Abstract
Because children are becoming overweight, unhealthy, and unfit, understanding the neurocognitive benefits of an active lifestyle in childhood has important public health and educational implications. Animal research has indicated that aerobic exercise is related to increased cell proliferation and survival in the hippocampus as well as enhanced hippocampal-dependent learning and memory. Recent evidence extends this relationship to elderly humans by suggesting that high aerobic fitness levels in older adults are associated with increased hippocampal volume and superior memory performance. The present study aimed to further extend the link between fitness, hippocampal volume, and memory to a sample of preadolescent children. To this end, magnetic resonance imaging was employed to investigate whether higher- and lower-fit 9- and 10-year-old children showed differences in hippocampal volume and if the differences were related to performance on an item and relational memory task. Relational but not item memory is primarily supported by the hippocampus. Consistent with predictions, higher-fit children showed greater bilateral hippocampal volumes and superior relational memory task performance compared to lower-fit children. Hippocampal volume was also positively associated with performance on the relational but not the item memory task. Furthermore, bilateral hippocampal volume was found to mediate the relationship between fitness level (VO2 max) and relational memory. No relationship between aerobic fitness, nucleus accumbens volume, and memory was reported, which strengthens the hypothesized specific effect of fitness on the hippocampus. The findings are the first to indicate that aerobic fitness may relate to the structure and function of the preadolescent human brain.
Keywords: Brain, Children, Exercise, Hippocampus, MRI, Physical activity
1. Introduction
Children in today’s industrial and technological society are becoming increasingly sedentary and unfit, leading to an increase in the incidence of obesity and illness (Olshansky et al., 2005; Baker et al., 2007; Ludwig, 2007). A sedentary lifestyle also influences neurocognitive function and academic performance. For example, children with low physical activity levels show poorer academic achievement scores, diminished neuroelectric activity, and inferior cognitive performance compared to physically fit children (Sibley and Etnier, 2003; Hillman et al., 2005, 2009; Castelli et al., 2007; Buck et al., 2008; Chomitz et al., 2009). This evidence is consonant with a growing research initiative in older adults which indicates that increased aerobic fitness can be neuroprotective and can enhance brain structure and function (Kramer et al., 1999; Colcombe and Kramer, 2003; Colcombe et al., 2004, 2006; Heyn et al., 2004; Etnier et al., 2006; Pereira et al., 2007; Erickson et al., 2009). In one recent study, higher levels of aerobic fitness in older adults were associated with larger hippocampal volumes and superior spatial memory performance (Erickson et al., 2009). The present study applies these findings to a youth population by exploring the association between aerobic fitness, hippocampal volume, and memory function in preadolescent 9- and 10-year-old children.
Rodent and human studies provide a number of reasons to explore the link between aerobic fitness levels and hippocampal structure and function. To begin, rodent models have unequivocally demonstrated that voluntary aerobic exercise positively affects the hippocampus. Specifically, wheel-running has been found to increase cell proliferation and survival in the dentate gyrus of the hippocampus in young adulthood through old age (van Praag et al., 1999, 2005; Eadie et al., 2005), enhance hippocampal-dependent learning and memory processes (Forordyce and Wehner, 1993; Vaynman et al., 2004; van Praag et al., 2005), and increase hippocampal levels of brain-derived neurotrophic factor (BDNF), insulin-like growth factor (IGF), and vascular endothelial-derived growth factor, which are molecules involved in neuronal survival, synaptic development, learning, and angiogenesis (Barde, 1994; Neeper et al., 1995; Lu and Chow, 1999; Cotman and Berchtold, 2002; Lopez-Lopez et al., 2004; Vaynman et al., 2004; Berchtold et al., 2005). Although many of the molecular and cellular details for exercise-induced changes in the human brain remain to be discovered, the broad hippocampal effects observed with exercise training in rodent populations suggest that greater aerobic fitness levels may be associated with increased hippocampal volume and superior hippocampal function during childhood.
Furthermore, exercise has been shown to impact memory function across the human life span (Pereira et al., 2007; Erickson et al., 2009; Chaddock et al., in press). During childhood, high levels of aerobic fitness have been associated with a superior ability to employ effective encoding and retrieval processes for relational material, a finding which suggests that physically fit 9- and 10-year-olds may exhibit stronger executive control abilities and flexible use of memory via prefrontal–hippocampal interactions (Chaddock et al., in press). No preadolescent fitness effects were found for items studied nonrelationally. This conclusion highlights the role of the hippocampus in the formation of new relational memories and in the “relational binding” process involved in successful retrieval while memory for single objects or items (i.e., item memory which requires little relational binding) is said to depend on the perirhinal cortex of the middle temporal lobe, prefrontal regions, or parahippocampal circuits (Cohen and Eichenbaum, 1993; Henke et al., 1997; Maguire et al., 1997; Cohen et al., 1999; Rombouts et al., 1999; Eichenbaum and Cohen, 2001; Brassen et al., 2006). The current study extends the behavioral results of Chaddock et al. (in press) in important ways by using a task more suitable for studying hippocampal function and by employing magnetic resonance imaging (MRI) techniques to examine the relationships among aerobic fitness, memory performance, and hippocampal volume.
Most imaging investigations of the developing brain focus on the structural development of the cortex rather than subcortical regions (Giedd et al., 1999; Gogtay et al., 2004). However, medial temporal lobe gray matter structures, including the hippocampus, are said to increase in volume during childhood and adolescence (Durston et al., 2001; Toga et al., 2006). In terms of memory performance, most developmental neuroscientists have explored how changes in dorsolateral prefrontal cortex and parietal regions map onto working memory abilities (Bunge and Wright, 2007) rather than the link between the developing hippocampus and memory abilities. The present investigation extends previous neurocognitive investigations by specifically exploring childhood hippocampal structure and function.
Given (1) the positive impact of physical activity and aerobic fitness on cognition in children; (2) the link between aerobic exercise, memory, and the hippocampus in rodent and human populations; and (3) the maturational trajectory of hippocampal development, the present study hypothesized that children with higher aerobic fitness levels would show larger bilateral hippocampal volumes and superior relational memory performance compared to lower-fit children. In addition, a mediation model was used to test the hypothesis that hippocampal volume mediated the relationship between fitness and memory such that greater bilateral hippocampal volume engendered by higher fitness levels was related to an improvement in relational memory performance. While a mediation analysis is designed to test causal hypotheses, the present study’s cross-sectional design precluded strong causal interpretations. Nonetheless, the results of a mediation analysis can provide an important framework for reported associations as well as guide future research and hypotheses.
To further explore the hypothesized specificity of the effect of childhood aerobic fitness on hippocampal volume and function, the relationships among fitness, nucleus accumbens volume, and memory task performance were examined. The nucleus accumbens was chosen as a “control” region because, like the hippocampus, it is a subcortical structure located in the midbrain, which can be demarcated from surrounding tissue by the employed segmentation technique. In addition, no previous investigations have reported an effect of aerobic fitness on the structure or function of the nucleus accumbens, and it is not known to play a role in memory performance but rather reinforcement learning and motivational states (Casey et al., 2008; Graybiel, 2008; Aron et al., 2009).
2. Results
2.1. Participant demographics
Participant demographic and fitness data are provided in Table 1. Demographic variables (i.e., age, IQ, SES, ADHD) did not differ between fitness groups. Furthermore, higher-fit participants (M = 51.51 mL/kg/min, SD = 4.31 mL/kg/min) had higher maximal oxygen consumption (VO2 max) scores than lower-fit children (M = 36.40 mL/kg/min, SD = 4.03 mL/kg/min) as revealed by an independent t-test (t(47) = 12.61, p < 0.001).
Table 1.
Participant mean demographic and fitness data (SD) by aerobic fitness group.
| Variable | Lower-fit | Higher-fit |
|---|---|---|
| n | 28 (10 males) | 21 (10 males) |
| Age (years) | 10.0 (0.6) | 10.0 (0.6) |
| VO2 max (mL/kg/min) * | 36.4 (4.0) | 51.5 (4.3) |
| K-BITa composite score (IQ) | 115.0 (15.1) | 114.4 (6.9) |
| K-BITa crystallized score (vocabulary) | 111.0 (11.9) | 108.5 (6.0) |
| K-BITa fluid score (matrices) | 115.8 (17.8) | 117.4 (8.9) |
| SESb (median) | 2.8 (0.6) | 2.6 (0.7) |
| ADHDc | 5.9 (3.9) | 6.7 (4.2) |
Significant difference between higher-fit and lower-fit groups at p < 0.001.
Kaufman Brief Intelligence Test (Kaufman and Kaufman, 1990).
Socioeconomic status. SES was determined by the creation of a trichotomous index based on three variables: child participation in a free or reduced-price lunch program at school, the highest level of education obtained by the child’s mother and father, and the number of parents who worked full-time (Birnbaum et al., 2002).
Scores on the ADHD Rating Scale V (DuPaul et al., 1998).
2.2. Aerobic fitness and memory performance
Item and relational memory task performance as a function of aerobic fitness was analyzed using both accuracy (percent correct) and d-prime (d′) scores. The pattern of results was the same for both performance measures. There were no fitness-based differences in response speed for either the relational memory (t(44) = 0.47, p = 0.64) or item memory (t(44) = 0.37, p = 0.71) task (Table 2).
Table 2.
Participant mean relational and item memory task performance data (SD) by fitness group.
| Lower-fit | Higher-fit | |
|---|---|---|
| Relational memory | ||
| Accuracy (% correct) | 54.00 (12.06) | 61.11 (13.83) |
| Reaction time (ms) | 2369.30 (357.11) | 2417.30 (335.67) |
| Hits (n) | 5.04 (1.73) | 5.81 (1.94) |
| Misses (n) | 3.21 (1.61) | 2.57 (1.40) |
| False alarms (n) | 4.13 (1.60) | 3.29 (1.76) |
| Correct rejections (n) | 4.42 (1.41) | 5.29 (1.76) |
| d′ * | 0.35 (0.67) | 0.84 (0.68) |
| Item memory | ||
| Accuracy (% correct) | 73.78 (17.31) | 75.93 (19.11) |
| Reaction time (ms) | 2159.10 (312.27) | 2193.40 (313.95) |
| Hits (n) | 6.13 (1.65) | 5.71 (2.26) |
| Misses (n) | 2.42 (1.53) | 2.62 (1.80) |
| False alarms (n) | 1.25 (1.62) | 0.95 (1.63) |
| Correct rejections (n) | 7.54 (1.67) | 7.95 (1.88) |
| d′ | 2.18 (1.37) | 2.40 (1.73) |
Significant difference between higher-fit and lower-fit groups at p < 0.05.
2.2.1. Accuracy
The results of an independent t-test revealed a trend such that higher-fit children showed superior accuracy on the relational memory task compared to lower-fit children (t(44) = 1.86, p = 0.06). There were no fitness-based differences in accuracy on the item memory task (t(44) = 0.40, p = 0.69). Mean item and relational accuracies as a function of aerobic fitness group are provided in Table 2. Furthermore, although participants were recruited to create bimodal higher-fit and lower-fit groups based on extreme VO2 max percentiles (i.e., excluding middle-fit participants) (Shvartz and Reibold, 1990), VO2 max was positively correlated with relational memory accuracy via a Spearman correlation (r = 0.287, p = 0.05). Unless stated otherwise, Spearman correlations were employed in all subsequent analyses given Kolmogorov–Smirnov tests of normality which indicated that test variables of interest were not normally distributed (all D’s > 0.13, df = 45, p < 0.05).
2.2.2. d′
A d′ measure of memory performance was also computed given that it helps to account for response bias (Macmillan and Creelman, 2005). To calculate a d′ score for each subject, the responses for each of the 18 item memory and relational memory recognition trials were first categorized as a hit, miss, false alarm, correct rejection, or no response. A “hit” was defined as correctly identifying a previously studied stimulus as “old / studied,” a “miss” was defined as incorrectly identifying a previously studied stimulus as “new / not studied,” a “false alarm” was defined as incorrectly identifying an unstudied stimulus as “old / studied,” a “correct rejection” was defined as correctly identifying an unstudied stimulus as “new / not studied,” and a “no response” was defined as failing to make a response in the 4-second response time window. The mean numbers of hits, misses, false alarms, and correct rejections for item and relational recognition task conditions as a function of aerobic fitness group are presented in Table 2. “No response” trials were not used in subsequent calculations and therefore were not included in Table 2.
Next, a constant of 0.05 was added to each number of hits, misses, false alarms, and correct rejections to eliminate cases of 0 and 1 hits and false alarms which would result in an undefined value for d′. Then, for both item and relational memory conditions, each participant’s “hit rate” was determined by calculating “number of hits / (number of hits + number of misses),” and each subject’s “false alarm rate” was determined by calculating “number of false alarms / (number of false alarms + number of correct rejections).” Finally, item and relational d′ scores were calculated for each subject by subtracting the z-score of the false alarm rate from the z-score of the hit rate (with chance performance represented by a d′ score of 0). Mean item and relational d′ scores for both fitness groups are presented in Table 2. Note that accuracy can also be calculated from these values by computing [(number of hits + number of correct rejections)/(number of hits + number of misses + number of correct rejections + number of false alarms)] × 100.
The results of an independent t-test revealed that higher-fit children showed higher relational memory d′ scores compared to lower-fit children (t(43) = 2.40, p = 0.021). There were no fitness-based differences in d′ scores for the item memory task (t(43) = 0.47, p = 0.64).
2.3. Aerobic fitness and hippocampal and nucleus accumbens volume
Left and right hippocampal volumes were significantly correlated with each other (r = 0.671, p < 0.0001). A repeated-measures ANOVA with hemisphere (left and right hippocampal volumes) as the within-subjects variable and aerobic fitness group (higher-fit and lower-fit) as the between-subjects variable indicated a main effect of fitness on hippocampal volume (F(1,47) = 6.16, p = 0.017), with higher-fit children showing greater volumes than lower-fit children (see Fig. 1). The effect of fitness on hippocampal volume, however, did not differ by hemisphere since the interaction between fitness and hemisphere did not reach significance (F(1,47) = 0.096, p = 0.758). This was confirmed by univariate ANCOVAs (with total intracranial volume [the sum of total gray matter, white matter, and cerebrospinal fluid] (mm3) as a covariate to control for variation in head size) which indicated that higher-fit children showed significantly greater hippocampal volume for both the left (F(1,46) = 4.97, p = 0.031) and right hemispheres (F(1,46) = 5.62, p = 0.022) when compared to lower-fit children. Together, these results justify the use of a bilateral measure of hippocampal volume (i.e., the sum of left and right hippocampal volumes) in subsequent analyses.
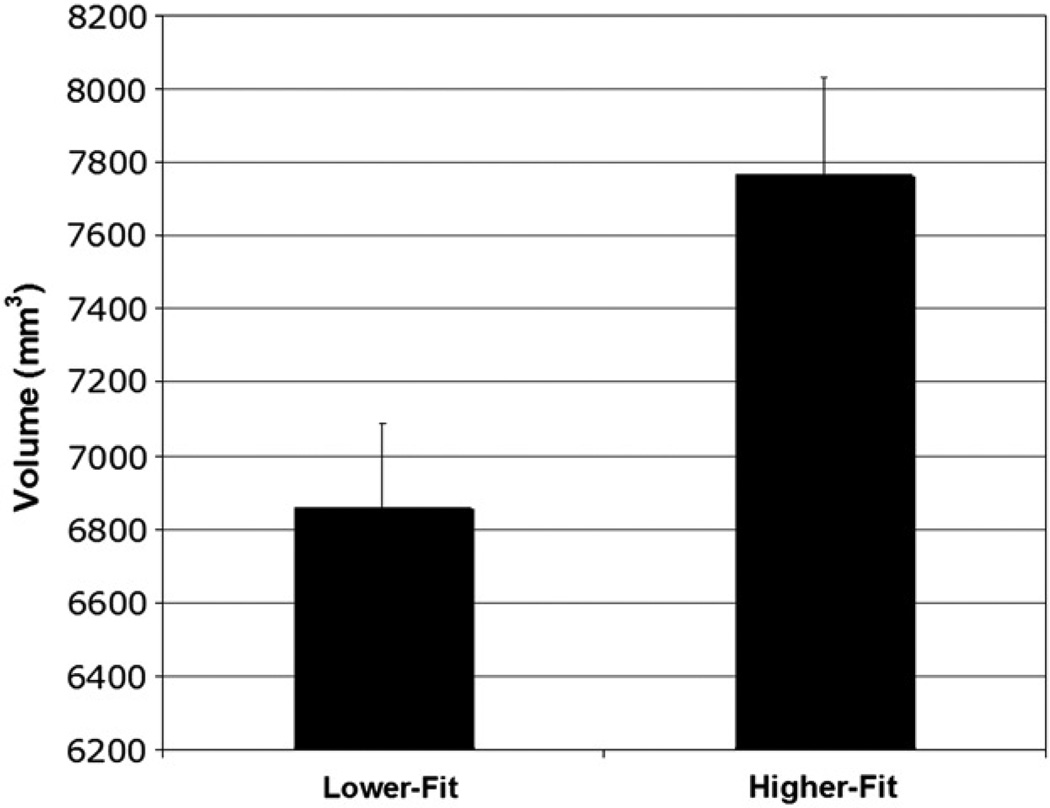
Fig. 1.
Bilateral hippocampal volume as a function of aerobic fitness group. Error bars represent standard error.
The results support the hypothesis that aerobic fitness influences bilateral hippocampal volume. A univariate ANCOVA was conducted to compare bilateral hippocampal volume as a function of fitness group, with total intracranial volume (mm3) as a covariate. Higher-fit children (M = 7772.60 mm3, SD = 899.57 mm3) showed greater bilateral hippocampal volumes compared to lower-fit children (M = 6854.09 mm3, SD = 1503.32 mm3) (F(1,46) = 6.63, p = 0.013) (see Fig. 1).
To explore the specificity of the association between aerobic fitness and hippocampus, three univariate ANCOVAs (with total intracranial volume [mm3] as a covariate) were performed to compare nucleus accumbens volume as a function of fitness group. The volume of the left nucleus accumbens, right nucleus accumbens, and bilateral nucleus accumbens did not differ for higher-fit and lower-fit participants (all F’s < 3.8, p > 0.05).
2.4. Hippocampal and nucleus accumbens volume and memory performance
To simplify the analysis of hippocampal volume and memory performance (i.e., to reduce the number of multiple comparisons by half), the sum of left and right hippocampal volumes was used as a predictor variable. Again, this was justified given the high correlation between left and right hemisphere volumes and the consistent association between volume and fitness group across both hemispheres. The results support the predicted dissociation between item and relational memory performance with regard to hippocampal volume.
2.4.1. Accuracy
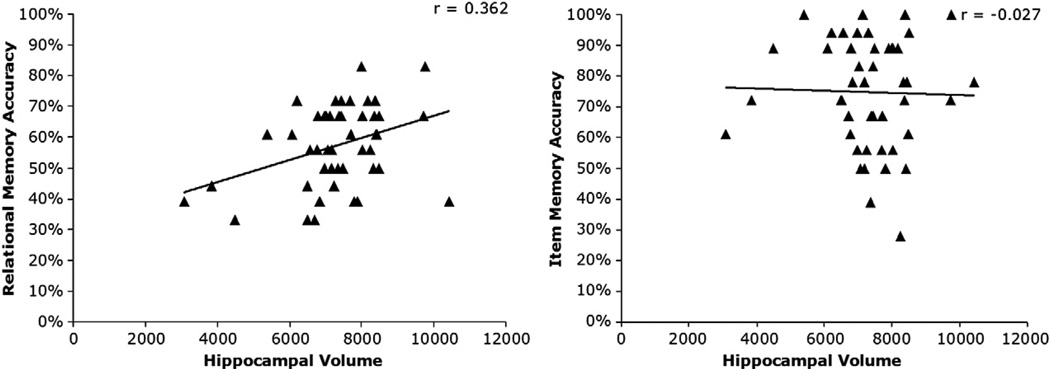
Bilateral hippocampal volume was positively correlated with accuracy (percent correct) on the relational memory task (r = 0.333, p = 0.024). There was no significant association between hippocampal volume and item memory accuracy (r = −0.050, p = 0.743) (See Fig. 2).
Fig. 2.
Scatter plots. Bilateral hippocampal volume was positively correlated with relational memory accuracy. There was no relationship between hippocampal volume and item memory accuracy. The pattern of results was the same using d′ scores.
2.4.2. d′
Bilateral hippocampal volume was positively correlated with d′ scores on the relational memory task (Pearson: r = 0.311, p = 0.038; Spearman: r = 0.283, p = 0.06). There was no significant association between hippocampal volume and item memory d′ (Pearson and Spearman: r < 0.06, p > 0.7). Kolmogorov–Smirnov tests of normality indicated that the distribution of bilateral hippocampal volume values was not normal (D = 0.138, df = 45, p < 0.05), whereas item and relational d′ scores were normally distributed (p > 0.05).
There were no significant correlations between hippocampal volume and response speed for either the item or relational memory condition (all r < −0.07, p > 0.65). Furthermore, no relationship was found between nucleus accumbens volume and item or relational memory task performance (accuracy, d′ scores, or reaction time) (all r’s < ±0.1; all p > 0.6).
2.5. Mediation analyses
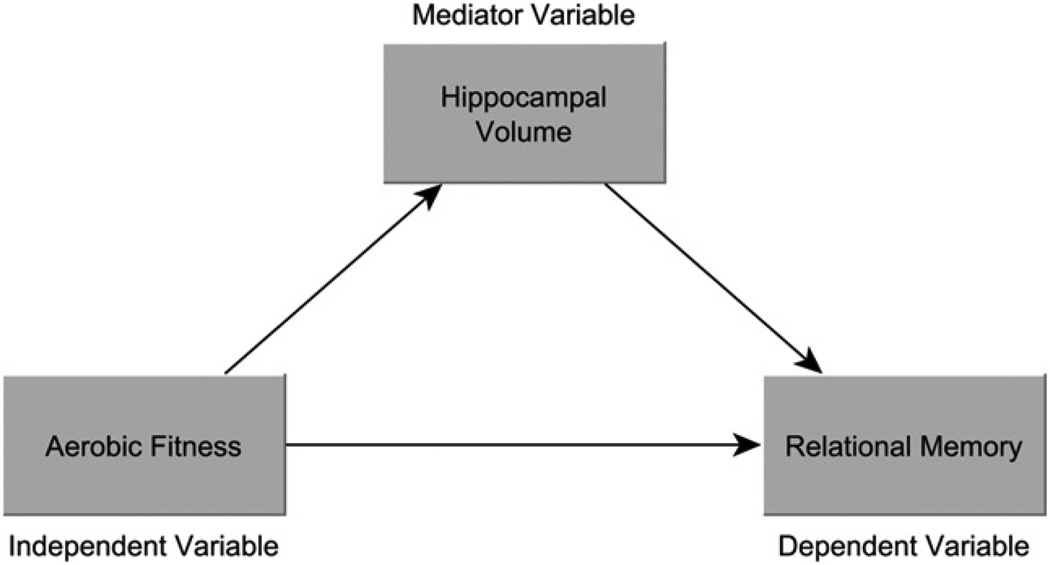
Given the above results which link childhood aerobic fitness, relational memory task performance, and bilateral hippocampal volume, a mediation analysis was conducted to determine if greater hippocampal volume mediated the relationship between fitness and memory (see Fig. 3). Mediation is a hypothesis about a causal relation among variables (Judd and Kenny, 1981; Baron and Kenny, 1986; MacKinnon et al., 2007), and three conditions must be met to conduct a Sobel test which establishes a significant mediation (Sobel, 1982; Baron and Kenny, 1986). Firstly, the independent variable (aerobic fitness) must be associated with the dependent variable (relational memory). Secondly, the independent variable must be associated with the mediator (bilateral hippocampal volume). Thirdly, the mediator variable must be associated with the dependent variable. The results described above reveal an association between aerobic fitness group and relational memory performance, aerobic fitness and bilateral hippocampal volume, and hippocampal volume and relational memory, thereby providing support for the three conditions necessary to perform a mediation analysis.
Fig. 3.
Figural representation of the mediation model. Bilateral hippocampal volume was shown to mediate the relationship between childhood aerobic fitness level (VO2 max) and relational memory.
Correlations between VO2 max, bilateral hippocampal volume and relational memory performance (accuracy and d′) were also computed to further test the requirements of potential mediation. Although the use of bimodal higher-fit and lower-fit groups designates VO2 max as a noncontinuous variable, VO2 max was used in all subsequent mediation analysis steps. VO2 max was positively correlated with relational memory accuracy (r = 0.287, p = 0.05), VO2 max was positively correlated with bilateral hippocampal volume (r = 0.351, p = 0.01), and bilateral hippocampal volume was positively correlated with relational memory accuracy (r = 0.333, p = 0.02). In terms of d′, VO2 max was positively correlated with relational memory d′ (Pearson: r = 0.358, p = 0.016; Spearman: r = 0.331, p = 0.026), and bilateral hippocampal volume was positively correlated with relational memory d′ (Pearson: r = 0.311, p = 0.038; Spearman: r = 0.283, p = 0.06). The correlations provide further confirmation for the three conditions necessary to test mediation. Because similar results were found when using accuracy and d′ scores, only the mediation analysis using accuracy as the dependent variable is reported below.
Mediation was tested by conducting two linear regression analyses to obtain raw regression coefficients and the standard errors of the coefficients for the variables of interest and then inserting these coefficients into the Sobel test (Sobel, 1982; Baron and Kenny, 1986). Specifically, the first linear regression analysis was conducted to determine the association between the independent variable (VO2 max) and the mediator variable (bilateral hippocampal volume) (b = 39.56, SE = 22.123, p = 0.08). A second linear regression was performed to examine the association between the mediator variable and the dependent variable (relational memory accuracy), while adjusting for the independent variable (b = 0.000031, SE < 0.001, p = 0.03).
The Sobel test (Sobel, 1982; Baron and Kenny, 1986) analyzed if the effect of the mediator on the dependent variable was significantly different from zero using a one-tailed z-test given the directional hypothesis. The regression coefficient and standard error of the coefficient for the relationship between the independent variable and the mediator variable as well as the regression coefficient and standard error of the coefficient for the relationship between the mediator variable and the dependent variable were entered into the Sobel test to calculate the significance of mediation (http://www.danielsoper.com/statcalc/calc31.aspx). The Sobel test reached significance (z = 1.79; p < 0.038, one-tailed), suggesting that greater hippocampal volume mediated the relationship between aerobic fitness and relational memory.
3. Discussion
Prior research has demonstrated that elderly adults with higher aerobic fitness levels have larger hippocampal volumes compared to older adults with lower fitness levels (Erickson et al., 2009). The results from the present cross-sectional study demonstrate that children with higher aerobic fitness levels also have larger hippocampal volumes compared to lower-fit children. Furthermore, larger hippocampal volumes were associated with superior relational memory task performance. No association between aerobic fitness, hippocampal volume, and item memory performance was observed, a finding consistent with the hypothesized specificity of the hippocampal–memory relationship (Cohen and Eichenbaum, 1993; Eichenbaum and Cohen, 2001). Nucleus accumbens volume was also not associated with aerobic fitness group or memory performance which reinforces the specificity of the fitness–brain relationship to the hippocampus. Finally, for the relational memory task condition, higher-fit children showed higher d′ scores and a trend for superior accuracy compared to lower-fit peers, and VO2 max was positively correlated with both measures of performance. No fitness differences were reported for the item memory task.
Together, the structural imaging and behavioral correlational findings suggest that higher-fit and lower-fit children exhibit differential hippocampal structure which may impact relational memory function. A mediation analysis supported this claim by demonstrating that bilateral hippocampal volume mediated the relationship between childhood VO2 max and relational memory. The results are important because they provide a starting point for a greater understanding of the neural underpinnings of cognitive enhancement through physical activity in preadolescent children as well as a potential neural correlate of the fitness–memory performance link in children (Chaddock et al., in press).
The results are consistent with animal models that indicate aerobic activity positively impacts hippocampal structure and function (e.g., Cotman and Berchtold, 2002). Given that the neurochemical processes involved in hippocampal changes with exercise in rodents are also involved in human brain development and organization, it seems possible that aerobic fitness may impact the brain during childhood, a period of significant cognitive and neural development (Caviness et al., 1996; Casey et al., 2005). For example, changes in gray and white matter during brain development are said to reflect the interplay among changes in cell proliferation / apoptosis, dendritic branching / pruning, synaptic formation / elimination, growth factors (e.g., BDNF, IGF), and myelination (Giedd et al., 1996; Giedd et al., 1999; Anderson, 2003; Gogtay et al., 2004). These cellular underpinnings parallel exercise-induced neural effects including changes in cell number, dendritic complexity, synaptic plasticity, and growth factors (e.g., Cotman and Berchtold, 2002). The current study provides initial evidence for the impact of exercise on the childhood brain by revealing that greater aerobic fitness level in preadolescents is related to greater hippocampal volume. Importantly, the results also suggest that hippocampal volume differences are associated with cognitive ability, and in particular relational memory. The mediation effects further solidify the strong association between fitness, hippocampus and memory.
3.1. Future directions and conclusions
The results provide a foundation for future developmental research by suggesting that physical activity may relate to the brain and cognition of children. While the present cross-sectional study provides a first step in understanding the relationship between fitness and childhood neurocognition, a cross-sectional design raises the possibility that the observed behavioral and structural fitness-related differences were caused by another factor (e.g., genes, motivation, personality characteristics, nutrition, intellectual stimulation, etc.). Thus, randomized, controlled trials are necessary to account for potential selection bias and to establish a direct relationship between aerobic fitness and hippocampal structure and function in children. Future research should explore how a physical activity intervention relates to hippocampal structure and memory performance over time to gain a deeper understanding of cognitive development, neural organization, and factors which impact developing neurocognitive function. Nevertheless, the promising results of the mediation analysis help provide preliminary insight into the causal association among childhood fitness, hippocampal volume, and memory.
The current study focused on a 9- and 10-year-old preadolescent population. During this age range, children are undergoing a critical phase of brain growth when brain circuitry is being fine-tuned to support the operations of the adult brain (Caviness et al., 1996). Future explorations should examine the effects of fitness at different ages across development as well as track changes in cognition and brain patterns in the same individuals across time. Given the evidence that physical activity is positively associated with preadolescent neurocognition, it is possible that high levels of fitness may affect the adolescent brain as well as the number of suboptimal, impulsive behaviors associated with this developmental stage (e.g., violence, drug abuse, unprotected sexual activity) (Casey et al., 2008). In addition, given that the present study recruited healthy children without learning disabilities or ADHD, it is important to examine the impact of fitness on children with cognitive and social disorders.
Finally, the current study limited its hypotheses and conclusions to subcortical structures to test the link between fitness and hippocampus in a youth population as well as to expand the developmental memory literature to new brain structures. The role of cortical volume in the aerobic fitness–memory relationship is an important avenue for additional investigations, especially given the behavioral study of Chaddock et al. (in press) that suggested a relationship between executive control processes, relational memory function, and childhood fitness. Future investigators should also employ tasks with graded levels of difficulty to gain a stronger understanding of the link between aerobic fitness and memory abilities given that, across all subjects, average item memory accuracy was 75% (SD = 0.18) while average relational memory accuracy was 57% (SD = 0.13). Nevertheless, despite relatively low accuracies, the results indicated higher relational d′ scores for higher-fit children as well as a significant association between VO2 max and relational memory accuracy.
In conclusion, the present investigation is the first to jointly employ MRI and behavioral methodologies to examine the link between aerobic fitness, brain structure, and cognition in preadolescent children. A clear association between aerobic fitness, hippocampal volume, and relational memory in children is demonstrated. The results extend previous research which has focused on how fitness impacts the brain and cognition in elderly adults. Not only does fitness protect against age-related brain tissue loss (Colcombe et al., 2006), it also relates to preadolescent brain and cognition. According to Davis et al. (2007), exercise may have a more long-lasting effect on brains that are still developing. To strengthen this claim, the effect size of Sibley and Etnier’s (2003) children–fitness–cognition meta-analysis (0.32) was slightly larger than the effect size of a meta-analysis of the effects of physical activity on cognition across the life span (6–90 years) (0.25) (Etnier et al., 1997), a finding which suggests that physical activity may be especially beneficial for children. Thus, although physical activity seems to be beneficial at all stages of life, early intervention might be important for the improvement and/or maintenance of cognitive health and function throughout the adult life span (Sibley and Etnier, 2003). Moreover, it is plausible that developing a love of sport and exercise as a child will encourage an active lifestyle during adulthood and old age.
The findings carry significant educational and public health implications. Educators are under increased pressure to improve the standardized test scores of their pupils. This pressure, coupled with the popular belief that physical education is of less educational value than academic work, has led to the elimination of physical education classes and recess in favor of “core academic subjects.” However, as children are becoming increasingly sedentary, overweight, and unfit, recent estimates have indicated that younger generations may live less healthy and shorter lives than their parents (Olshansky et al., 2005). Furthermore, inactivity during childhood can increase the prevalence of obesity as well as a number of diseases and disorders throughout the life span (e.g., depression, anxiety, cardiovascular disease, colon cancer, type-2 diabetes) (Olshansky et al., 2005; Baker et al., 2007; Ludwig, 2007).
The present results suggest that physical fitness programs should be integrated into educational curriculums not only for obesity and public health purposes but also because exercise may benefit brain structure and function. Although speculative and requiring future research, it is possible that physical activity during childhood encourages optimal cortical development and results in long-term changes in brain structure and function. Hopefully, the present findings will encourage modifications of educational and health care policies which emphasize the importance of physical activity on physical and cognitive health.
4. Experimental procedures
4.1. Participants
Preadolescent 9- and 10-year-old children were recruited from East-Central Illinois. Children were screened for several factors that influence physical activity participation or cognitive function. To begin, the Kaufman Brief Intelligence Test (K-BIT; Kaufman and Kaufman, 1990) was administered to each child to obtain a composite intelligence quotient (IQ) score including both crystallized and fluid intelligence measures. Subjects were excluded if their scores were more than 1 standard deviation below the mean (85%). Next, a guardian of the child completed the Attention-Deficit Hyperactivity Disorder (ADHD) Rating Scale IV (DuPaul et al., 1998) to screen for the presence of attentional disorders. Participants were excluded if they scored above the 85th percentile. Pubertal timing was also assessed using a modified Tanner Staging System (Tanner, 1962; Taylor et al., 2001) with all included prepubescent participants at or below a score of 2 on a 5-point scale of developmental stages. In addition, socioeconomic status (SES) was determined by creating a trichotomous index based on three variables: participation in a free or reduced-price lunch program at school, the highest level of education obtained by the child’s mother and father, and the number of parents who worked full-time (Birnbaum et al., 2002).
Furthermore, eligible participants were required to (1) qualify as higher-fit or lower-fit (see Aerobic fitness assessment section); (2) demonstrate right-handedness (as measured by the Edinburgh Handedness Questionnaire) (Oldfield, 1971); (3) report no adverse health conditions, physical incapacities, or neurological disorders; (4) report no use of medications that influenced central nervous system function; (5) have a corrected visual acuity of 20/20 and no color-blindness; (6) successfully perform a “mock MRI” session to test for body size compatibility with an MRI machine and to screen for claustrophobia; and (7) sign an informed assent approved by the University of Illinois at Urbana-Champaign. A legal guardian also provided written informed consent in accordance with the Institutional Review Board of the University of Illinois at Urbana-Champaign. Subjects were compensated for participation.
4.2. Aerobic fitness assessment
The aerobic fitness level of each child was determined by measuring VO2 max using a computerized indirect calorimetry system (ParvoMedics True Max 2400) during a modified Balke protocol (American College of Sports Medicine, 2006). Specifically, participants ran on a motor-driven treadmill at a constant speed with increases in grade increments of 2.5% every 2 minutes until volitional exhaustion. Averages for oxygen uptake (VO2) and respiratory exchange ratio (RER) (the ratio between carbon dioxide and oxygen percentage) were assessed every 30 seconds. In addition, heart rate was measured throughout the fitness test (using a Polar heart rate monitor [Polar WearLink® + 31, Polar Electro, Finland]), and ratings of perceived exertion were assessed every 2 minutes using the children’s OMNI scale (Utter et al., 2002).
VO2 max was defined when oxygen consumption remained at a steady-state despite an increase in workload. Relative peak oxygen consumption was based upon maximal effort as evidenced by (1) a peak heart rate greater than 185 beats per minute (American College of Sports Medicine, 2006) accompanied by a heart rate plateau (i.e., an increase in work rate with no concomitant increase in heart rate) (Freedson and Goodman, 1993), (2) RER greater than 1.0 (Bar-Or, 1983), and/or (3) ratings on the children’s OMNI scale of perceived exertion greater than 8 (Utter et al., 2002). Relative peak oxygen consumption was expressed in milliliters per kilogram per minute.
Fitness group assignments (i.e., higher-fit and lower-fit) were based on whether a child’s VO2 max value fell above the 70th percentile or below the 30th percentile according to normative data provided by Shvartz and Reibold (1990). Children who did not qualify as higher-fit or lower-fit were excluded from participation.
4.3. Sample
Fifty-nine subjects were initially eligible for the present study (after exclusions due to K-BIT scores, ADHD, pubertal timing, VO2 max criteria, etc). Additional subjects were excluded due to poor scan quality because of excessive motion (n = 4), hippocampal volume outliers (n = 1), and less than chance memory performance (less than 30% accuracy on either the item or relational memory task) (n = 5).
Analyses were conducted on a total of 49 subjects, including 21 higher-fit children (10 boys and 11 girls) with an average age of 10.0 years (SD = 0.6; range 9–10) and 28 lower-fit children (10 boys and 18 girls) with an average age of 10.0 years (SD = 0.6; range 9–10). No statistically reliable differences in age, gender, socioeconomic status, or K-BIT scores existed between the fitness groups. Table 1 provides a list of demographic and fitness information for the final sample.
4.4. MR imaging protocol and image processing
For all participants, high-resolution (1.3 mm × 1.3 mm × 1.3 mm) T1-weighted structural brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol with 144 contiguous axial slices, collected in ascending fashion parallel to the anterior and posterior commissures (echo time = 3.87 ms, repetition time = 1800 ms, field of view = 256 mm, acquisition matrix 192 mm × 192 mm, slice thickness = 1.3 mm, and flip angle = 8º). All images were collected on a 3-T head-only Siemens Allegra MRI scanner.
Segmentation and volumetric analysis of the left and right hippocampus and nucleus accumbens was performed using a semiautomated, model-based subcortical tool (FIRST; FMRIB’s Integrated Registration and Segmentation Tool) in FMRIB’s Software Library (FSL) version 4.1.4 (Patenaude, 2007; Patenaude et al., 2007a; Patenaude et al., 2007b). To begin, a two-stage affine registration to a standard space template (MNI space) with 1 mm resolution using 12-degrees of freedom and a subcortical mask to exclude voxels outside the subcortical regions was performed on each subject’s MPRAGE.
Next, the left and right hippocampi and nucleus accumbens were segmented with 30 and 50 modes of variation for each structure, respectively. To achieve accurate segmentation, the FIRST methodology models 317 manually segmented and labeled T1 brain images from normal children, adults, and pathological populations (obtained from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston) as a point distribution model with the geometry and variation of the shape of each structure submitted as priors. Volumetric labels are parameterized by a 3D deformation of a surface model based on multivariate Gaussian assumptions. FIRST searches through linear combinations of shape modes of variation for the most probable shape (i.e., brain structure) given the intensity distributionin the T1-weighted image, and specific brain regions are extracted (see Patenaude et al., 2007a,b for further description of the method). Modes of variation are optimized based on leave-one-out cross-validation on the training set, and they increase the robustness and reliability of the results (Patenaude et al., 2007b).
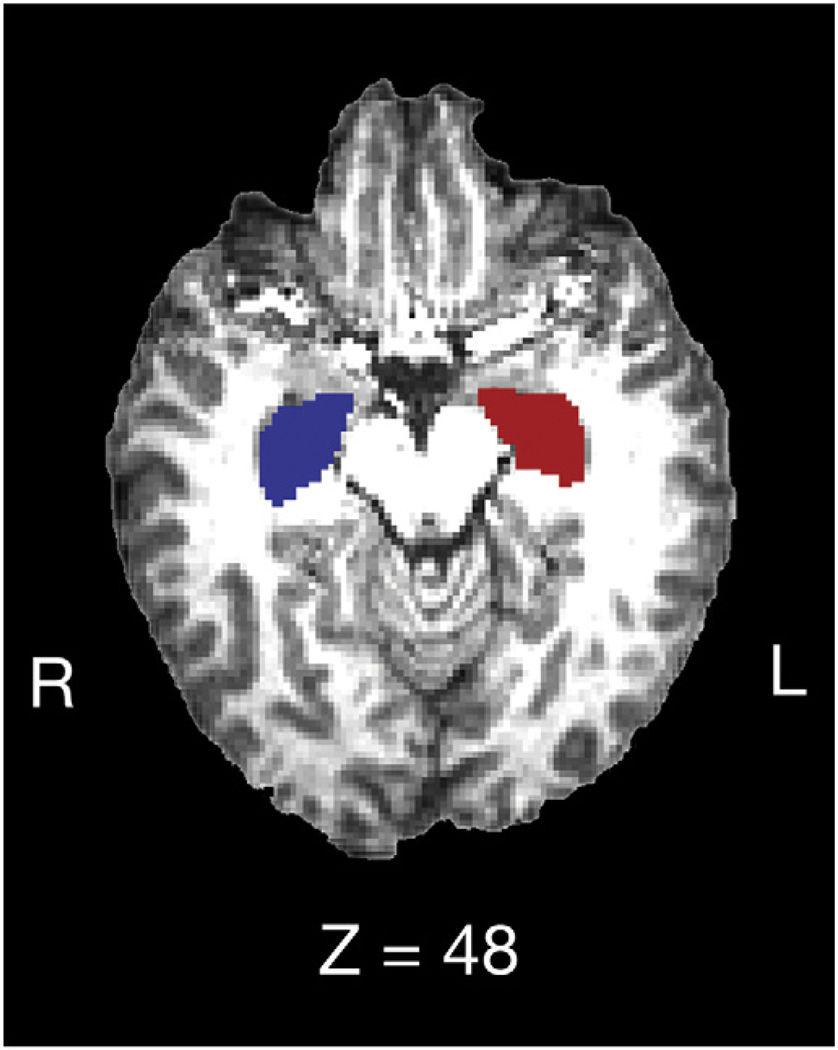
The hippocampus included the dentate gyrus, the ammonic subfields (CA1–4), the prosubiculum, and the subiculum and did not include the fimbria/fornix behind the posterior commissure. Hippocampal and nucleus accumbens segmentations were visually checked for errors, and no errors were noted. Finally, boundary correction was run, a process which classifies boundary voxels as belonging to the structure (or not) based on a statistical probability (z-score > 3.00, p < 0.001). The volume of each participant’s left and right hippocampus and nucleus accumbens was measured in cubic millimeters, and the volume of each subject’s bilateral hippocampus (i.e., sum of left and right hippocampal volume) and bilateral nucleus accumbens was used in the majority of analyses. See Fig. 4 for a sample FIRST segmentation of the left and right hippocampus.
Fig. 4.
FIRST segmentation of the left (red) and right (blue) hippocampus on a structural brain reconstruction.
4.5. Item and relational memory paradigm
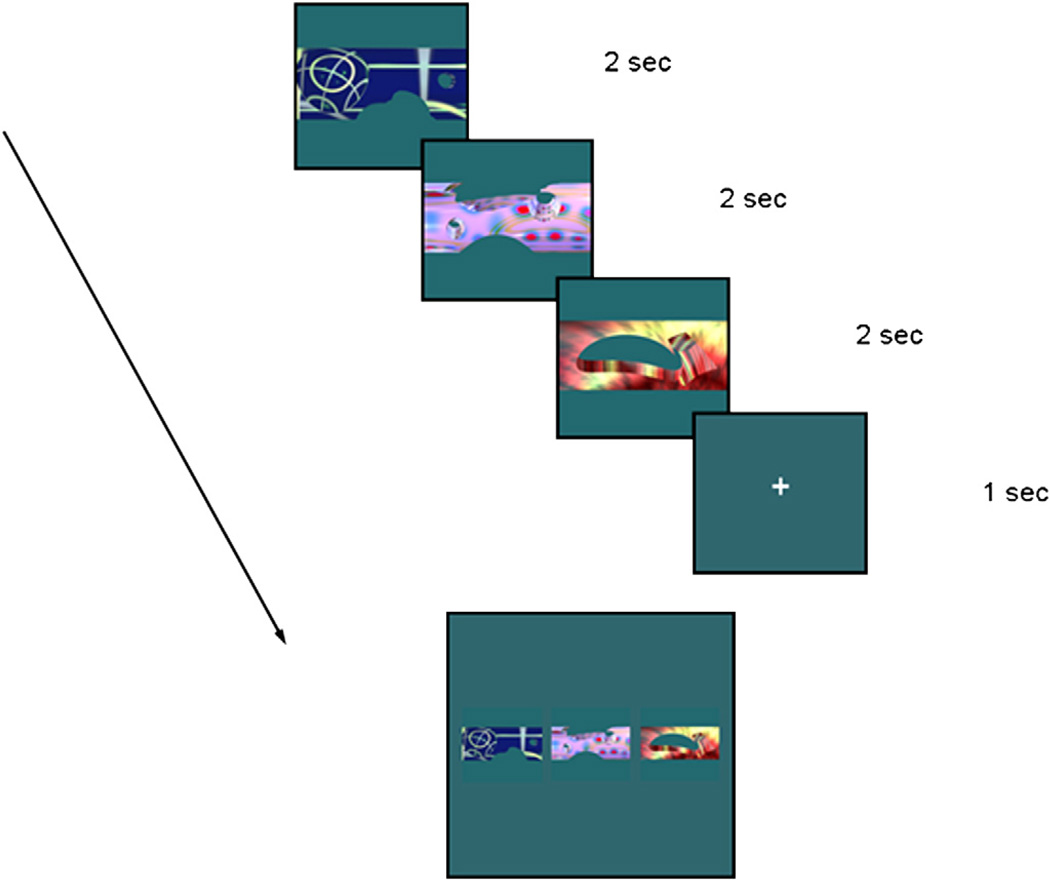
The paradigm examined memory in successive encoding-then-recognition phases and was administered to participants in the MRI machine. Each block included an encoding phase followed by a recognition phase. Six blocks were included in one paradigm in the following order for all participants: “item (encoding and recognition),” “relational (encoding and recognition),” “relational,” “item,” “item,” and “relational.” The stimuli were novel visual objects (created using Bryce software; used in Konkel et al., 2008) to ensure that participants had no prior exposure to the images or previous representations of the stimuli. See Fig. 5 for an illustration of the stimuli and task.
Fig. 5.
Figural representation of the item and relational memory task. Sample encoding and recognition stimuli are presented. Each image was presented individually and sequentially during encoding trials, and a fixation cross only separated encoding triplets during relational memory encoding trials. Three images were presented simultaneously during item and relational recognition trials.
During encoding, each participant was presented with a series of trials consisting of “scrambled stimuli” (not to be recognized later and only incorporated to serve as a baseline for a functional MRI protocol not to be discussed in this article) or “encoding stimuli” (to be recognized later). Participants viewed 18 scrambled stimuli (during which they were asked to “watch the snow”) followed by 18 encoding stimuli during both item and relational encoding conditions. Each image was presented on an MRI back projection for 2000 ms. The identical sequence of scrambled and encoding item images was presented twice.
“Relational encoding” blocks were distinguished from “item encoding” blocks in two ways. Firstly, in terms of subject instructions, for item blocks, subjects were instructed to “remember as many shapes as possible,” while for relational blocks, participants were instructed to “remember which shapes were in each group of 3.” Secondly, in terms of stimulus presentation, item stimuli were presented sequentially and individually, without intermixed fixation crosses, whereas an additional 1000-ms fixation cross separated the relational scrambled and encoding stimuli into triplets (i.e., three stimuli were presented individually and sequentially, then a fixation cross appeared for 1000 ms, followed by three new stimuli presented individually and sequentially).
During recognition, memory was probed for either individual test items (“item recognition”) or associative relations of stimuli within a triplet (“relational recognition”). For both item and relational recognition, three test items were displayed simultaneously during the probe period, and participants were given 4 seconds to respond. Each trial was separated by a fixation cross presented for 1 second. Six recognition trials (i.e., six groups of 3) were presented. Subjects used MRI-compatible response boxes for a yes/no response during recognition trials.
Specifically, during “item recognition,” participants read the following instructions: “You will see 3 shapes appear at the same time. If all 3 shapes were seen in the previous block of shapes, press your right index finger. If any of the 3 shapes was not seen in the previous block, press your left index finger.” Right index finger responses (i.e., all three shapes were seen during item encoding) contained three studied stimuli (i.e., stimuli that had occurred in the 18 stimuli presented during encoding), while left index finger responses (i.e., all three shapes were not seen during item encoding) contained two never-studied stimuli and one studied stimulus.
During “relational recognition,” participants read the following instructions: “You will see 3 shapes appear at the same time. If all 3 shapes are from the same group, press your right index finger. If any of the shapes do not belong in the group, press your left index finger.” Right index finger responses (i.e., the three shapes were in the same triplet) contained three stimuli that had occurred as a triplet (i.e., enclosed with two fixation crosses) during encoding, while left index finger responses (i.e., if any of the three shapes were not seen together as a triplet) contained stimuli that had occurred in the 18 stimuli presented during encoding but were not presented as a sequential triplet. Each recognition condition was designed to probe one type of memory or form of representation (i.e., item memory or relational memory), unconfounded by the other.
Acknowledgments
We would like to thank Nancy Dodge and Holly Tracy for their help in data collection.
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- BDNF
brain-derived neurotrophic factor
- d′
d prime
- FIRST
FMRIB’s Integrated Registration and Segmentation Tool
- IGF
insulin-like growth factor
- IQ
intelligence quotient
- K-BIT
Kaufman Brief Intelligence Test
- M
mean
- MPRAGE
magnetization prepared rapid gradient echo imaging
- MRI
magnetic resonance imaging
- RER
respiratory exchange ratio
- SD
standard deviation
- SE
standard error
- SES
socioeconomic status
- VO2 max
maximal oxygen consumption
REFERENCES
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed. New York: Lippincott Williams & Wilkins; 2006. p. 366. [Google Scholar]
- Anderson SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA, Wise SP. Cognition: basal ganglia role. Encyclopedia Neurosci. 2009;2:1069–1077. [Google Scholar]
- Baker JL, Olsen LW, Sorensen TIA. Childhood body-mass index and risk of coronary heart disease in adulthood. N Engl J. Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Pediatric Sports Medicine for the Practitioner: From Physiologic Principles to Clinical Applications. New York: Springer-Verlag; 1983. p. 376. [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Birnbaum AS, Lytle LA, Murray DM, Story M, Perry CL, Boutelle KN. Survey development for assessing correlates of young adolescents’ eating. Am. J. Health Behav. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Brassen S, Weber-Fahr W, Sommer T, Lehmbeck JT, Braus DF. Hippocampal–prefrontal encoding activation predicts whether words can be successfully recalled or only recognized. Behav. Brain Res. 2006;171:271–278. doi: 10.1016/j.bbr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Buck SM, Hillman CH, Castelli DM. The relation of aerobic fitness to Stroop task performance in preadolescent children. Med. Sci. Sports Exerc. 2008;40:166–172. doi: 10.1249/mss.0b013e318159b035. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third- and fifth-grade students. J. Sport Exerc. Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb. Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Med. Sci. Sports Exerc. doi: 10.1249/MSS.0b013e3181e9af48. in press. [DOI] [PubMed] [Google Scholar]
- Chomitz VR, Slining MM, McGowan RJ, Mitchell SE, Dawson GF, Hacker KA. Is there a relationship between physical fitness and academic achievement?: positive results from public school children in the northeastern United States. J. Sch. Health. 2009;79:30–37. doi: 10.1111/j.1746-1561.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge: MIT Press; 1993. p. 326. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl Acad. Sci. USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Davis CL, Tomporowski PD, Boyle CA, Waller JL, Miller PH, Naglieri JA, Gregoski M. Effects of aerobic exercise on overweight children’s cognitive functioning: a randomized controlled trial. Res. Q. Exerc. Sport. 2007;78:510–519. doi: 10.1080/02701367.2007.10599450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos A, Reid R. ADHD Rating Scale–IV: Checklists, Norms, and Clinical Interpretation. Guilford, New York: 1998. [Google Scholar]
- Durston S, Hulshoff HE, Casey BJ, Giedd JN, Buitelaar JK, Engeland HV. Anatomical MRI of the developing human brain: what have we learned? J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redilla VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York: Oxford University Press; 2001. p. 600. [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. J. Sport Exerc. Psychol. 1997;19:249–277. [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006;52:119–130. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Goodman TL. Measurement of oxygen consumption. In: Rowland TW, editor. Pediatric Laboratory Exercise Testing: Clinical Guidelines. Champaign, IL: Human Kinetics; 1993. pp. 91–113. [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus A, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzid AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. PNAS. 2004;101:8147–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Heyn P, Abreu B, Ottenbacher K. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch. Phys. Med. Rehabil. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Castelli DM, Buck SM. Aerobic fitness and neurocognitive function in healthy preadolescent children. Med. Sci. Sports Exerc. 2005;37:1967–1974. doi: 10.1249/01.mss.0000176680.79702.ce. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: event-related brain potential and task performance of executive control in preadolescent children. Dev. Psychol. 2009;45:114–129. doi: 10.1037/a0014437. [DOI] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Process analysis: estimating mediation in treatment evaluations. Eval. Rev. 1981;5:602–619. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. Circle Pines, MN: AGS; 1990. [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum. Neurosci. 2008;2:1–15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison C, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Aging, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl Acad. Sci. USA. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J. Neurosci. Res. 1999;58:176–180. [PubMed] [Google Scholar]
- Ludwig DS. Childhood obesity: the shape of things to come. N Engl J. Med. 2007;357:2325–2327. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Ann. Rev. Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. New Jersey: M Lawrence Erlbaum Associates; 2005. p. 445. [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: activation of the right hippocampus in taxi drivers. J. Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper S, Gomez-Pinilla F, Choi J, Cotman CW. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J. Med. 2005;352:1138–1143. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Patenaude B. D. Phil. Thesis. University of Oxford; 2007. Bayesian statistical models of shape and appearance for subcortical brain segmentation. [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. FIRST-FMRIB’s integrated registration and segmentation tool. Human Brain Mapping Conference. 2007a [Google Scholar]
- Patenaude B, Smith SM, Kennedy D, Jenkinson M. Technical report TR07BP1. FMRIB Center, University of Oxford; 2007b. Bayesian shape and appearance models. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Scheltens P, Machielson WC, Barkhof F, Hoogenraad FG, Veltman DJ, Valk J, Witter MP. Parametric fMRI analysis of visual encoding in the human medial temporal lobe. Hippocampus. 1999;9:637–643. doi: 10.1002/(SICI)1098-1063(1999)9:6<637::AID-HIPO4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat. Space Environ. Med. 1990;61:3–11. [PubMed] [Google Scholar]
- Sibley BA, Etnier JL. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr. Exerc. Sci. 2003;15:243–256. [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol. Methodol. 1982;13:290–312. [Google Scholar]
- Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Publications; 1962. p. 340. [Google Scholar]
- Taylor SJC, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Roberson RJ, Nieman DC, Kang J. Children’s OMNI scale of perceived exertion: walking/running evaluation. Med. Sci. Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Neurobiology. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]