Abstract
Transcriptional coactivator with PDZ-binding motif (TAZ) is known to bind to a variety of transcription factors to control cell differentiation and organ development. We examined TAZ protein levels in 146 stage II–IV gastric cancer using immunohistochemistry (IHC), while TAZ mRNA was confirmed by quantitative reverse-transcription polymerase chain reaction (QRT-PCR) in 84 samples with enough tissue. TAZ protein expression was positive in 113 out of 146 (77.4%) gastric cancer samples. In parallel, TAZ mRNA expression was successfully detected in 81 of the 84 (96.4%) samples. Protein levels of TAZ were positively correlated with its mRNA levels (P = 0.018). High expression of TAZ protein was observed with higher percentage in gastric cancer samples with histology of signet ring cell carcinoma (SRCC) than adenocarcinoma (85.7% versus 60.2%, P = 0.001). Similarly, TAZ mRNA level was higher in SRCC than in adenocarcinoma (P = 0.003). When correlated with survival, the median overall survival (OS) is 14 months (95% CI: 12.2–15.8 months) in all patients. There was no significant association between survival and other clinical characteristics or TAZ expression levels. Our results show that TAZ is highly expressed in SRCC. TAZ might be considered as a target for the treatment of gastric SRCC in future.
1. Introduction
Gastric cancer is the fourth most common cancer with the leading causes of cancer death in East Asian countries and some western countries [1, 2]. Signet ring cell carcinoma (SRCC) is characterized by cells with abundant mucin in the cytoplasm and nuclei located at the cell periphery. This type of carcinoma appears to be relatively frequent in women and young patients [3, 4]. It has long been thought to have a worse prognosis than other forms of gastric cancer. Recently, studies have begun to question this idea. Several studies find that the survival of patients with early SRCC was not significantly different from other types of gastric carcinoma [5]. This was because SRCC of the stomach is less likely to lymph node metastasis and it had a higher proportion in the early stage of gastric carcinoma than other carcinomas [6, 7]. The genetic background of SRCC has rarely been investigated, and the molecular basis of their growth, differentiation, and metastasis still remains unclear. Therefore, studies of the molecular profile of gastric SRCC and identification of new molecular markers are both relevant to improve the diagnosis and the prognosis of the tumor.
Transcriptional coactivator with PDZ-binding motif (TAZ), also called WW-domain containing transcription regulator 1 (WWTR1), has been defined for its role in the nucleus [8–10]. It functions directly as a transcriptional regulator by interacting with several nuclear factors and plays a central role in the Hippo pathway, which regulates the size and shape of organ development [8–12]. TAZ was described as controlling gene important for muscle differentiation, lung and respiratory epithelia differentiation, cardiac and limb development, adipogenesis and osteogenesis, and tumorigenesis. Most human tissues, except thymus and peripheral blood leucocytes, express TAZ mRNA, with the highest levels in kidney, heart, placenta, and lung [8–12]. TAZ has been identified as an oncogene and has an important role in tumorigenicity of many cancers, such as non-small cell lung cancer [13, 14], papillary thyroid carcinoma [15], and colon cancer [16]. They found that TAZ gene expression signature was over-represented in poorly differentiated tumors compared with well-differentiated low-grade tumors. Importantly, TAZ confers cancer stem cell-related traits in breast cancer cells [17–19], further highlighting its importance in tumor initiation and progression. According to present studies, TAZ is significantly associated with poor survival of cancer, so TAZ may be a novel prognostic indicator for cancer progression. But so far, no report has been published concerning the relationships between TAZ expression and clinicopathological features and prognosis of gastric cancer patients. Therefore, the objectives of this study were to evaluate the relationships between TAZ expression and the clinicopathological parameters of gastric cancer and to evaluate its potential role as a prognostic biomarker and an anticancer target.
2. Materials and Methods
146 gastric samples were collected from patients of the Comprehensive Cancer Center, Drum Tower Hospital Affiliated to Medical School of Nanjing University, from November 2007 to August 2011. All samples have been pathologically proven to be cancer. TAZ protein levels were examined by IHC in 146 samples. Meanwhile, TAZ mRNA levels were confirmed by quantitative reverse-transcription polymerase chain reaction (QRT-PCR) in 84 samples with enough tissue. This project has been approved by Institutional Review Board of Drum Tower Hospital.
2.1. Immunohistochemical Staining for TAZ
After dewaxing in xylene and rehydrating stepwise in ethanol, sections were subjected to heat-induced antigen retrieval. The endogenous peroxidase activity was inactivated in a solution containing 3% hydrogen peroxide (H2O2) in methanol. In the negative control, the primary antibody was omitted. Skeletal muscle was used as positive control. Pretreated sections were incubated with rabbit polyclonal TAZ antibody (T3467, 1 : 50, Epitomics) at 4°C overnight, followed by secondary antibody. Immunohistochemical staining was evaluated independently by two pathologists without knowledge of patient characteristics, and discrepancy was resolved by consensus review. Tissue was scored (H score) based on the total percentage of positive cells ((≤5%) = 0, (6%~25%) = 1, (26%~50%) = 2, (51%~75%) = 3, and (>75%) = 4) and the intensity of the staining (0, 1, 2, or 3), where H is the percentage of positive score multiply intensity score. The sample was considered negative if H = 0 and positive if H was more than 0. Positive samples were also categorized as weak (1+) if H = 1 to 4, middle (2+) if H = 5 to 8, and strong (3+) if H was more than 8 [20]. A minimum of 100 cells were evaluated in calculating the H score. Patients with negative or weak staining were considered as lower group, while patients with middle and strong staining were considered as higher group.
2.2. Quantitative Reverse-Transcription Polymerase Chain Reaction (QRT-PCR) Assessment of TAZ Expression
Three 5 μm sections were prepared from FFPE tumor blocks that contained at least 80% tumor cells. After hematoxylin-eosin staining, RNA was isolated in accordance with a proprietary procedure as we published before [21]. Briefly, paraffin was removed by xylene, and macrodissected tissues were lysed in a proteinase K-containing buffer at 60°C for 16 h. RNA was purified by phenol and chloroform extractions followed by precipitation with isopropanol in the presence of sodium acetate at −20°C. The RNA pellet was washed in 70% ethanol and resuspended in RNase-free water followed by DNase. M-MLV Reverse Transcriptase Kit (Ambion, Carlsbad, CA) was used to generate cDNA for quantitative reverse-transcription polymerase chain reaction (QRT-PCR) to detect the expression of β-actin (used as endogenous control) and TAZ. Commercial human total RNA was used for each RT reaction as calibrator. Template cDNA was amplified with specific primers and probes (Table 1) for β-actin and TAZ using TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA). The QRT-PCR was performed to quantify gene expression using ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The PCR conditions were 50°C for 2 min and 95°C for 15 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Relative gene expression quantifications were calculated according to the comparative Ct method [21] and analyzed with the Applied Biosystems analysis software. TAZ mRNA levels were further divided into three groups according to tercile levels.
Table 1.
Primers and probes of TAZ and β-actin.
| Primer | TAZ | β-actin |
|---|---|---|
| Forward primer | 5′ CCAGTGCCTCAGAGGTCCA 3′ | 5′ TGAGCGCGGCTACAGCTT 3′ |
| Reverse primer | 5′ ATCTGCTGCTGGTGTTGGTG 3′ | 5′ TCCTTAATGTCACGCACGATTT 3′ |
| Probe | 6FAM 5′ CCAAATCTCGTGATGAAT 3′ MGB | 6FAM 5′ ACCACCACGGCCGAGCGG 3′ TAMRA |
2.3. Statistical Analysis
Correlations between TAZ protein expression and clinicopathological parameters were analyzed by χ 2 test. Correlations between TAZ protein expression and mRNA were also analyzed by χ 2 test. The Mann-Whitney U test and the Kruskal-Wallis test were used to test the associations between TAZ mRNA levels and clinical characteristics. Survival curves were assessed by the Kaplan-Meier method. Two-sided P < 0.05 was considered statistically significant. All analyses were performed with the SPSS 17.0 software package (SPSS Inc., Chicago, USA).
3. Results
A total of 111 males and 35 females were included with ages ranging from 24 to 92 years (median, 61 years). Eighty-three patients (56.8%) with the histology of adenocarcinoma and 63 patients (43.2%) were confirmed as signet ring cell carcinoma. There were 6 patients (4.1%) with stage II (4 stage IIA and 2 stage IIB), 136 patients (93.2%) with stage III (22 stage IIIA, 36 stage IIIB, and 80 stage IIIC), and 4 patients (2.7%) with stage IV disease. 40 patients received 5-FU and/or oxaliplatin-based chemotherapy. The median follow-up time was 14.3 months (95% CI = 2.53 to 27.5 months).
3.1. Relationship between TAZ Protein Expression and mRNA Expression
TAZ protein expressions were positive in 113 of 146 (77.4%) samples. TAZ had nuclear and cytoplasmic expression (Figure 1). In parallel, 84 samples had enough tissue to detect TAZ mRNA. TAZ mRNA expression was found in 81 of the 84 (96.4%) samples. In TAZ mRNA low expression group, 44.8% of patients had low level of TAZ protein. Protein levels of TAZ were correlated with its mRNA levels (P = 0.018). There were 88.5% of patients with high TAZ protein levels in TAZ mRNA high group, 76.9% of patients with high TAZ protein levels in mRNA intermediate group, and 55.2% of patients with high TAZ protein levels in mRNA low group (Table 2).
Figure 1.
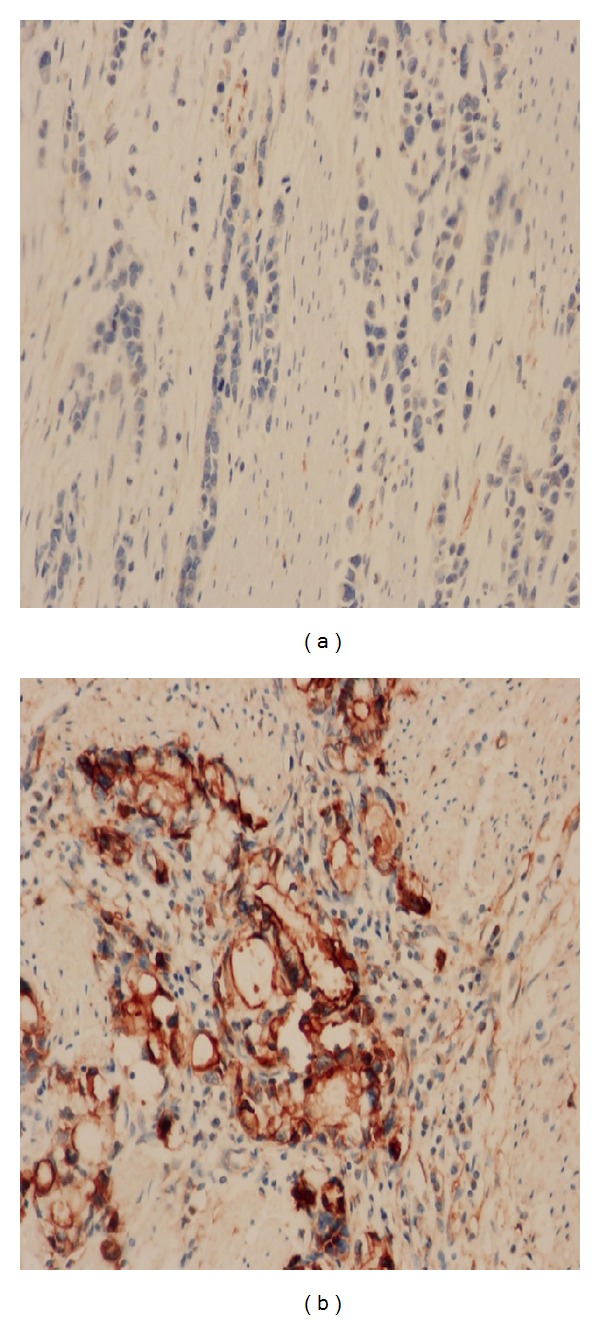
TAZ protein expression in gastric cancer. (a) Negative case at high magnification (×400). (b) Positive case (H score = 12) with signet ring cell phenotype at high magnification (×400).
Table 2.
TAZ protein levels and mRNA levels.
| TAZ mRNA | Protein low expression (IHC: 0-1+) | Protein high expression (IHC: 2+-3+) | P |
|---|---|---|---|
| Low expression | 13 (44.8%) | 16 (55.2%) | 0.018 |
| Intermediate expression | 6 (23.1%) | 20 (76.9%) | |
| High expression | 3 (11.5%) | 23 (88.5%) |
3.2. Relationship between TAZ Protein Expression and Clinicopathological Characteristics
TAZ protein levels were higher in SRCC than in adenocarcinoma (P = 0.001) and higher in Grade 3 cancer than in Grade 2 cancer (P = 0.004). However, there was no difference between TAZ protein levels and age (P = 0.294), gender (P = 0.376), tumor site (P = 0.159), lymph node metastasis (P = 0.232), or stage (P = 0.785) (Table 3).
Table 3.
The relationship between TAZ and clinicopathological characteristics.
| Characteristics | TAZ protein levels | P | TAZ mRNA levels | P | |
|---|---|---|---|---|---|
| low IHC (0~1+) | High IHC (2+~3+) | ||||
| Age | 0.294 | 0.374 | |||
| <60 | 15 (24.2%) | 47 (75.8%) | 4.45 ± 4.84 | ||
| ≥60 | 27 (32.1%) | 57 (67.9%) | 3.92 ± 4.93 | ||
| Sex | 0.376 | 0.696 | |||
| Female | 8 (22.9%) | 27 (77.1%) | 4.71 ± 6.44 | ||
| Male | 34 (30.6%) | 77 (69.4%) | 3.99 ± 4.32 | ||
| Histology | 0.001 | 0.003 | |||
| Adenocarcinoma | 33 (39.8%) | 50 (60.2%) | 3.15 ± 3.25 | ||
| SRCC | 9 (14.3%) | 54 (85.7%) | 6.71 ± 7.02 | ||
| Tumor site | 0.159 | 0.069 | |||
| Distal stomach | 13 (26.5%) | 36 (73.5%) | 5.43 ± 6.02 | ||
| Proximal stomach | 17 (41.5%) | 24 (58.5%) | 2.2 ± 2.31 | ||
| Whole stomach | 11 (22.9%) | 37 (77.1%) | 4.37 ± 4.82 | ||
| Unknown | 1 (12.5%) | 7 (87.5%) | 2.94 ± 1.51 | ||
| Lymph node | 0.232 | 0.899 | |||
| N0-1 | 8 (40%) | 12 (60%) | 4.8 ± 6.99 | ||
| N2-3 | 34 (27%) | 92 (73%) | 4.04 ± 4.45 | ||
| Stage | 0.785 | 0.492 | |||
| II | 1 (16.7%) | 5 (83.3%) | 3.60 ± 2.30 | ||
| III | 40 (29.4%) | 96 (70.6%) | 4.24 ± 5.05 | ||
| IV | 1 (25%) | 3 (75%) | 2.80 ± 2.59 | ||
| Histological grade | 0.004 | 0.375 | |||
| G2 | 16 (48.5%) | 17 (51.5%) | 4.05 ± 5.86 | ||
| G3 | 26 (23%) | 87 (77%) | 4.19 ± 4.54 | ||
3.3. Relationship between mRNA Expression and Clinicopathological Characteristics
TAZ mRNA level in signet ring cell carcinoma was higher than adenocarcinoma (median levels: 4.64 versus 2.02, P = 0.003). However there was no difference between TAZ mRNA levels and patients' age (P = 0.374), gender (P = 0.696), tumor site (P = 0.069), lymph node metastasis (P = 0.899), p-TNM stage (P = 0.492), or histological grade (P = 0.375) (Table 3).
3.4. Survival for Gastric Cancer Patients According to TAZ Protein and mRNA Levels
The median overall survival (OS) is 14 months (95% CI = 12.2 to 15.8 months) in all patients. The median OS is longer in younger patients (16.9 months, 95% CI = 11.9–22.6 months) than in elder patients (12.4 months, 95% CI = 9.3–14.9). Patients with stage II had a longer OS (23.25 months, 95% CI = 10.4–36.1 months) than stage III (14 months, 95% CI = 12.4–15.7 months) and stage IV (5.1 months, 95% CI = 4.6-5.6 months). There was no significant association between OS and gender (P = 0.652), tumor site (P = 0.312), differentiation (P = 0.477), lymph node metastasis (P = 0.294), TAZ protein levels (P = 0.481), or TAZ mRNA levels (P = 0.132) (Table 4).
Table 4.
The median overall survival for patients according to TAZ levels.
| Characteristics | Number of patients | Median overall survival (months) (95% CI) | P |
|---|---|---|---|
| Age | 0.029 | ||
| <60 | 61 (41.8%) | 16.9 (11.9–22.6) | |
| ≥60 | 85 (58.2%) | 12.4 (9.3–14.9) | |
| Sex | |||
| Female | 35 (24.0%) | 14.8 (8.3–21.4) | |
| Male | 111 (76.0%) | 14.0 (12.6–15.5) | |
| Tumor site | 0.312 | ||
| Distal stomach | 49 (33.6%) | 14.1 (10.4–17.9) | |
| Proximal stomach | 40 (27.3%) | 13.7 (12.8–14.6) | |
| Whole stomach | 49 (33.6%) | 14.1 (9.8–18.3) | |
| Unknown | 8 (5.5%) | 6.7 (4.8–8.5) | |
| Lymph node | 0.294 | ||
| N0~1 | 20 (13.7%) | 18.1 (8.7–27.5) | |
| N2~3 | 126 (86.3%) | 14.0 (12.2–15.8) | |
| Stage | 0.029 | ||
| II | 6 (4.1%) | 23.25 (10.4–36.1) | |
| III | 136 (93.2%) | 14.0 (12.4–15.7) | |
| IV | 4 (2.7%) | 5.1 (4.6–5.6) | |
| Histological grade | 0.477 | ||
| G2 | 33 (22.6%) | 12.9 (10.1–15.7) | |
| G3 | 113 (77.4%) | 14.2 (11.9–16.5) | |
| TAZ protein expression | 0.481 | ||
| Low expression | 42 (28.8%) | 13.7 (10.9–16.5) | |
| High expression | 104 (71.2%) | 14.1 (11.4–16.7) | |
| TAZ mRNA expression | 0.132 | ||
| Low expression | 29 (35.8%) | 8.1 (5.2–10.9) | |
| Intermediate expression | 26 (32.1%) | 14.0 (12.6–15.5) | |
| High expression | 26 (32.1%) | 9.6 (6.5–12.6) |
4. Discussion
The Hippo pathway plays an important role in cell proliferation, organ size control, and cancer development and progression. TAZ is a transcriptional coactivator that is inhibited by Hippo pathway [22, 23]. Aberrant inactivation of the Hippo pathway and/or overexpression of TAZ results in transcriptional activation of their downstream targets. TAZ overexpression induces cell proliferation and epithelial-mesenchymal transition (EMT) and inhibits apoptosis and contact inhibition [24, 25]. EMT is a process in which cells lose epithelial-like characteristics, such as cell-cell adhesion and polarity, and acquire mesenchymal properties that include increased motility. Most carcinomas exhibit a partial EMT, which is thought to promote the formation of cell populations that are enriched in cancer stem cells (CSCs). Cordenonsi et al. [19] found that TAZ was required to sustain self-renewal of breast CSCs and to induce their tumorigenic potential. And most interestingly, TAZ was overrepresented in poorly differentiated breast tumors compared with well-differentiated ones. TAZ protein levels increase during EMT and that this is required for mammosphere formation, which is also promoted by EMT. Bhat et al. [26] found that TAZ expression was lower in proneural glioblastomas (GBMs) and lower grade gliomas compared with GBMs that had a mesenchymal phenotype. TAZ expression in GBMs is positively correlated with the expression for mesenchymal genes and is also predictive of poor overall survival. Moreover, TAZ is significantly associated with poor survival of colon cancer patients in two independent colon cancer datasets, comprising 522 patients [16]. In present study, we successfully detected and compared TAZ protein and mRNA expressions in gastric tumor tissues (Table 2) and correlated TAZ levels with clinicopathological parameters and survival (Table 3).
We also found that TAZ was higher expressed in SRCC than adenocarcinoma in either protein or mRNA levels (Table 3). SRCC has long been thought to have a worse prognosis than other forms of gastric cancer. Recently, SRCC has been known to have different biologic characteristics between early stage and advanced stage gastric cancer. In early gastric cancer, SRCC has been reported to have better prognosis than others because of less lymph node metastasis and a more grossly depressed type, which is helpful for diagnosis. However, in advanced gastric cancer, SRCC has been characterized to be a more grossly infiltrative type, although the reason is still unclear. Few molecular markers had been proven to have relationship with SRCC, such as the M2 isoform of pyruvate kinase (PKM2), bone morphogenetic proteins (BMP-7), and transcriptional factor forkhead box P3 (FoxP3). PKM2 was identified as a driver of aerobic glycolysis and has been shown to be the isoform preferentially overexpressed in tumor cells. Well and moderately differentiated adenocarcinoma showed significantly higher expression of PKM2 than SRCC. PKM2 protein expression was found to negatively correlate with survival in SRCC patients [27]. BMP-7 is signaling molecule belonging to the transforming growth factor (TGF) superfamily. Recent studies demonstrated that BMP-7 expression is found in various human cancers and regulates cell differentiation, proliferation, migration, invasion, and apoptosis [28]. BMP-7 expression was significantly higher in the differentiated histology group than in the undifferentiated group. And the BMP-7 positive group had significantly poorer survival than the BMP-7 negative group in the undifferentiated group. The key role of FoxP3 is induction of immunesuppressive function to maintain self-tolerance. It is widely accepted that FoxP3 is expressed not only in mice and humans but also in tumor cells such as melanoma stomach and might have relationship with immunosuppressive effect. Yoshii, et al [29] demonstrated that FoxP3 was expressed in SRCC. FoxP3 would allow them to escape from immune surveillance, thereby resulting in cancer progression such as lymph node metastasis. But the molecular pathogenesis of SRCC remains largely unknown. In present study, we find that TAZ expression was higher in SRCC than in adenocarcinoma for the first time. We hypothesize that TAZ might participate in tumorigenesis and development of signet ring cells. However, future studies were more needed. Our results show the way for future studies aiming to reveal additional insights into the molecular mechanisms of signet ring cell. Since TAZ was reported to bind to a variety of transcription factors to control cell differentiation and organ development, such as p73 (p53 family member), Runx2 (runt family member 2), PPAR γ (peroxisome prolif-erator-activated receptor γ), TTF-1 (thyroid transcription factor-1), Pax3 (paired box 3), Tbx5 (T-box 5), Smad2/3/4 (SMAD family member 2/3/4), and TEAD [8–10]. In present study, the TAZ protein is mainly accumulated in the nucleus with a less cytoplasmic presence. TAZ might be considered as a novel target for the treatment of gastric cancer, especially in SRCC. However, in the present study, the sample size is rather limited and the distribution between different stages is also scattered, which might be the reason that we did not find any correlations between TAZ and stage or prognosis. Further studies with larger number of patients were warranted to valid utility of TAZ in gastric cancer patients.
Acknowledgments
This work was funded by grants from the National Natural Science Foundation of China (Grant nos. 81000980, 81220108023, and 81370064), Jiangsu Provincial Program of Medical Science (BL2012001), and the Distinguished Young Investigator Project of Nanjing (JQX12002). The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Author's Contribution
Guofeng Yue and Xia Sun contributed equally to this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal for Clinicians. 2011;61(2):p. 134. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cho JY, Lim JY, Cheong JH, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clinical Cancer Research. 2011;17(7):1850–1857. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hass HG, Smith U, Jäger C, et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): single-center experience of 160 cases. Onkologie. 2011;34(12):682–686. doi: 10.1159/000334545. [DOI] [PubMed] [Google Scholar]
- 4.Taghavi S, Jayarajan SN, Davey A, et al. Prognostic significance of signet ring gasrtric cancer. Journal of Clinical Oncology. 2012;30(28):3493–3498. doi: 10.1200/JCO.2012.42.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. Journal of Gastrointestinal Surgery. 2010;14(4):601–606. doi: 10.1007/s11605-009-1127-9. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Kim S, Lai JF, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72(1-2):64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 7.Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Annals of Surgery. 2009;250(6):878–887. doi: 10.1097/SLA.0b013e3181b21c7b. [DOI] [PubMed] [Google Scholar]
- 8.Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO Journal. 2000;19(24):6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(50):18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Palma T, D’Andrea B, Liguori GL, et al. TAZ is a coactivator for Pax8 and TTF-1, two transcription factors involved in thyroid differentiation. Experimental Cell Research. 2009;315(2):162–175. doi: 10.1016/j.yexcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Varelas X, Sakuma R, Samavarchi-Tehrani P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biology. 2008;10(7):837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 12.Lei Q-Y, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Molecular and Cellular Biology. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Hao Y, Liu N, Raptis L, Tsao M-S, Yang X. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011;30(18):2181–2186. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 14.Xie M, Zhang L, He CS, et al. Prognostic significance of TAZ expression in resected non-small cell lung cancer. Journal of Thoracic Oncology. 2012;7(5):799–807. doi: 10.1097/JTO.0b013e318248240b. [DOI] [PubMed] [Google Scholar]
- 15.De Cristofaro T, Di Palma T, Ferraro A, et al. TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. European Journal of Cancer. 2011;47(6):926–933. doi: 10.1016/j.ejca.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Yuen HF, McCrudd CM, Huang YH, et al. TAZ Expression as prognostic indicator in colorectal cancer. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054211.54211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao D, Zhi X, Zhou Z, Chen C. TAZ antagonizes the WWP1-mediated KLF5 degradation and promotes breast cell proliferation and tumorigenesis. Carcinogenesis. 2012;33(1):59–67. doi: 10.1093/carcin/bgr242. [DOI] [PubMed] [Google Scholar]
- 18.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Research. 2011;71(7):2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 19.Cordenonsi M, Zanconato F, Azzolin L, et al. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann KC, Sarbia M, Weber A-A, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Research. 1999;59(1):198–204. [PubMed] [Google Scholar]
- 21.Wei J, Costa C, Ding Y, et al. MRNA expression of BRCA1, PIAS1, and PIAS4 and survival after second-line docetaxel in advanced gastric cancer. Journal of the National Cancer Institute. 2011;103(20):1552–1556. doi: 10.1093/jnci/djr326. [DOI] [PubMed] [Google Scholar]
- 22.Pan D. The hippo signaling pathway in development and cancer. Developmental Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, Li L, Lei Q, Guan K-L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes and Development. 2010;24(9):862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siew WC, Chun JL, Guo K, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Research. 2008;68(8):2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Liu C-Y, Zha Z-Y, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. Journal of Biological Chemistry. 2009;284(20):13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat KPL, Salazar KL, Balasubramaniyan V, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes and Development. 2011;25(24):2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim JY, Yoon SO, Seol SY, et al. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World Journal of Gastroenterology. 2012;18(30):4037–4043. doi: 10.3748/wjg.v18.i30.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoki M, Ishigami S, Uenosono Y, et al. Expression of BMP-7 in human gastric cancer and its clinical significance. British Journal of Cancer. 2011;104(4):714–718. doi: 10.1038/sj.bjc.6606075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshii M, Tanaka H, Ohira M, et al. Expression of Forkhead box P3 in tumor cells causes immunregulatory function of signet ring cell carcinoma of the stomach. British Journal of Cancer. 2012;106(10):1668–1674. doi: 10.1038/bjc.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]


