 3% normal goat serum in experiments using SynII antibodies) for 30 minutes, then in primary antibody solution (antibody dilutions given in Table 1), diluted in PBS with 1% BSA, 1% Triton X-100 and 0.05% NaN3 at room temperature for 3 days. Sections for light microscopy were rinsed in PBS, and incubated in 1:100 dilutions of secondary antibodies (anti-rabbit Cy2 (PA42004, Amersham), anti-rabbit Cy3 (PA43004, Amersham), or anti-guinea pig Cy5 (106-175-003, Jackson) for 2 hours. Then the sections were rinsed three times 10 minutes in PBS, mounted on glass slides and cover slipped using Prolong mounting medium (Invitrogen). The SynI and SynII antibodies were not applied on the same tissue because each synapsin antibody worked optimally with a different fixative; 4% paraformaldehyde was optimum for SynI, and the mixture of 4% paraformaldehyde and 0.1% glutaraldehyde was optimum for SynII.
Table 1.
Primary antibodies and dilutions used for protein labeling
| Primary antibody | Light microscopy | Electron microscopy |
|---|---|---|
| Synapsin I (Stressgen VAP-SV060E) | 1:1000 | 1:5000 |
| Synapsin II (BD biosci. 611392) | 1:1000 | 1:1000 |
| VGluT1 (Chemicon AB5905) | 1:1000 | 1:5000 |
| VGluT2 (Chemicon AB5907) | 1:1000 | 1:5000 |
Confocal microscopy
Primary visual cortex was imaged on a Zeiss LSM 510 confocal microscope using 63x objective (NA = 1.4) and a confocal aperture setting of 102 μm, corresponding to the width of 1 Airy disc for the shortest wavelength channel (Cy2). An argon laser was used for excitation at 488 nm (Cy2), a diode-pumped solid-state laser at 561 nm (Cy3) and a HeNe laser at 633 nm (Cy5); all were used in conjunction with optical barrier filters. Scanning was performed sequentially to avoid cross-talk. Scan speed and laser settings were kept constant for all images for each combination of antibodies. Given the theoretical resolution of the objective (approximately 0.14 μm in the xy-direction, and 0.43 μm in the z-direction) when scanning confocally at the shortest wavelength, 488 nm, a 133.6 μm square field with a resolution of 1476 × 1476 pixels was chosen. Correspondingly, z-stacks of 5 consecutive depths were made at a z-step size of 0.37 μm. The first optical slice was always scanned 5 μm below the slice surface to avoid cutting artifacts and debris. The cortical layers were determined based on tissue landmarks, namely pial surface, white matter/Layer 6 transition, and cellular density.
Colocalization analyses
Acquired images were opened in “Image J” (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997-2011), and then analyzed for colocalization using the “JACoP” plug-in (Bolte and Cordelieres, 2006). The degree of overlap between pairs of synapsin (SynI or Syn II) and VGluT (type 1 or type 2) channels was computed using two algorithms: 1. Pearson Correlation Coefficient (PC): An estimate for the goodness of the rate of association of two fluorophores was obtained using Pearson Correlation Coefficient (PC) analysis included in the JACoP worktool (Bolte and Cordelieres, 2006). PC is a measure of the linearity when plotting pixel-intensities between two channels in unprocessed images without thresholding. Its value can range from 1 to −1; the values of 1, −1 and 0 stand for complete positive correlation, a negative correlation, and no correlation, respectively. Although values between +0.5 and −0.5 are attributable to noise and near-threshold events, thus do not allow conclusions to be drawn (Bolte and Cordelieres, 2006), PC is considered a simple way to measure dependency of pixels in dual channels. 2. Object-based analysis: This method determined whether the geometrical center of a synapsin-stained voxel cluster fell within the area of a VGluT2 stained cluster. In order to exclude random or nonspecific fluorophores from the analysis, minimum and maximum limits of clusters to be considered as an ‘object’ (putative terminals) were set to 25 and 2500 voxels, respectively, which corresponds to 0.075 and 7.5 μm3, including fluorescence spread. The algorithm was used to estimate the percentage of VGluT2 positive pixel clusters that were colocalized with another marker (Jaskolski et al., 2005). The Object-Based and Pearson Correlation analyses for pairs of immunostains for synapsin and VGluT proteins in cortical Layers 4 and 6, and in control areas were performed for each sample, mean and standard deviation values were obtained for statistics.
Electron microscopy
Sections from GA fixed specimens were incubated 30 min in blocking solution (1% BSA in PBS), then overnight in primary antibody solution (antibodies, Table 1; PBS with 1% BSA and 0.05% NaN3). After a 3 × 3 min rinse in PBS, incubation in 1:100 diluted, secondary, biotinylated antibody lasted for 2 hours followed by a new 3 × 3 min rinse in PBS and incubation for 2 hours in ABC reagent (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA). Sections where then rinsed 3 × 3 min in PBS and incubated in a 1% diaminobenzidine (DAB) solution with a 1:10000 dilution of 30% H2O2 and gently agitated for approximately 5 minutes while staining became visible, after which the process was stopped by rinsing 3 × 3min in PBS. Next, sections were post-fixed in osmium tetroxide and embedded as described previously (Erisir, 2001). In short, post-fixation were done by incubating in 1% osmium tetroxide for one hour, followed by a 3 × 3 min rinse and gradual dehydration using: 50% ethanol for 3 min, 4% uranyl acetate in 70% EtOH overnight at 4° C, 70% EtOH for 1 min, 90% EtOH for 5 min and 2 × 5 min in 100% EtOH. Next, sections were submerged in a 1:1 acetone to resin (Epon 812; Electron Microscopy Sciences) solution for 2 hours, then full resin for another 2 hours and flat embedding in an oven at 60° C overnight between 2 acetate sheets (Aclar, Ted Pella). Flat-embedded sections were drawn for reference using a Camera Lucida; a strip of tissue containing Layers 1-6 from V1 was excised, placed in plastic capsules filled with Epon and left in an oven at 60° C for two days. Ultrathin sections (~70nm) were cut and collected on 400 mesh copper grids (Ted Pella) using an ultra-microtome (Ultracut UCT, Leica).
Antibody penetration is restricted to the first micrometers inwards from the tissue surface. Hence, cutting was done at a near parallel tissue-surface plane to maximize the area that had staining while taking care to include the tissue-Epon interface for reference. Ultrathin sections typically contained tissue-Epon transition region (i.e., the surface of immuno-stained thick sections) through all cortical layers. A region of the tissue to be analyzed was identified and imaged based on the following criteria: sufficient depth from the surface to, (1) preserve tissue without cutting artifacts, and (2) without losing labeling due to insufficient antibody penetration. A JEOL 1010 EM equipped with a 16 megapixel SIA-12C (sia-cam.com) camera and “MaxIm DL CCD” software (Diffraction Limited) was used at a primary magnification of 10000x, yielding a pixel size of 2.75 nm. The size of labeled boutons was analyzed blind with respect to staining type (for VGluT2/SynI/SynII staining).
The criteria to identify asymmetric and symmetric synapses were as follows: The presence of electron dense strip at the postsynaptic membrane categorized the synapse as asymmetric. Lack of inconspicuous electron-dense strip at the postsynaptic site of the synaptic cleft categorized the synapse as symmetric. While serial sampling through the same synapse may reveal that a synapse which was previously categorized as symmetrical was, in fact, asymmetrical, that conformation was not routinely sought for. This is because asymmetrical/asymmetrical classification is an imprecise method to attribute functional specification to terminals: Asymmetric synapses can be glutamatergic, as well as cholinergic or catecholaminergic; postsynaptic density thickness is widely variable, even within asymmetric group; without GABA staining, morphology alone is not sufficient to categorize inhibitory terminals. Based on these ambiguities of the symmetrical/asymmetrical criteria, morphological categorization of synapses was not used for quantitative analysis, yet, qualitative observations for encounter of asymmetric and symmetric synapses were reported.
Statistics
For confocal microscope images, percentage of object-based colocalization and the Pearson Coefficient was computed using each image as a sample, and these were averaged across samples. Numbers of images per animal and number of animals for each comparison are provided in the Results. ANOVA and Student’s T-test were used for comparison of means. For electron microscopy analysis, the distributions of terminal area of synapsin and VGluT positive terminals were compared using the Kruskall-Wallis test with a Dunn post-test across cases.
RESULTS
Staining pattern for VGluT1, VGluT2 and synapsins in visual cortex
Immunostaining with synapsins, VGluT1 and VGluT2 antibodies resulted in punctate labeling in visual cortex, and the patterns of labeling were consistent with selectivity for terminal boutons. VGluT1 staining appeared uniform in all layers of the visual cortex, while VGluT2 staining pattern was not uniform across different cortical layers (Fig. 1) consistent with previous findings (Fremaeu et al., 2001; 2004; Nahmani and Erisir, 2005; Coleman et al., 2010). Layer 4 contained the densest VGluT2 labeled fibers, followed by Layer 6 (Figs. 1A, and 1D). The SynI and SynII, on the other hand, labeled all cortical layers with the weakest labeling in Layer 4 (Figs. 1B and 1E). When synapsin (green) and VGluT2 (red) channels were merged (Figs. 1C and 1F), the dense VGluT2 band in Layer 4 overlapped with the weak SynI (Fig. 1C) and SynII (Fig. 1F) bands.
Fig. 1.
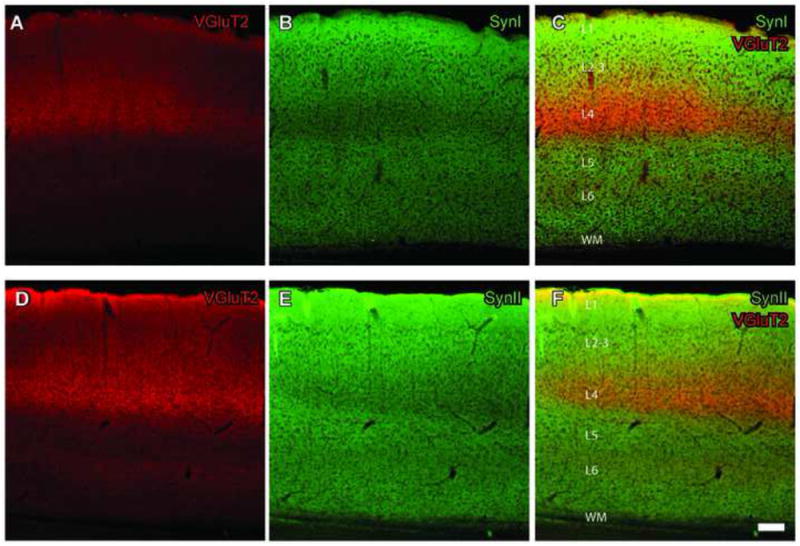
SynI and SynII staining patterns in visual cortex are interdigitated with that of VGluT2. A, VGluT2 staining using a Cy5 coupled secondary antibody. A strong band of staining appears in Layer 4. B, Image of SynI staining using a Cy2 coupled secondary antibody. A band of weaker staining corresponds to Layer 4. C, Merged images with both SynI and VGluT2 labeling. D,E, VGluT2 staining as in (A), but staining with VGluT2 (Cy2 secondary) and SynII (Cy3 secondary) on a slice fixed with GA-containing fixative. Similar to SynI, staining intensity is the weakest in Layer 4. F, Merged images with both SynII, and VGluT2 labeling. Scale bar = 100μm (applies to all panels).
Colocalization of synapsins in VGluT1 and VGluT 2 fibers
In order to quantify the extent of colocalization of synapsins in VGluT fibers in visual cortex Layers 4 and 6, a confocal microscope with a 63x objective (1.4 NA) was used to scan dually stained tissue. At this resolution, the labeling appeared as distinct clusters of voxels, in various sizes. The degree of overlap between pairs of synapsin (SynI or Syn II) and VGluT (type 1 or type 2) channels was computed using two separate algorithms; object based colocalization (OBC) algorithm, and Pearson Coefficient (PC; see Methods)
The OBC analyses revealed that there was a high degree of overlap between SynI and VGluT1 (Figs. 2A, 2C), and between SynII and VGluT1 (Figs. 2B, 2D), in both Layer 4 and Layer 6 (Table 2). Out of all VGluT1 positive objects in Layer 4, 61 ± 9% were also positive for SynI, and 33 ± 13% were positive for SynII. In Layer 6, 55 ± 20% and 31 ± 9% of all VGluT1 positive objects were also positive for SynI and SynII, respectively. The PC analysis corroborated these findings: PC for VGluT1 and SynI labels were 0.78 ± 0.03 in Layer 4, and 0.71 ± 0.08 in Layer 6 (Fig. 2E). Similarly, PC values for SynII were 0.67 ± 0.04 in Layer 4 and 0.71 ± 0.07 in Layer 6 (Fig. 2E). These results suggest that VGluT1 containing axons in visual cortex Layer 4 and 6 contain Synapsin I and (or) Synapsin II.
Fig. 2.
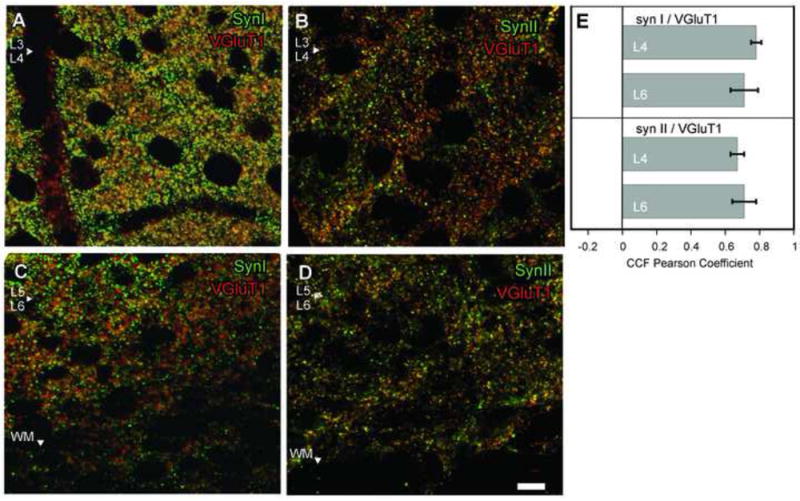
In primary visual cortex (V1) Layers 4 and 6, VGluT1 (red puncta) labeled fibers also contain SynI and SynII (green puncta). Overlapping pixels appear yellow. A, VGluT1-SynI colocalization in Layer 4 (L4), area V1. Putative Layer 4 (L4) / Layer 3 (L3) border indicated by white arrowhead. B, VGluT1- SynII colocalization in Layer 4. C, VGluT1 and SynI colocalization in Layer 6. Arrowhead marks putative Layer 5 (L5) and Layer 6 (L6) border. D, VGluT1 and SynII coloalization in L6. Scale bar =10 μm (applies to all panels). E, Pearson correlation coefficient (PC) analysis between VGluT1 and synapsin containing pixels in subsequent scans with each fluorophore (avg ± SD). PC values between -1 and 1 indicate the strength of colocalization; an index value of 1 can only be attained between two identical scans. A value of 0 indicates two randomly distributed sets of pixels, that is, chance level of pixel overlap between two scans. A PC value of -1 indicates that the pixels obtained in two scans are systematically non-overlapping. Values between -0.5 and 0.5 occur as an outcome of strong background and subthreshold fluorescence, thus inconclusive for colocalization.
Table 2.
Percentage of VGluT1 terminals that contain synapsins ± SD, mean number of terminals per labeling per image in visual cortex, and number of corresponding images that were analyzed.
| Visual Cortex Layer 4 |
Visual Cortex Layer 6 |
|||
|---|---|---|---|---|
| Syn I | Syn II | Syn I | Syn II | |
| %Coloc | 61 ± 9 | 33 ± 13 | 55 ± 20 | 31 ± 9 |
| VGluT1+ | 892 ± 88 | 533 ± 72 | 684 ± 95 | 605 ± 113 |
| Synapsin+ | 1768 ± 103 | 921 ± 17 | 1877 ± 188 | 618 ± 76 |
| Images | 6 | 6 | 6 | 6 |
In contrast, VGluT2 containing visual axons were notably devoid of synapsins (Fig. 3). In Layer 4, only 3 ± 1% and 7 ± 4% of all VGluT2 positive objects colocalized with SynI (Figs. 3A, 3C; Table 3) and SynII positive objects (Figs. 3B-3D), respectively, and these values were statistically different from the degree of colocalization of VGluT1 and SynI or SynII. Consistently, PC averages for SynI and SynII colocalization with VGluT2 in Layer 4 were 0.16 ± 0.06 and 0.17 ± 0.05 (Fig. 3E) respectively, thus even this less conservative measure did not yield any evidence for colocalization of SynI or SynII labels within VGluT2 positive fibers in Layer 4 of visual cortex. Similarly in Layer 6, only 2 ± 1% of all VGluT2 positive clusters were also positive for SynI, yielding a PC average of 0.14 ± 0.05 (Fig. 3E), indicating that VGluT2 axons in Layer 6 are also devoid of SynI. Surprisingly, OBC analysis for VGluT2-SynII colocalization in Layer 6 revealed that 36 ± 10% of VGluT2 positive clusters were positive for SynII. This is a significantly higher percentage than any of the other OBC colocalization analyses performed for VGluT2. However, PC analysis of VGluT2 and SynII in Layer 6 was inconclusive, for it yielded a correlation less than 0.5 (0.30 ± 0.07), indicating that the label in these cases might include high background and subthreshold labeling. The combination of results obtained with OBA and PC analyses suggest that SynI is not located in VGluT2 terminals in Layer 6. However, a subpopulation of VGluT2 terminals in Layer 6 may contain SynII.
Fig. 3.
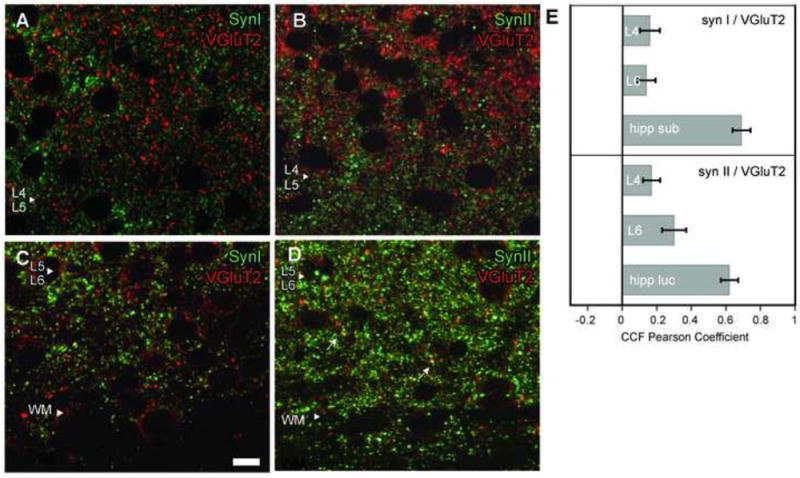
In primary visual cortex (V1) Layer 4, VGluT2 (red puncta) labeled fibers do not contain SynI or SynII (green puncta). A, Lack of colocalization between VGluT2 and SynI positive puncta in Layer 4. B, Lack of colocalization between VGluT2 and SynII positive puncta in Layer 4. C, Lack of colocalization between VGluT2 and SynI positive puncta in Layer 6. Scale bar = 10 μm (applies to all panels). D, In Layer 6, some VGluT2 positive puncta also contained SynII (yellow puncta). E, Cross-correlation function analysis between VGluT2 and synapsin containing pixels in subsequent scans with each fluorophore, averaged from 18 sections (±SD).
Table 3.
Percentage of VGluT2 terminals that contain synapsins ±SD, mean number of terminals per labeling per image in visual cortex Layer 4 and Layer 6, and the number of corresponding images that were analyzed. Positive Controls for Syn I and Syn II staining were obtained from hippocampus.
| Visual Cortex Layer 4 |
Visual Cortex Layer 6 |
Hippocampus (Positive Control) |
||||
|---|---|---|---|---|---|---|
| Syn I | Syn II | Syn I | Syn II | Syn I | Syn II | |
| % Coloc | 3 ± 1 | 7 ± 4 | 2 ± 1 | 36 ± 10 | 24 ± 6 | 86 ± 18 |
| VGluT2+ | 1803 ± 152 | 804 ± 77 | 759 ± 103 | 290 ± 26 | 655 ± 63 | 459 ± 171 |
| Synapsin+ | 1467 ± 119 | 1536 ± 140 | 1262 ± 96 | 1601 ± 95 | 756± 74 | 630 ± 198 |
| Images | 18 | 18 | 18 | 18 | 7 | 3 |
In order to control for antibody specificity, transgenic SynI or SynII knockout mice were tested. When the same acquisition settings were used as in control mice, no detectable SynI staining was obtained in SynI knockouts (Fig. 4A), while VGluT2 labeling on the same sections was qualitatively unaffected. Similarly, SynII knockouts do not display any fiber staining with SynII antibody (Fig. 4B).
Fig. 4.
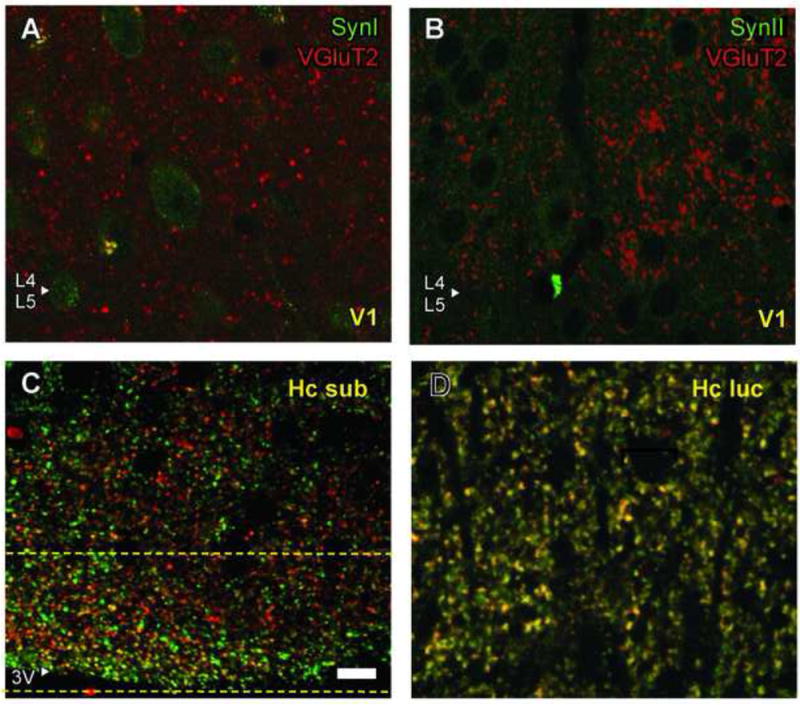
Control experiments. A,B, Transgenic mouse lacking SynI or SynII proteins are used as negative controls. Genetic deletion of SynI or SynII proteins eliminated SynI (A) and SynII (B) labeling in cortical fibers, while VGluT2 stain was unaffected. C,D, Colocalization of synapsin and VGluT2 occurs in other brain areas. C, In hippocampal subiculum, VGluT2 and SynI were colocalized in a narrow band close to the medial border (Hc sub). PC analysis yielded high positive correlation within a band that was marked within two yellow stippled lines. Scale bar =10 μm (applies to all panels). D, VGluT2 and SynII were colocalized in a region apical to the pyramidal cells of hippocampal lucidum (Hc luc).
To test the methodological power of our protocol to detect the overlap of VGluT2 and synapsin positive structures, we looked for other areas in the brain that have strong colocalization of VGluT2 and SynI, which thereby could serve as a positive control. Dense staining with VGluT2 has been described at the inferior border of hippocampal subiculum (Fremeau et al., 2001). In our material, this region contained VGluT2 labeled structures that also stained positively for SynI (Fig. 4C). In this control area, 24 ± 6% of VGluT2 labeled structures were colocalized with SynI (Table 3), and this degree of overlap was significantly higher than the overlap we found in visual cortex Layer 4 (p < 0.01). The PC average in hippocampal subiculum was 0.69 ± 0.05 (p < 0.01). As a positive control for VGluT2 and SynII colocalization, we used the region of CA3 pyramidal cells apical dendrites in hippocampus lucidum, which contains VGluT2 (Varoqui et al., 2002). This region displayed an almost complete confocal object overlap between SynII and VGluT2 positive structures (86 ± 18%, Fig. 4D; Table 3), and PC values of 0.62 ± 0.13 (p < 0.01). The extent of VGluT2 and SynII colocalization in hippocampus lucidum was significantly higher than in visual cortex Layer 4 (Fig. 3E; p < 0.01). Therefore, VGluT2 positive axons may contain SynI or SynII, although not in Layer 4 of visual cortex.
Synapsin positive terminals do not match TC terminals in size
To further confirm the lack of synapsins in Layer 4, the size of VGluT2 terminals (i.e., TC terminals) and the size of synapsin labeled terminals were compared by electron microscopy. The rationale for this analysis is as follows: TC axons provide the largest terminal boutons in Layer 4 of visual cortex (Ahmed et al., 1997; Erisir and Dreusicke, 2005). If synapsins are not utilized in TC terminals, then synapsin positive terminals in Layer 4 should not include large boutons.
In general, both SynI and SynII stained terminals displayed asymmetric and symmetric synapse morphology (Figs. 5A-B), while VGluT2 labeled terminals had asymmetric synapses and occasional unlabeled inclusions (Fig. 5C). Synaptic terminal cross-section area analysis revealed that VGluT2 positive terminals (N = 108) were significantly larger than SynI (N = 97) and SynII positive (N = 131) ones (Fig. 5D; Mann Whitney-U, p < 0.01), consistent with the hypothesis that the VGluT2 positive TC terminals in Layer 4 of visual cortex lack SynI and SynII.
Fig. 5.
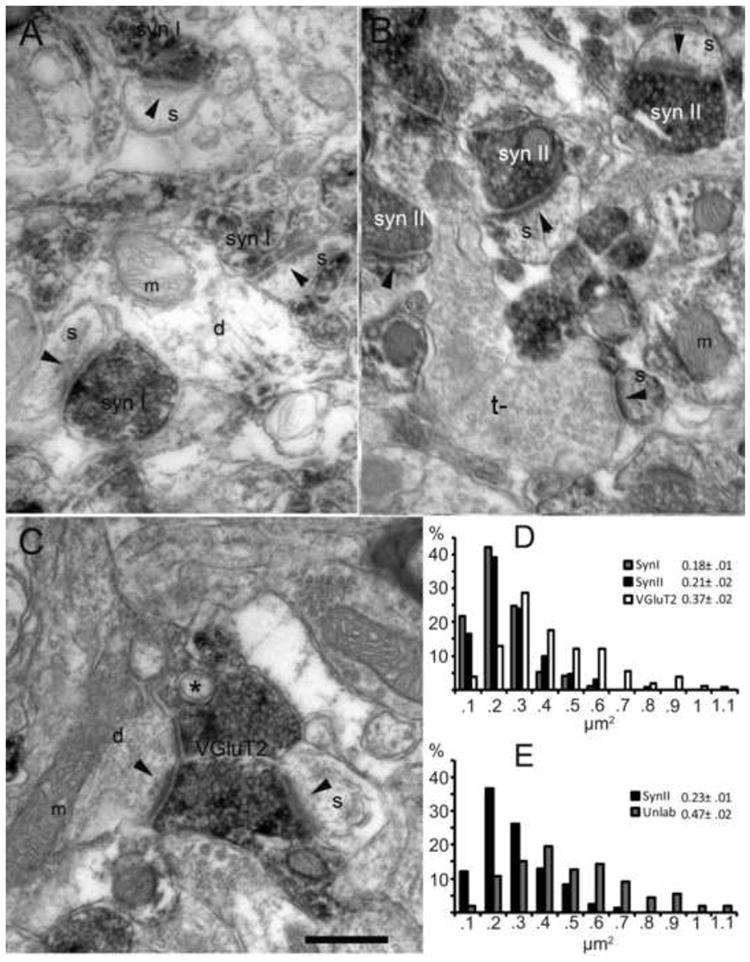
The size of synapsin labeled terminals do not match the size of thalamocortical terminals. A, Electron micrograph from primary visual cortex Layer 4. DAB-labeled, SynI positive terminals appear grey, apposed to a dendrite (d), mitochondria (m), or spine (s). Synaptic specializations indicated by black arrowheads. B, Similar section treated with SynII antibodies. An unstained, larger terminal (t-) forms an asymmetric synapse onto a spine. C, A large, VGluT2 positive terminal in primary visual cortex, forms synapses onto both a dendrite (d) and a spine (s). The terminal contains an unlabeled inclusion (*), a characteristic of TC terminals. Such inclusions are not encountered in synapsin-positive terminals. Scale bar = 250 nm. D, Terminal cross-section area distributions of terminals labeled for SynI, SynII or VGluT2. SynI and SynII terminals are similar in size, whereas VGluT2 positive terminals are significantly larger. While non-parametric comparisons were used for analysis, descriptive statistics for each population are indicated in the inset (mean ±SE). E, Histogram comparing cross-section areas of SynII positive terminals and unlabeled terminals that are found in the same regions as SynII terminals. Terminals that were labeled for SynII were significantly smaller than neighboring synapsin-unlabeled terminals.
We also investigated the properties of terminals that were left unstained with SynII. In over 30 minimally overlapping photographs (each capturing about 20 μm2 area) we analyzed all terminals, that is, both the terminals labeled for SynII and those that remained unlabeled within the same region. Out of 278 synaptic terminals encountered 161 (58%) were SynII positive. The terminals that were SynII negative were significantly larger than SynII positive terminals (0.47 μm vs. 0.23 μm2; Fig. 5E) suggesting that the population of morphologically larger cortical terminals were selectively devoid of SynII. Furthermore, terminal area distribution of SynII-unlabeled terminals were statistically indistinguishable from the terminal area distribution of VGluT2 positive terminals (Mann Whitney-U, p > 0.1) (Figs. 4D and 4E), supporting the hypothesis that TC terminals lack SynII.
It is conceivable that not all of the 117 synaptic terminals that did not contain SynII were TC boutons: no immunostaining protocol can claim that all binding sites in a tissue were visualized. Some proportion of our SynII unlabeled terminals should include false-negatives. In addition, there is no available evidence to indicate that TC terminals in Layer 4 are the only inputs that lack SynII. Therefore, these data do not provide evidence for the possibility that TC axons provide a relatively large proportion of terminals in mice Layer 4. There are no published quantifications for the ratio of cortical terminals originating in thalamus in rodents, but if it is similar to the ratios in primates and feline, TC axons are not expected to provide more than about 5-10% of all synapses (Ahmed et al., 1994). However, the observation that about 40% of terminals lacked SynII, and that these were as large as VGluT2 positive terminals, is consistent with unpublished quantifications that 35-42% of all Layer 4 asymmetric terminals in mice contain VGluT2 (unnormalized quantification from Fig. 5 of Coleman et al., 2010).
DISCUSSION
Our results indicate that TC terminals giving the driver input to visual cortex from dLGN do not utilize SynI or SynII. This conclusion is supported by several findings. First, the level of synapsins was low in Layer 4 of visual cortex, the major geniculate recipient layer. Second, synapsin antibodies and the TC terminal marker VGluT2 did not colocalize in Layer 4. Third, morphometric properties of synapsin-labeled terminals were different from those of TC terminals and VGluT2-labeled terminals. This, combined with previous results (Mandell et al. 1990; 1992; Kielland et al., 2006), implies that synapsins are lacking in the driver synapses at each step of the visual pathway from photoreceptors to Layer 4 in primary visual cortex. Both in dLGN and visual cortex the driver inputs have conventional terminals rather than an unusual structure like in photoreceptors and bipolar cells in retina. Thus, lack of synapsins in the visual pathway appears to be a characteristic of driver synapses rather than a particularity of morphologically specialized synapses like the ribbon synapse (Mandell et al., 1990). Furthermore, we found colocalization between synapsin- and VGluT1-labelled terminals, terminals presumably related to intracortical neurons (Nahmani and Erisir, 2005), indicating that synapsins in visual cortex are linked to terminals of modulator inputs like in retina and dLGN. Moreover, in other brain structures, VGluT2 and synapsins were colocalized, suggesting that the lack of synapsins is not a characteristic of all VGluT2 containing terminals, but rather a distinct property of the primary driver terminals in visual cortex.
In the somatosenory and auditory pathways, recent evidence has suggested that there is direct input from the respective thalamic relay nuclei also to cortical Layer 2/3 and Layer 5 (Viaene et al., 2011a; Viaene et al., 2011b), and a similar organization might exist in visual cortex. This could suggest that a significant driver input is present also in these other cortical layers. However, the physiological data on the thalamic input to Layers 2/3 and 5 indicated that this input had modulatory rather than driver characteristics (Viaene et al., 2011a; Viaene et al., 2011b).
Our main conclusion is based on the assumption that VGluT2 is a reliable marker for TC terminals in Layer 4 of visual cortex. This assumption is based on a series of previous studies which showed that VGluT2 is predominantly expressed in diencephalic and other subcortical brain regions, whereas VGluT1 is predominantly expressed in neocortex and other telencephalic regions (Ni et al., 1995; Hisano et al., 2000; Fremeau et al., 2001; Fujiyama et al., 2001; Herzog et al., 2001). This differential expression pattern of VGluT1 and VGluT2 may vary through the postnatal development (De Gois et al., 2005; Barroso-Chinea et al., 2007; Nakamura et al., 2007), and there is evidence for a period around P7-P10 when some terminals in neocortex of mice have coexpression of VGluT1 and VGluT2, especially in Layer 4 of barrel cortex (Nakamura et al., 2007). However, conclusive evidence for colocalization of the two transporter proteins in the same cortical terminals is lacking, as is evidence for VGluT2-positive terminals of cortical neurons in visual cortex of mouse after P21. On the contrary, it has been demonstrated that VGluT2 can be used as a reliable marker for TC terminals in Layer 4 of visual cortex in the adult ferret (Nahmani and Erisir, 2005) and in mice after P21 (Coleman et al., 2010). The age of our animals was between P38 and P90. Thus, we regard VGluT2 as a valid marker for TC terminals in Layer 4 of visual cortex in our experiments. Moreover, antibody specificity for the synapsin antibodies was verified using genetically modified mice without the synapsin subtype in question, excluding unspecific staining as a factor in our experiments. However, it is possible that not all terminals containing synapsin protein were labeled in our material, i.e., false negative labeling due to limited antigenicity or tissue penetration. If that is the case, false negative labeling would occur across the board, not selectively for one type of terminal. Furthermore, the number of labeled clusters (putative terminals) was high in all experiments, and, in positive controls a very high degree of overlap could be achieved. Positive controls were also used to verify that the laser lines were properly aligned, and that chromatic aberration was within tolerable limits. Crosstalk and bleed-through were minimized by choosing separable fluorochromes and by sequential scanning of the closest laser lines. The theoretical resolution of the objective and lasers used was 0.14 μm, whereas the ultrastrucural studies of VGluT2 positive terminals revealed an average size of 0.37μm. This means that the size of the terminals were within the resolution of our detection system. Thus, we think our conclusion concerning a lack of SynI and SynII in TC terminals in Layer 4 of visual cortex is well founded.
In Layer 6 of visual cortex we found sparse punctate labeling of VGluT2 reflecting the relative weak input from dLGN. Moreover, we found no colocalization between VGluT2 and SynI. However, we found colocalization between SynII and VGluT2 in about 1/3 of the tested terminals, indicating a subpopulation of VGluT2-labeled terminals that contain SynI. These terminals could be TC terminals, or they might be glutamatergic terminals from another type of subcortical neurons distinct from the geniculate afferents. One candidate site of origin for these terminals is claustrum. At least in cat, claustrum receive excitatory input from a small group of Layer 6 neurons in visual cortex, distinct from the neurons projecting to dLGN, and claustrum sends a projection back to visual cortex, partly to Layer 6 (Katz, 1987; Grieve and Sillito, 1995; Baughman and Gilbert, 1981; Da Costa et al., 2010). Moreover, it is known that neurons in claustrum that project to sensory cortex in rat express VGluT2 (Hur and Zaborszky, 2005). The finding that the lack of synapsins in VGluT2 positive terminals is specific to thalamocortical terminals but not to VGluT2 positive terminals in general is also consistent with a recent study that demonstrated that both Synapsin I and Synapsin II colocalize with VGluT2 positive terminals in tree shrew pulvinar (Wei et al., 2011).
As described above, our results support the hypothesis that SynI and SynII are expressed in modulator synapses but not in driver synapses, at least in the visual system. The functional implication of this is unclear. Several lines of evidence suggest that these proteins play an important role in synaptic plasticity, particularly in short-term plasticity (e.g. Rosahl et al., 1993; 1995; Humeau et al., 2001; Kushner et al., 2005; Kielland et al., 2006; Chiappalone et al., 2009; Owe et al., 2009; review in Cesca et al., 2010). One possibility is that less complex regulation of synaptic transmission is required in the driver synapses which are primarily designed for fast and powerful transmission to get the basic message across, whereas the repertory of plasticity provided by the synapsins is important for the regulation and fine tuning of signal transmission executed at the modulator synapses. For instance, in TC neurons in dLGN the driver input from retina lacks synapsins (Kielland et al., 2006), but the effect of this input on the signal processing in the TC neuron is modified by inputs from local interneurons and feedback from visual cortex that modulate their effect through synaptic short-term plasticity mechanisms in which synapsins play an important role. Thus, repetitive firing in corticothalamic afferents facilitates the transmission of signals from TC-neurons to visual cortex, and this facilitation is markedly reduced by inactivation of synapsins (Kielland et al. 2006). Moreover, the cortical feedback can also evoke post-tetanic potentiation of the output from the TC-neurons, and also this plasticity effect is markedly reduced by inactivation of synapsins (Kielland et al. 2006). Presumably, synapsins in visual cortex have similar roles such that direct synaptic input to cortical neurons in Layer 4 is modified by input from other cortical neurons where synapsin-dependent synaptic short-term plasticity play an important role in fine-tuning of the local processing.
Highlights.
-
►
Synapsins, regulators of synaptic release and plasticity, are not ubiquitous to all synapses
-
►
In retina and visual thalamus, synapsins are utilized in local inputs, but not in primary afferents
-
►
We show that the primary afferents to visual cortex, thalamocortical terminals also lack synapsins
-
►
Thus, in visual pathway synapsins are selectively linked to functions of modulatory neuron inputs
Acknowledgments
The project was financially supported by the Research Council of Norway (PH, SGO), and by NIH EY12138 (AE).
Abbreviations
- BSA
bovine serum albumin
- DAB
diaminobenzidine
- dLGN
dorsal lateral geniculate nucleus
- GA
glutaraldehyde
- OBC
object based colocalization
- PBS
phosphate buffered saline
- PC
Pearson correlation coefficient
- SynI
Synapsin I
- SynII
Synapsin II
- TC
thalamocortical
References
- Ahmed B, Anderson JC, Douglas RJ, Martin KA, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. doi: 10.1002/cne.903410105. [DOI] [PubMed] [Google Scholar]
- Ahmed B, Anderson JC, Martin KA, Nelson JC. Map of the synapses onto layer 4 basket cells of the primary visual cortex of the cat. J Comp Neurol. 1997;380:230–242. [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Pérez-Manso M, Erro E, Tuñon T, Lanciego JL. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol. 2007;501:703–715. doi: 10.1002/cne.21265. [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–32. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Baughman RW, Gilbert CD. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J Neurosci. 1981;1:427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, Benfenati F. Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex. 2009;19:1422–39. doi: 10.1093/cercor/bhn182. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Jeffrey P, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30:9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa NM, Fürsinger D, Martin KAC. The synaptic organization of the claustral projection to the cat’s visual cortex. J Neurosci. 2010;30:13166–13170. doi: 10.1523/JNEUROSCI.3122-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Benfenati F, Valtorta F, Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- De Gois S, Schäfer MK-H, Defamie N, Chen C, Ricci A, Weihe E. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Dreusicke M. Quantitative morphology and postsynaptic targets of thalamocortical axons in critical period and adult ferret visual cortex. J Comp Neurol. 2005;485:11–31. doi: 10.1002/cne.20507. [DOI] [PubMed] [Google Scholar]
- Etholm L, Heggelund P. Seizure elements and seizure element transitions during tonic-clonic seizure activity in synapsin I/II double knockout mouse: a neuroethological description. Epilepsy Behav. 2009;14:582–590. doi: 10.1016/j.yebeh.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Chin LS, Li L, Lanier LM, Kosik KS, Greengard P. Distinct roles of synapsin I and synapsin II during neuronal development. Mol Med. 1998;4:22–28. [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Kao HT, Feng J, Rapoport M, Greengard P. Synapsin III: developmentalexpression, subcellular localization, and role in axon formation. J Neurosci. 2000;20:3736–3744. doi: 10.1523/JNEUROSCI.20-10-03736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–9. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Furuta T, Kaneko T. Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex. J Comp Neurol. 2001;435:379–387. doi: 10.1002/cne.1037. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Grieve KL, Sillito AM. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J Neurosci. 1995;15:4868–4874. doi: 10.1523/JNEUROSCI.15-07-04868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vescicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:1–6. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. RC181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano S, Hoshi S, Ikeda Y, Maruyama D, Kanemoto M, Ichijo H, Kojima I, Takeda J, Norami H. Regional expression of a gene encoding a neuron-specific Na+-dependent inorganic phosphate cotransporter (DNPI) in the rat forebrain. Mol Brain Res. 2000;83:34–43. doi: 10.1016/s0169-328x(00)00194-7. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Südhof TC. Synapsin III, a novel synapsin with an unusual regulation by Ca2. J Biol Chem. 1998;273:13371–13374. doi: 10.1074/jbc.273.22.13371. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Doussau F, Vitiello F, Greengard P, Benfenati F, Poulain B. Synapsin controls both reserve and releasable synaptic vesicle pools during neuronal activity and short-term plasticity in Aplysia. J Neurosci. 2001;21:4195–4206. doi: 10.1523/JNEUROSCI.21-12-04195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. VgluT2 afferents to the medial prefrontal and primary somatosensory cortices: A combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Mulle C, Manzoni OJ. An automated method to quantify and visualize colocalized fluorescent signals. J Neurosci Methods. 2005;146:42–49. doi: 10.1016/j.jneumeth.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, Haring M, Benfenati F, Greengard P. A third member of the synapsin gene family. Proc Natl Acad Sci USA. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987;7:1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland A, Erisir A, Walaas SI, Heggelund P. Synapsin utilization differs among functional classes of synapses on thalamocortical cells. J Neurosci. 2006;26:5786–5793. doi: 10.1523/JNEUROSCI.4631-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, Pozzo-Miller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell JW, Townes-Anderson E, Czernik AJ, Cameron R, Greengard P, De Camilli P. Synapsins in the vertebrate retina-absence from ribbon synapses and heterogeneous distribution among conventional synapses. Neuron. 1990;5:19–33. doi: 10.1016/0896-6273(90)90030-j. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Czernik AJ, De Camilli P, Greengard P, Townes-Anderson E. Differential expression of synapsin-I and synapsin-II among rat retinal synapses. J Neurosci. 1992;12:1736–1749. doi: 10.1523/JNEUROSCI.12-05-01736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGluT2 Immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Watakabe A, Hioki H, Fujiyama F, Tanaka Y, Yamamori T, Kaneko T. Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur J Neurosci. 2007;26:3054–3067. doi: 10.1111/j.1460-9568.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- Ni B, Wu X, Yan GM, Wang J, Paul SM. Regional expression and cellular localization of the Na+-dependent inorganic phosphate cotransporter. J Neurosci. 1995;15:5789–5799. doi: 10.1523/JNEUROSCI.15-08-05789.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owe SG, Jensen V, Evergren E, Ruiz A, Shupliakov O, Kullmann DM, Storm-Mathisen J, Walaas SI, Hvalby Ø, Bergersen LH. Synapsin- and actin-dependent frequency enhancement in mouse hippocampal mossy fiber synapses. Cereb Cortex. 2009;19:511–23. doi: 10.1093/cercor/bhn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Porton B, Rendon B, Feng J, Greengard P, Kao HT. Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J Comp Neurol. 2002;454:105–14. doi: 10.1002/cne.10417. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsin-I and synapsin-II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Czernik AJ, Kao HT, Takei K, Johnston PA, Horiuchi A, Kanazir SD, Wagner MA, Perin MS, De CP. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MK-H, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/Pi transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to the subgranualar layers of primary somatosensory and auditory cortices in the mouse. J Neurosci. 2011a;31:12738–12747. doi: 10.1523/JNEUROSCI.1565-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. J Neurophysiol. 2011b;105:279–292. doi: 10.1152/jn.00747.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Masterson SP, Petry HM, Bickford ME. Diffuse and specific tectopulvinar terminals in the tree shrew: synapses, synapsins, and synaptic potentials. PLoS ONE. 2011;6:e23781. doi: 10.1371/journal.pone.0023781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991;43:299–349. [PubMed] [Google Scholar]
- Zurmöhle U, Herms J, Schlingensiepen R, Brysch W, Schlingensiepen KH. Changes in the expression of synapsin I and II messenger RNA during postnatal rat brain development. Exp Brain Res. 1996;108:441–449. doi: 10.1007/BF00227267. [DOI] [PubMed] [Google Scholar]


