Abstract
Glucagon-like peptide-1 (GLP-1) secretion is greatly enhanced after Roux-en-Y gastric bypass (RYGB). While intact GLP-1exerts its metabolic effects via the classical GLP-1 receptor (GLP-1R), proteolytic processing of circulating GLP-1 yields metabolites such as GLP-1(9–36)amide/GLP-1(28–36)amide, that exert similar effects independent of the classical GLP-1R. We investigated the hypothesis that GLP-1, acting via these metabolites or through its known receptor, is required for the beneficial effects of RYGB using two models of functional GLP-1 deficiency – α-gustducin-deficient (α-Gust−/−) mice, which exhibit attenuated nutrient-stimulated GLP-1 secretion, and GLP-1R-deficient mice. We show that the effect of RYGB to enhance glucose-stimulated GLP-1 secretion was greatly attenuated in α-Gust−/− mice. In both genetic models, RYGB reduced body weight and improved glucose homeostasis to levels observed in lean control mice. Therefore, GLP-1, acting through its classical GLP-1R or its bioactive metabolites, does not seem to be involved in the effects of RYGB on body weight and glucose homeostasis.
Abbreviations: α-Gust−/−, α-gustducin deficient mice; GLP-1, glucagon-like peptide-1; GLP-1R, glucagon-like peptide-1 receptor; Glp1r−/−, glucagon-like peptide-1 receptor deficient mice; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; PF-sham, pair-fed sham; RYGB, Roux-en-Y gastric bypass; WM-sham, weight-matched sham; WT, wild-type
Keywords: Weight-loss surgery, Gut hormones, Mouse model, Taste perception
1. Introduction
Bariatric surgery is the most effective treatment for weight loss and randomized, controlled trials demonstrate its superiority compared to medical therapy for diabetes control [1,2]. RYGB is the most commonly performed bariatric procedure in the U.S. and one of the most efficacious [3,4]. Several clinical observations suggest that the gut-derived peptide hormone glucagon-like peptide-1 (GLP-1) is responsible for the beneficial effects of RYGB on glucose and energy homeostasis. First, GLP-1 secretion is substantially enhanced early after RYGB, independent of weight loss and calorie restriction [5,6]. Second, enhanced meal-stimulated insulin secretion and β-cell glucose sensitivity observed after RYGB are suppressed by the GLP-1R antagonist Exendin (9–39) [7–10]. Third, administration of octreotide to patients after RYGB inhibits the secretion of GLP-1 (and other gut hormones), and also decreases satiety and increases food intake [5]. In rodents undergoing RYGB and other models of bariatric surgery, food intake is also increased by octreotide and glucose tolerance worsened by Exendin (9–39) [11–15]. Also in rodents, GLP-1 responsiveness predicts improved glucose tolerance after RYGB [16]. An understanding of the role of GLP-1 during RYGB will facilitate the development of less-invasive therapies for weight loss and metabolic control that can be more broadly applied than surgery.
GLP-1 is a peptide hormone secreted by entero-endocrine L-cells of the intestine and colon. In addition to its incretin effect (i.e., increased insulin secretion in response to oral versus intravenous glucose delivery), GLP-1 delays gastric emptying, enhances satiety, reduces food intake, suppresses glucagon secretion, and regulates hepatic and peripheral glucose flux [17,18]. These effects occur via the GLP-1 receptor (GLP-1R), which is widely expressed in the intestine, pancreatic islets, and central and peripheral nervous systems [19], and are clinically meaningful as GLP-1 mimetics are used to treat type 2 diabetes and also confer weight loss [20]. GLP-1 circulates as two equipotent forms, GLP-1(7–37) and GLP-1(7–36)amide [18]. Intact GLP-1(7–36)amide is rapidly metabolized to GLP-1(9–36)amide, which in turn can be further cleaved to GLP-1(28–36)amide, and both of these peptides also improve glucose homeostasis and control body weight in experimental models of diabetes and obesity [21,22]. GLP-1(9–36)amide and GLP-1(28–36)amide mediate their effects independent of the classical GLP-1R [21,23]. Thus, GLP-1 exerts its beneficial effects through both classical GLP-1R (via intact GLP-1) and GLP-1R-independent mechanisms (via these bioactive GLP-1 derivatives) suggesting that loss of function experimental studies should address both.
We hypothesized that GLP-1 is required for the effects of RYGB on body weight, body composition, and glucose homeostasis. GLP-1 is one of several peptide hormones of known metabolic function derived by post-translational processing of the precursor proglucagon [18], complicating the generation of specific GLP-1 deficient mice. We therefore chose to test our hypothesis by performing RYGB in two models of functional GLP-1 deficiency, α-gustducin deficient (α-Gust−/−) mice and GLP-1R deficient (Glp1r−/−) mice. The G-protein α-subunit α-gustducin couples sweet and bitter taste receptors of the lingual epithelium to intracellular signaling and taste perception [24]. α-Gustducin is also co-expressed with GLP-1 in L-cells and has been implicated in GLP-1 secretion in response to extracellular glucose, bile acids, and fatty acids [25,26]. In α-Gust−/− mice, the αβγ-gustducin heterotrimer does not form and any signals mediated by its coupled receptors are lost [27]. In response to oral glucose, α-Gust−/− mice do not exhibit elevated serum GLP-1 observed in wild-type mice resulting in blunted glucose-stimulated plasma insulin and abnormal glucose tolerance [25]. In addition, ghrelin secretion and octanoylation induced by bitter taste receptor agonists is attenuated and glucose-dependent insulinotropic polypeptide secretion occurs earlier in response to glucose in α-Gust−/− mice compared to wild-type [25,28,29]. The roles of these hormones in the effects of bariatric surgery are controversial [30,31]. However, their altered secretions could compensate for the attenuated GLP-1 secretion of α-Gust−/− mice. Nonetheless, as GLP-1(9–36)amide and GLP-1(28–36)amide are derived exclusively from intact GLP-1, α-Gust−/− mice afford the unique opportunity to investigate classical GLP-1R-independent functions of GLP-1 occurring via these bioactive GLP-1 peptide derivatives. Glp1r−/− mice lack the classical GLP-1R and exhibit impaired glucose tolerance and impaired glucose-stimulated insulin secretion [32]. We therefore used α-Gust−/− mice to investigate the functional consequences of blunted GLP-1 secretion, thereby lowering levels of intact GLP-1 and consequently its bioactive metabolites, and Glp1r−/− mice to investigate the consequences of the absence of GLP-1 signaling via its classical GLP-1R function on the effects of RYGB.
2. Materials and methods
2.1. Animals
All studies were conducted in accordance with the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee. Lean and diet-induced obese C57BL/6 (WT) mice were obtained from Taconic. Glp1r−/− and α-Gust−/− mice were generated as previously described [32,33]. Animals were provided high fat diet (D12492; Research Diets, Inc.) or regular chow (Teklad), as specified. All mice were back-crossed into a pure C57BL/6 background, housed individually, and maintained on a 12-hr light-dark cycle.
2.2. Surgery
RYGB and sham procedures were performed after an overnight fast as previously described [34]. RYGB involved reconstruction connecting a restricted gastric pouch to a jejunal alimentary limb. The proximal gut was excluded from alimentary flow using a hemostasis clip (Ethicon Endosurgery) placed immediately distal to the gastrojejunostomy. The sham procedure involved gastrotomy, enterotomy and repair. Anesthesia was provided using a scavenged circuit of isoflurane and time-standardized between groups. Mice were maintained on a standardized re-feeding protocol during which liquid diet was provided on post-operative days 2–7. On days 6 and 7, .25 g of high fat diet was also provided. From day 8 through the end of the study, high fat diet was provided ad libitum (RYGB and sham mice) or calorie-restricted where indicated.
2.3. Study design
Male mice were maintained on high fat diet from 6 weeks of age and, upon reaching 45 g, randomized to RYGB or sham procedures. The pre-operative weights of mice from all intervention groups are presented in Supplementary Table 1 for comparison. Where specified, subsets of sham-operated mice were pair-fed (PF-sham) or weight-matched by calorie restriction (WM-sham) to RYGB counterparts of the same genotype. Non-operated, age-matched, C57BL/6 mice maintained on regular chow were evaluated in parallel as lean controls. Energy balance was evaluated from post-operative week 1 through 5. As we have previously published [34], RYGB and sham operations both induce substantial post-operative weight loss due to the combined effects of peri-operative stress and calorie restriction. Sham-operated mice do not regain their full pre-operative body weight and obtain the weight of non-operated diet-induced obese mice until approximately week 5. Thus, studies conducted before week 5 are confounded by the metabolic effects of post-operative convalescence. For this reason, glucose homeostasis was evaluated during weeks 6–8. To circumvent this issue, WM-sham mice were studied in parallel to evaluate for weight-independent gluco-regulatory effects of RYGB. Stimulated GLP-1 levels were measured in WT and α-Gust−/− mice from tail vein blood during week 9; fasted levels were measured from blood collected at the time of sacrifice. All animals were sacrificed during week 10.
2.4. Energy balance
Total 24-h energy expenditure was estimated using an energy balance technique in which change in energy stores are subtracted from energy intake over time. This methodology has been validated versus indirect calorimetry for the accurate measure of long-term changes in energy expenditure [35]. Over a period of 27 days (from the beginning of post-operative week 2 through the end of week 5), body weight and food intake were measured daily. Body composition was measured during weeks 2 and 5 using nuclear magnetic resonance (Bruker Minispec mq10, Bruker Optics). So as not to disturb the energy balance experiment, stool from a separate cohort of RYGB-treated and sham-operated α-Gust−/− mice was collected, dried, and weighed on a daily basis during weeks 2 and 5 to measure total fecal calories by bomb calorimetry, as previously described [34].
Metabolizable energy intake, defined as grams of high fat diet ingested per day multiplied by 22.0 kJ/g, was calculated from the food intake measurements. Feeding efficiency was calculated by dividing weight change (in mg) by food consumed (in kJ). The contribution of reduced absorption to energy balance was evaluated using adjusted feeding efficiency, in which total intake was multiplied by the percentage of total calorie absorption. Total calorie absorption used to calculate adjusted feeding efficiency in WT mice was previously determined [34]. In this methodology, mean total 24-h energy expenditure (kJ/d) represents the consumed energy remaining for physical activity and basal metabolism after accounting for changes in body mass [35–38]. The energetic cost of fat mass and lean mass deposition was estimated using values of 55.3 kJ/g and 9.2 kJ/g, respectively and the energy contents of fat and lean mass were estimated as 37.7 kJ/g and 4.2 kJ/g, respectively [36,39]. When animals lost weight, there was no energetic cost of deposition and the body energy lost was subtracted from the energy equation [35,37,38].
2.5. Glucose homeostasis
2.5.1. Basal
Fasting blood glucose was measured from the tail vein of mice fasted overnight (1700–1000) using a hand-held glucometer (Bayer Healthcare). Plasma insulin was also measured from tail vein blood using an ultra-sensitive mouse insulin ELISA (Crystal Chem). Homeostasis Model Assessment-Insulin Resistance (HOMA-IR), a mathematical model approximating treatment effects on insulin homeostasis that has been validated in humans and rodents, was calculated as previously described [40].
2.5.2. Glucose tolerance
In fasted mice (1700–1000), blood glucose level was measured from tail vein blood collected before and at the indicated times after administration of 1 g/kg d-glucose (Sigma-Aldrich) by oral gavage with a feeding tube.
2.5.3. Glucose-stimulated plasma insulin
Tail vein blood was collected before, 15 and 30 min after oral glucose gavage with a feeding tube to measure plasma insulin as described above.
2.5.4. Insulin tolerance
Four hour fasted mice (0900–1300) were administered .75 U/kg insulin (Eli Lilly) by intraperitoneal injection. Blood glucose was measured from tail vein blood using a hand-held glucometer before and at the indicated times after injection.
2.6. GLP-1 measurement
Peripheral plasma active GLP-1 was measured after an overnight fast (1700–1000) and five minutes after oral gavage of 1.5 g/kg d-Glucose [41]. Glucose was administered via a feeding tube to avoid stimulation of the taste buds of the lingual epithelium. Blood was collected into EDTA coated Microvettes (SARSTEDT) preloaded with enzyme inhibitor cocktail containing .5 M EDTA (Fisher), 283 μM aprotinin (Sigma-Aldrich), 10,000 U/mL heparin (Sigma-Aldrich), and 1.265 mM Diprotin A (Bachem) [14]. GLP-1 was measured using the Active GLP-1 (ver.2) Assay Kit from Meso Scale Discovery, which measures intact GLP-1 [GLP-1(7–36)amide and GLP-1(7–37)] to a lower limit of detection of .12 pg/mL and has <.1% cross reactivity with all other GLP-1 metabolites, including GLP-1(9–36)amide and GLP-1(28–36)amide.
2.7. Tissue chemistries
Triglyceride levels were measured from frozen liver tissue by the UT Southwestern Medical Center Mouse Metabolic Phenotyping Core.
2.8. Statistical analysis
All data are presented as mean±SEM. P-values less than .05 were considered statistically significant. Student's t-test was used to compare two sets of means with Welch’s correction, if required. One-way ANOVA, followed by Tukey–Kramer post-hoc analysis, was used to compare three or more means with one independent variable. Repeated measures two-way ANOVA was used to analyze body weight curves. Glucose and insulin excursion curves were analyzed by area-under-curve analysis using one-way ANOVA followed by Tukey–Kramer post-hoc analysis. Age-matched lean C57BL/6 control mice were compared to RYGB-treated α-Gust−/− and Glp1r−/− mice using Student's t-test, when appropriate.
3. Results
3.1. RYGB-enhanced GLP-1 secretion Is attenuated in α-Gust−/− mice
To first evaluate GLP-1 secretion in α-Gust−/− mice with and without RYGB treatment, we measured GLP-1 in the peripheral blood of WT and α-Gust−/− mice after RYGB or sham operations. Fasting GLP-1 was undetectable in sham-operated WT and α-Gust−/− mice (Table 1). By contrast, fasting GLP-1 was elevated in RYGB-treated mice of both genotypes (Table 1). However, this effect was attenuated in α-Gust−/− mice compared to WT (Table 1). After glucose administration via oral gavage with a feeding tube, GLP-1 secretion was greatly enhanced in RYGB-treated WT mice compared to sham (117.6±32.8, WT RYGB versus 21.6±3.2 pg/mL, WT sham, P<.05) and this effect was also attenuated in α-Gust−/− mice (117.6±32.8, WT RYGB versus 14.3±2.4 pg/mL, α-Gust−/− RYGB, P<.05; Figure 1 and Table 1). In fact, glucose-stimulated GLP-1 was actually lower in RYGB-treated α-Gust−/− mice than in sham-operated WT mice (14.3±2.4, α-Gust−/− RYGB versus 21.6±3.2 pg/mL, WT sham; Figure 1 and Table 1). Since glucose-stimulated GLP-1 levels are lower in RYGB-treated α-Gust−/− than in sham-operated WT mice, RYGB-induced effects occurring in α-Gust−/− mice are unlikely to be related to enhanced glucose-stimulated GLP-1 levels.
Table 1.
Peripheral GLP-1 levels in WT and α-Gust−/− mice. Fasting plasma GLP-1 in WT and α-Gust−/− mice was collected from tail vein after an overnight fast. Glucose-stimulated plasma GLP-1 was collected 5 min after administration of 1.5 g/kg of d-glucose via oral gavage through a feeding tube in both genotypes. (n=6, sham; n=6, RYGB; n=6, WM-sham). Values are expressed as mean±SEM. One-way ANOVA was used to compare surgical interventions within a genotype. Student's t-test was used to compare the effect of surgical intervention across genotype.
| Fasting GLP-1 (pg/mL) |
Stimulated GLP-1 (pg/mL) |
|||||
|---|---|---|---|---|---|---|
| Sham | RYGB | WM-Sham | Sham | RYGB | WM-Sham | |
| WT | <assay | 13.0±4.3 | <assay | 21.6±3.2 | 117.6±32.8⁎ | 8.8±2.3# |
| α-Gust−/− | <assay | 2.7±0.4 | <assay | 8.1±1.2 | 14.3±2.4⁎ | 1.6±0.2⁎# |
bolding, P<.05 α-Gust−/− versus WT.
P<.05 versus sham.
P<.05 versus RYGB.
Figure 1.
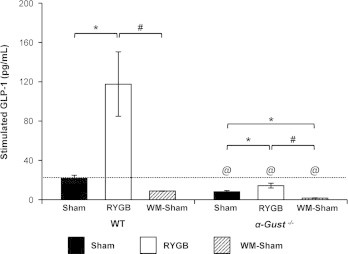
RYGB-enhanced GLP-1 secretion is attenuated in α-Gust−/− mice. RYGB greatly enhanced glucose-stimulated GLP-1 in WT mice and this effect was attenuated in α-Gust−/− mice. Stimulated GLP-1 was reduced in α-Gust−/− WM-sham compared to sham mice. (n=6, sham; n=6, RYGB; n=6, WM-sham). Values are expressed as mean±SEM. One-way ANOVA was used to compare surgical interventions within a genotype. Student's t-test was used to compare the effect of surgical intervention across genotype. ⁎P<.05 versus sham; #P<.05 versus RYGB; @P<.05 α-Gust−/− versus WT.
3.2. RYGB reduces body weight and improves body composition in α-Gust−/− mice despite their attenuated GLP-1 secretion
To investigate the consequence of attenuated GLP-1 secretion on RYGB-induced effects, we examined body weight and body composition after RYGB or sham operations in α-Gust−/− mice. RYGB and sham operations were performed in WT mice in parallel. RYGB-induced effects on body weight and composition were compared to age-matched, non-operated lean controls to assess the magnitude of RYGB-induced effects. RYGB reduced the body weight of α-Gust−/− mice compared to sham mice (Figure 2A, left). Expressed as a percentage of pre-operative weight, shams weighed 102.8±1.7% during post-operative week 5 (Figure 2A, left). In contrast, RYGB mice weighed only 62.6±3.1% (Figure 2A, left). As total body weight, shams weighed 47.2±1.0 g while RYGB mice weighed 28.9±1.5 g, a reduction of 39% (Figure 2B). RYGB reduced fat mass by 70% and lean mass by 15% (Figure 2, C and D). Body weight, fat mass, and lean mass were reduced to a comparable extent by RYGB in WT mice (Figure 2, A–D) and these parameters in RYGB-treated α-Gust−/− mice were comparable to non-operated, age-matched lean C57BL/6 mice (Figure 2, B-D).
Figure 2.
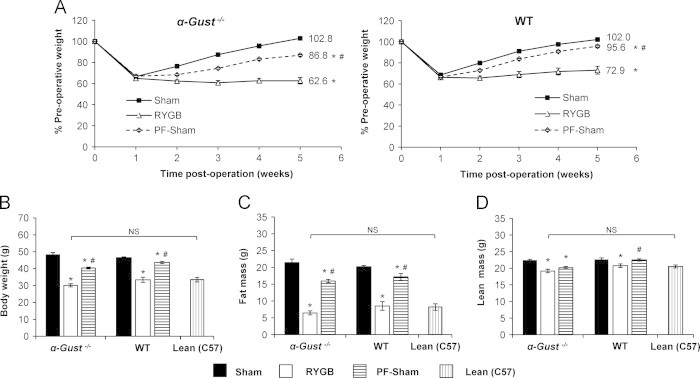
RYGB reduces body weight and improves body composition in α-Gust−/− mice. (A) Body weight, expressed as a percentage of pre-operative values, was reduced in RYGB-treated α-Gust−/− mice (left) compared to sham and PF-sham mice. RYGB induced a comparable reduction in WT mice (right). (B) Total body weight, (C) fat mass, and (D) lean mass (as measured during post-operative week 5) were reduced after RYGB in α-Gust−/− mice to levels observed in non-operated, age-matched lean C57BL/6 control mice (Lean (C57)). (n=6–11, sham; n=7–12, RYGB; n=7, PF-sham; n=7, Lean (C57)). Values are expressed as mean±SEM. Two-way ANOVA with repeated measures was used to compare weight over time among surgical interventions within a genotype. One-way ANOVA was used to compare surgical interventions within a genotype. Student's t-test was used to compare RYGB-treated α-Gust−/− mice and Lean (C57) controls. ⁎P<.05 versus sham; #P<.05 versus RYGB; NS=not significant.
3.3. RYGB reduces food intake and increases energy expenditure in α-Gust−/− mice
In α-Gust−/− mice, daily food intake was reduced after RYGB from post-operative day 8 (the first day of ad libitum feeding) through 24 after which it was equivalent to shams (Figure 3A). As measured through post-operative week 5 (day 35), cumulative intake was reduced by 18% in RYGB-treated mice compared to shams (Figure 3B). In WT mice after RYGB, daily food intake was reduced from day 8 through 11 after which it was equivalent to shams (Figure 3C). As a result of this more contracted period of early reduced intake, cumulative intake through week 5 was not reduced (Figure 3D).
Figure 3.
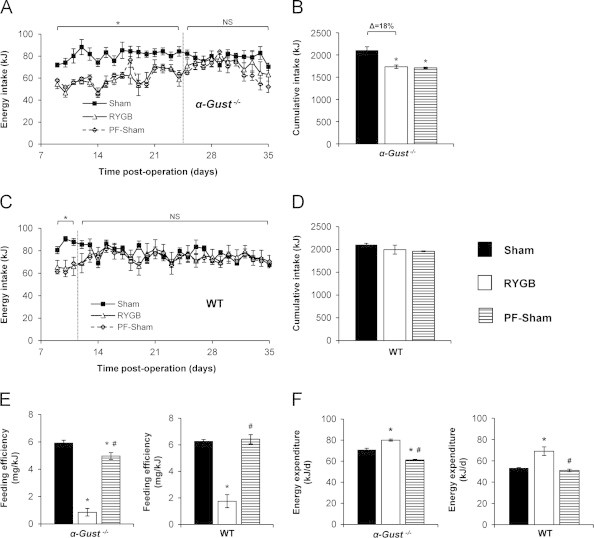
RYGB reduces food intake and increases total energy expenditure in α-Gust−/− mice. (A) RYGB reduced daily intake in α-Gust−/− mice from post-operative day 8 through 24. Thereafter, daily intake in RYGB and sham mice was equivalent. (B) RYGB reduced cumulative intake through week 5 in α-Gust−/− mice. (C) RYGB reduced daily intake in WT mice from day 8 through day 11. (D) Cumulative intake through week 5 was not reduced in WT mice after RYGB. (E) RYGB reduced feeding efficiency and increased (F) total energy expenditure in α-Gust−/− (left) and WT (right) mice compared to sham and PF-sham. (n=6–7, sham; n=7–9, RYGB; n=7, PF-sham). Values are expressed as mean±SEM. One-way ANOVA was used to compare surgical interventions within a genotype. Student's t-test was used to compare the effect of RYGB on daily intake compared to shams. ⁎P<.05 versus sham; #P<.05, versus RYGB; NS=not significant.
To investigate the contribution of this pattern of reduced intake to RYGB-induced weight reduction, a cohort of sham-operated α-Gust−/− mice were pair-fed to RYGB mice from day 8 through the end of week 5. Compared to the 40.2% reduction of normalized body weight observed in RYGB mice, the reduction observed in PF-shams was only 16% (Figure 2A, left). This difference in body weight was predominately due to a difference in fat mass (Figure 2C). A similar pattern was observed in WT mice except that the body weight difference between PF-sham and sham mice was only 6.4% (Figure 2A, right) consistent with their more protracted interval of early reduced intake.
Consistent with the greater weight reduction observed in RYGB mice compared to PF-Shams, RYGB also reduced feeding efficiency (weight gain per kJ consumed) in α-Gust−/− mice by 86.4% and 84.0% compared to sham and PF-sham mice, respectively (Figure 3E, left). We have previously demonstrated that RYGB in mice reduces total calorie absorption to a small extent [34] and this effect was also observed in α-Gust−/− mice (Supplementary Figure 1A). However, even after adjusting for this reduced absorption, feeding efficiency was still reduced by 84.6% (Supplementary Figure 1B) demonstrating a relatively insignificant contribution of fecal calorie losses to RYGB-induced weight loss. We have also previously demonstrated that RYGB has no effect on physical activity in mice [34]. Substantially reduced feeding efficiency in the setting of insignificant fecal calorie losses would therefore suggest the predominant mechanism of weight loss is increased energy expenditure. To estimate the relative contribution of increased energy expenditure to the observed weight loss, we calculated total 24-h energy expenditure. In α-Gust−/− mice, RYGB increased total energy expenditure by 12.8% and 30.9% compared to sham and PF-sham mice, respectively (Figure 3F, left). In WT mice, RYGB induced a similar reduced feeding efficiency and increased total energy expenditure as in α-Gust−/− mice (Figure 3E and F). These data demonstrate that despite attenuated GLP-1 secretion, RYGB still reduced body weight, fat mass, lean mass, food intake, and feeding efficiency as well as increased energy expenditure in α-Gust−/− mice.
3.4. RYGB improves glucose homeostasis in α-Gust−/− mice
In α-Gust−/− mice, RYGB improved glucose and insulin tolerance and reduced fasting blood glucose, fasting plasma insulin, HOMA-IR and glucose-stimulated plasma insulin (Figure 4A–F). Notably, these measures were improved to levels observed in lean, age-matched C57BL/6 control mice (Figure 4A–F). RYGB also reduced fasting hepatic triglyceride content (Supplementary Figure 2). Consistent with our previous observations [34], RYGB induced similar improvements in glucose homeostasis and hepatic triglyceride in WT mice (data not shown).
Figure 4.
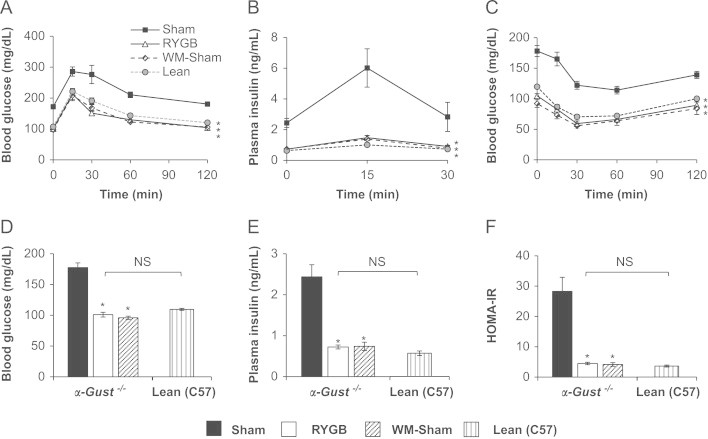
RYGB improves glucose homeostasis in α-Gust−/− mice. (A) Oral glucose tolerance, (B) glucose-stimulated plasma insulin, (C) insulin tolerance, (D) fasting blood glucose, (E) fasting plasma insulin, and (F) HOMA-IR were improved after RYGB in α-Gust−/− mice. These parameters in RYGB mice were comparable to WM-shams and lean C57BL/6 controls. (n=4–11, sham; n=6–12, RYGB; n=6–7, WM-sham; n=6–7, Lean (C57)). Values are expressed as mean±SEM. Curves were analyzed by area under the curve analysis, using trapezoidal rule. One-way ANOVA was used to compare surgical interventions within a genotype. Student's t-test was used to compare RYGB-treated α-Gust−/− mice and Lean (C57) controls. ⁎P<.05, versus sham; NS=not significant.
To control for gluco-regulatory effects of RYGB-induced weight loss, we also studied α-Gust−/− and WT WM-sham mice. In these mice, post-operative body weight (Supplementary Figure 3A) and composition (data not shown) were equivalent to RYGB mice of the same genotype. As expected, fasting GLP-1 was undetectable in WM-shams (Table 1). Compared to shams, glucose-stimulated GLP-1 was slightly reduced in WM-shams of both genotypes, reaching significance in only α-Gust−/− mice (Figure 1). As seen in sham and RYGB mice, glucose-stimulated GLP-1 was attenuated in α-Gust−/− WM-shams compared to their WT counterparts (Figure 1). Despite reduced GLP-1 compared to shams, WM-shams of both genotypes exhibited improved fasting blood glucose and insulin, HOMA-IR, glucose tolerance, insulin tolerance, and fasting hepatic triglyceride content to an equivalent extent as in RYGB-treated mice (Figure 4, Supplementary Figure 2, and data not shown). These data demonstrate that despite attenuated GLP-1 secretion, RYGB induced the expected improvement in glucose homeostasis and reduction of hepatic triglyceride in α-Gust−/− mice.
To investigate if the enhanced GLP-1 secretion is involved in the augmented glucose-stimulated plasma insulin observed after RYGB, we compared plasma insulin levels 15 min after administration of oral glucose in WT and α-Gust−/− RYGB and WM-sham mice. This time-point was chosen as it represents the peak of the plasma insulin curve observed after oral glucose administration (Figure 4B). Responses in RYGB and WM-sham mice were compared to control for the effect of weight reduction on plasma insulin. In WT mice, RYGB increased glucose-stimulated plasma insulin compared to WM-shams and this effect was lost in α-Gust−/− mice (Supplementary Figure 3B, left and middle panels). As glucose tolerance was comparably improved in WT and α-Gust−/− mice, this suggests that augmented glucose-stimulated insulin secretion is not required for improved glucose tolerance after RYGB.
3.5. GLP-1R is dispensable for the beneficial effects of RYGB on body weight and energy balance
While RYGB-induced GLP-1 secretion was substantially attenuated in α-Gust−/− mice, which would lead to diminished levels of circulating intact GLP-1 and GLP-1(9–36)amide/GLP-1(28–36)amide, it was not completely absent (Figure 1). Thus, the possibility still exists that enhanced GLP-1R signaling, perhaps reflecting receptor/post-receptor sensitization, contributes to an effect of GLP-1 during RYGB. We investigated this hypothesis using Glp1r−/− mice. Despite complete absence of the classical GLP-1R receptor, RYGB reduced body weight, fat mass, and lean mass in Glp1r−/− mice (Figure 5A–C) and these parameters were reduced to levels observed in lean, age-matched C57BL/6 controls (Figure 5A–C). The effects of RYGB to transiently reduce daily energy intake, reduce feeding efficiency, and increase total energy expenditure were all preserved in Glp1r−/− mice (Figure 5D–F).
Figure 5.
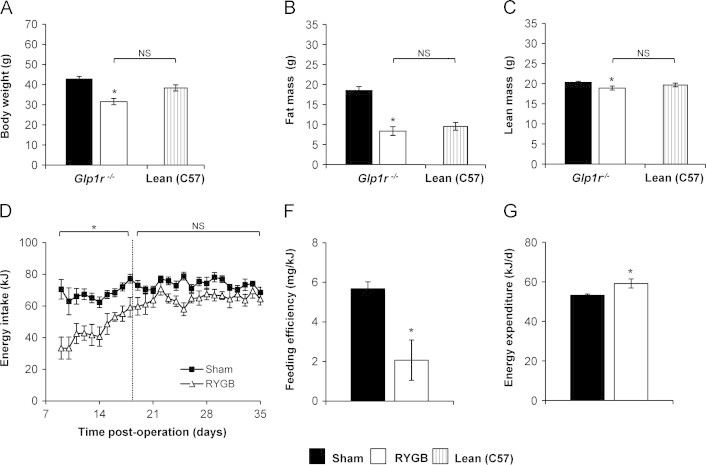
RYGB reduces body weight, improves body composition, and increases total energy expenditure in Glp1r−/− mice. (A) Body weight, (B) fat mass and (C) lean mass were all reduced in Glp1r−/− mice after RYGB to the level of age-matched lean C57BL/6 control mice [Lean (C57)]. (D) Daily intake was reduced in Glp1r−/− mice through post-operative day 18 compared to shams, but no difference was observed thereafter. (E) Feeding efficiency was decreased and (F) total energy expenditure was increased after RYGB in Glp1r−/− mice. [n=9, sham; n=9, RYGB; n=7, Lean (C57)]. Values are expressed as mean±SEM. Student's t-test was used to compare RYGB-treated Glp1r−/− mice to Glp1r−/− shams and Lean (C57) controls. ⁎P<.05, versus sham; NS=not significant.
3.6. GLP-1R is dispensable for the beneficial effects of RYGB on glucose homeostasis.
Again, despite complete absence of the classical GLP-1R, RYGB improved glucose tolerance and insulin tolerance in Glp1r−/− mice (Figure 6A and B). Glucose-stimulated plasma insulin was unchanged (Figure 6C). In the fasted state, blood glucose was reduced after RYGB, but not plasma insulin (Figure 6D and E). Nonetheless, HOMA-IR was still reduced (Figure 6F). These findings demonstrate an overall pattern of improved insulin sensitivity occurring in Glp1r−/− mice after RYGB. Glucose and insulin tolerance, fasting blood glucose, HOMA-IR, and fasting hepatic triglyceride content were comparably improved in RYGB-treated and WM-sham Glp1r−/− mice (Figure 6 and Supplementary Figure 2). As in α-Gust−/− mice, RYGB failed to increase plasma insulin 15-min after administration of oral glucose compared to WM-shams (Supplementary Figure 3B, right). Thus, GLP-1 function via its classical receptor is dispensable for the weight-reducing, energetic, and gluco-regulatory effects of RYGB.
Figure 6.
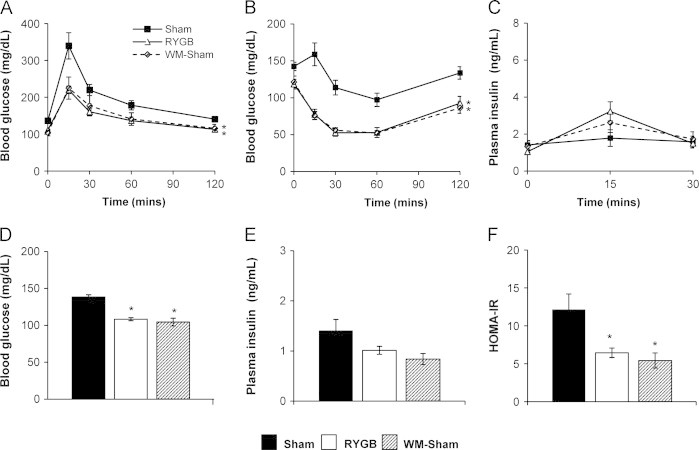
RYGB improves glucose homeostasis in Glp1r−/− mice. (A) Oral glucose tolerance (B) insulin tolerance, (C) glucose-stimulated plasma insulin, (D) fasting blood glucose, (E) fasting plasma insulin, and (F) HOMA-IR in RYGB, sham, and WM-sham Glp1r−/− mice. (n=8–10, shams; n=8–11, RYGB; n=4–7, WM-sham). Values are expressed as mean±SEM. Curves were analyzed by area under the curve analysis, using trapezoidal rule. One-way ANOVA was used to compare surgical interventions within a genotype. ⁎P<.05, versus sham.
4. Discussion
Herein, we demonstrate that attenuated secretion of GLP-1 (including that of its bioactive metabolites) and lack of classical GLP-1R signaling do not prevent the overall beneficial effects of RYGB in mice using two models of functional GLP-1 deficiency. We intentionally studied two distinct yet complementary mouse models exhibiting altered GLP-1 action, α-Gust−/− mice and Glp1r−/− mice, to delineate the importance of the classic GLP-1R versus the potential contribution of two smaller GLP-1-derived peptides, GLP-1(9–36)amide and GLP-1(28–36)amide. Both GLP-1 degradation metabolites improve glucose homeostasis, suppress hepatic glucose production, decrease hepatic steatosis, and attenuate weight gain through GLP-1R-independent mechanisms, actions overlapping, to a considerable extent, with those exhibited by degradation-resistant GLP-1R agonists acting through the classical GLP-1R [21,22]. Hence, genetic disruption or pharmacological blockade of the GLP-1R would not eliminate the contribution(s) of GLP-1(9–36)amide and GLP-1(28–36)amide to the phenotypes observed after RYGB. In contrast, α-Gust−/− mice exhibit markedly lower levels of RYGB-induced intact GLP-1 secretion, and consequently its most common degradation products, GLP-1(9–36)amide and GLP-1(28–36)amide.
First, we demonstrate that the effect of RYGB to enhance GLP-1 secretion is α-gustducin-dependent as GLP-1 secretion was significantly attenuated in α-Gust−/− mice. Nonetheless, RYGB still reduces body weight, reduces fat and lean mass, reduces food intake, increases total energy expenditure, improves glucose homeostasis, and reduces hepatic triglyceride in these mice. Thus, enhanced GLP-1 secretion (and generation of its bioactive metabolites) does not seem predominantly responsible for the beneficial effects of RYGB on body weight, body composition, energy balance, or glucose homeostasis.
While peripheral GLP-1 levels are substantially attenuated in α-Gust−/− mice, they are not entirely lost. In addition, GLP-1 is also expressed in the central nervous system where it is believed to play important roles in feeding, weight regulation, and glucose homeostasis [18,42,43]. To investigate the possibility that central GLP-1 and/or enhanced GLP-1 function, perhaps as a consequence of receptor/post-receptor sensitization, are involved in effects of RYGB, we performed RYGB in Glp1r−/− mice. We found that RYGB still reduces body weight, improves body composition, reduces food intake, increases total energy expenditure, improves glucose homeostasis, and reduces hepatic triglyceride content in Glp1r−/− mice. The magnitude of the effect of RYGB in both genetic models is demonstrated by the fact that RYGB improves body weight/composition and glucose homeostasis to the same degree as in WT diet-induced obese mice as well as to the levels observed in age-matched, lean C57BL/6 control mice despite different diets (ad libitum consumption of regular chow by lean controls versus high fat diet). Thus, enhanced GLP-1 function (central or peripheral) via the classical GLP-1R is not required for the beneficial effects of RYGB. The Glp1r−/− model represents the more robust dataset regarding the role of GLP-1 during RYGB as these mice lack the classical GLP-1R, through which most of the appetitive, gastrointestinal motility, insulinomimetic, insulinotropic, and gluco-regulatory functions of GLP-1 occur [18]. Our data are consistent with that from the Seeley group who recently found that GLP-1R is not required for weight loss and improved glucose homeostasis after vertical sleeve gastrectomy in mice. Our results extend this notion to RYGB, which is an important finding given studies in humans [40,44] and rats [45,46] suggesting that RYGB and vertical sleeve gastrectomy may differ mechanistically as well as anatomically (reviewed in [47]).
A seemingly minimal role for GLP-1 in the gluco-regulatory effects of RYGB is further supported by our findings in WM-sham mice. In WT and α-Gust−/− WM-sham mice, glucose-stimulated peripheral GLP-1 is actually reduced compared to shams. Despite this, glucose homeostasis was improved in WM-sham mice (of all three genotypes) compared to shams and to an equivalent extent as seen in RYGB-treated mice. Thus, the anti-diabetic effect conferred by comparable weight loss to that of RYGB is sufficient to improve glucose homeostasis in the settings of attenuated peripheral GLP-1 levels and lack of GLP-1R signaling. This is consistent with human studies in which patients undergoing equivalent weight loss via mechanisms that do not enhance gut hormone secretion (i.e., calorie restriction and restrictive bariatric procedures) exhibit comparable improved glucose homeostasis to RYGB [6,48–51]. In addition, despite the fact that WT WM-sham mice do not exhibit the enhanced glucose-stimulated plasma insulin observed in WT RYGB mice, glucose excursion in these mice is equivalent. Thus, this effect on plasma insulin is also dispensable for improved glucose homeostasis after RYGB. While the significance of this effect for improved glycemia is unclear, it requires α-gustducin and GLP-1R. These findings are consistent with reports in patients in which pharmacologic GLP-1R antagonism attenuates enhanced insulin secretion observed after RYGB yet has no or only a minimal effect on glycemia [8–10].
Our data do not eliminate the possibility that GLP-1, either via its intact peptide acting via the GLP-1R or through GLP-1(9–36)amide or GLP-1(28–36)amide, is involved in effects of RYGB on energy balance, glucose homeostasis, and hepatic triglyceride, but rather that it is not required. It remains possible that GLP-1 is sufficient for or only a minor contributor to the myriad of RYGB-induced beneficial effects given the effects of pharmacologic GLP-1R antagonism to attenuate enhanced insulin secretion in patients and improve glucose homeostasis in rodents after RYGB. Our data also do not eliminate the possibility that gut hormones other than GLP-1 are involved, as would be suggested by the effects of octreotide (an inhibitor of gut hormone secretion) to increase food intake in patients and rodents after RYGB [5,11]. It would be surprising that any single hormone could explain a complex mechanism of cross talk between the gut and the remaining viscera. Therefore, single molecule knock-outs will probably fail to explain a complete story of bariatric procedures' effects on metabolism. A more plausible theory is that multiple hormones or neuro-hormonal circuits relay the altered message from the gastrointestinal tract to other organs involved in energy and glucose homeostasis after RYGB. Definitive clarification of the possible role of other gut hormones in RYGB effects and the mechanism by which α-gustducin enhances GLP-1 secretion after RYGB remains an important goal.
Despite consuming an equivalent number of daily and cumulative calories, RYGB WT mice were more substantially weight-reduced than PF-shams (29%, RYGB versus 6.4%, PF-shams). As reduced absorption was a negligible contributor to RYGB-induced weight loss, we can estimate that reduced intake after RYGB accounts for 22% of the observed weight loss while increased total energy expenditure accounts for 78%. Similar contributions of reduced intake and increased energy expenditure were observed in α-Gust−/− and Glp1r−/− mice further demonstrating a minimal role of GLP-1 signaling in the energetic and weight-reducing effects of RYGB.
Notably, RYGB mice of all genotypes maintained a stable reduced body weight compared to shams during both the early period of increasing post-operative intake as well as once intake was equivalent to shams. In addition, RYGB reduced the body weight of mice of all three genotypes to that of lean, adult C57BL/6 control mice (e.g., WT RYGB mice weigh 33.7±1.6 g versus 33.1±1.3 g for lean adult C57BL/6 mice), which consume ~37% fewer calories per day (data not shown) of a lower energy diet (regular chow). These observations suggest that RYGB induces an energetic response to maintain (or “defend”) a lower body weight. This hypothesis is reinforced by the fact that total energy expenditure is increased by 46% in RYGB mice compared to WM-shams (data not shown), despite a comparable reduction of body weight and fasting leptin [34]. In other words, the same degree of weight reduction and leptin withdrawal capable of inducing a substantial compensatory anabolic response in WM-sham mice does not do so in RYGB mice. Instead, RYGB mice remain weight neutral due to an increased maintenance metabolic requirement. An understanding of these effects has implications for the development of adjunctive therapy for patients undergoing RYGB aimed at augmenting early post-operative weight loss as well as preventing substantial late post-operative weight regain by offsetting the associated increase in feeding [52].
The strengths of our study include the reproducibility of our robust model of Roux-en-Y gastric bypass in mice that recapitulates the beneficial effects of the procedure in humans, our careful phenotyping of loss-of-function genetic models to investigate the mechanisms of RYGB, and our identification of a mechanism whereby the altered gut anatomy after RYGB engages entero-endocrine cells of the gut via α-gustducin to enhance GLP-1 secretion. The limitations of our study include (1) that the secretion profiles of ghrelin and glucose-dependent insulinotropic polypeptide, and possibly other gut hormones, are altered in α-Gust−/− mice and that these alterations may affect the phenotype (or consequences) of GLP-1 deficiency observed in this model. (2) Despite fasting and glucose-stimulated GLP-1 levels being substantially attenuated in α-Gust−/− mice, they are not completely lost. As we did not assay residual GLP-1 activity, a conclusion of complete functional GLP-1 deficiency is therefore precluded. (3) As we did not measure portal venous levels of GLP-1 (we have found this technically impossible due to post-surgical scarring), the possibility exists that peripheral levels do not accurately reflect the consequences of α-gustducin deficiency on GLP-1 secretion. (4) The possibility of biological compensation always exists when using genetic models utilizing germline or early (i.e., pre-natal or neo-natal) deletion.
In summary, using two models of functional GLP-1 deficiency (i.e., secretory and receptor/post-receptor function), we demonstrate that intact GLP-1 (via the classical GLP-1R) and its degradation metabolites (via GLP-1R-independent mechanisms) are unlikely to mediate the major effects of RYGB on body weight, energy balance, body composition, glucose homeostasis, and hepatic triglyceride content. We further demonstrate that RYGB utilizes taste receptor signaling via α-gustducin to enhance peripheral GLP-1 secretion. In L-cells of the intestine and colon, α-gustducin couples cell surface receptors for luminal carbohydrates, bile acids, and fatty acids to GLP-1 secretion. How the rearranged post-RYGB gut engages this mechanism is currently unclear. Given the preserved response of α-Gust−/− mice to the beneficial effects of RYGB, the significance of this interaction is also unclear. We further demonstrate that RYGB-induced weight reduction alone (i.e., in the absence of enhanced GLP-1 secretion) is sufficient to confer the majority of the RYGB-induced anti-diabetic effect. Continued investigation to determine alternative afferent, gut-derived mechanisms induced by RYGB remains an important goal.
Conflict of interest
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank Luis Palomino and Kristen Dyer for administrative assistance; Eric Berglund, Naim Maalouf, Vishal Patel and Lama Noureddine for critical review of the manuscript; Aki Uchida, UT Southwestern Mouse Metabolic Phenotyping Core, and Linda Kirby (UARK-CEPS) for technical assistance; and Joel Elmquist for mentoring (V.A.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Grant Support: This work was supported by funds from DK007745 (MM), DK091511 (VA), UT Southwestern Department of Internal Medicine (VA), CIHR MOP 123391 (DJD), DC03055 (RFM), and DK081421 (RFM).
Author Contributions: Mohamad Mokadem, Juliet F. Zechner, and Vincent Aguirre (study design, execution, and data analysis); Mohamad Mokadem and Vincent Aguirre (drafted manuscript); Mohamad Mokadem, Juliet F. Zechner, and Vincent Aguirre (critical revision); Robert F. Margolskee and Daniel J. Drucker (animals and critical revision).
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2013.11.010.
Appendix A. Supporting information
Supplementary data
References
- 1.Schauer P.R., Kashyap S.R., Wolski K., Brethauer S.A., Kirwan J.P., Pothier C.E., Thomas S., Abood B., Nissen S.E., Bhatt D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. New England Journal of Medicine. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Leccesi L., Nanni G., Pomp A., Castagneto M., Ghirlanda G., Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. New England Journal of Medicine. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen N.T., Masoomi H., Magno C.P., Nguyen X.M., Laugenour K., Lane J. Trends in use of bariatric surgery, 2003–2008. Journal of the American College of Surgeons. 2011;213:261–266. doi: 10.1016/j.jamcollsurg.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J., Bantle J.P., Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. American Journal of Medicine. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 5.le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A., Lonroth H., Fandriks L., Ghatei M.A., Bloom S.R., Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of Surgery. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 6.Laferrere B., Teixeira J., McGinty J., Tran H., Egger J.R., Colarusso A., Kovack B., Bawa B., Koshy N., Lee H., Yapp K., Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi M., Prigeon R.L., D'Alessio D.A. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60:2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez A., Casamitjana R., Viaplana-Masclans J., Lacy A., Vidal J. GLP-1 action and glucose tolerance in subjects with remission of type 2 diabetes after gastric bypass surgery. Diabetes Care. 2013;36:2062–2069. doi: 10.2337/dc12-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen N.B., Dirksen C., Bojsen-Moller K.N., Jacobsen S.H., Worm D., Hansen D.L., Kristiansen V.B., Naver L., Madsbad S., Holst J.J. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–3052. doi: 10.2337/db13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah M., Law J.H., Micheletto F., Sathananthan M., Man C.D., Cobelli C., Rizza R.A., Camilleri M., Zinsmeister A.R., Vella A. The contribution of endogenous glucagon-like peptide-1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes. 2013 doi: 10.2337/db13-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenske W.K., Bueter M., Miras A.D., Ghatei M.A., Bloom S.R., le Roux C.W. Exogenous peptide YY3-36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. International Journal of Obesity (London) 2012;36:379–384. doi: 10.1038/ijo.2011.126. [DOI] [PubMed] [Google Scholar]
- 12.Kindel T.L., Yoder S.M., Seeley R.J., D'Alessio D.A., Tso P. Duodenal–jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. Journal of Gastrointestinal Surgery. 2009;13:1762–1772. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 13.Gaitonde S., Kohli R., Seeley R. The role of the gut hormone GLP-1 in the metabolic improvements caused by ileal transposition. Journal of Surgical Research. 2012;178:33–39. doi: 10.1016/j.jss.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers A.P., Jessen L., Ryan K.K., Sisley S., Wilson-Perez H.E., Stefater M.A., Gaitonde S.G., Sorrell J.E., Toure M., Berger J., D'Alessio D.A., Woods S.C., Seeley R.J., Sandoval D.A. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141:950–958. doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troy S., Soty M., Ribeiro L., Laval L., Migrenne S., Fioramonti X., Pillot B., Fauveau V., Aubert R., Viollet B., Foretz M., Leclerc J., Duchampt A., Zitoun C., Thorens B., Magnan C., Mithieux G., Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metabolism. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Habegger K.M., Heppner K.M., Amburgy S.E., Ottaway N., Holland J., Raver C., Bartley E., Muller T.D., Pfluger P.T., Berger J., Toure M., Benoit S.C., Dimarchi R.D., Perez-Tilve D., D'Alessio D.A., Seeley R.J., Tschop M.H. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2013 doi: 10.2337/db13-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala J.E., Bracy D.P., James F.D., Julien B.M., Wasserman D.H., Drucker D.J. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology. 2009;150:1155–1164. doi: 10.1210/en.2008-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drucker D.J. The role of gut hormones in glucose homeostasis. Journal of Clinical Investigation. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos R.V., Lee Y.C., Drucker D.J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 20.Drucker D.J., Nauck M.A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 21.Tomas E., Wood J.A., Stanojevic V., Habener J.F. Glucagon-like peptide-1(9–36)amide metabolite inhibits weight gain and attenuates diabetes and hepatic steatosis in diet-induced obese mice. Diabetes, Obesity and Metabolism. 2011;13:26–33. doi: 10.1111/j.1463-1326.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 22.Ip W., Shao W., Chiang Y.T., Jin T. GLP-1-derived nonapeptide GLP-1(28–36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high fat diet fed mice. American Journal of Physiology- Endocrinology and Metabolism. 2013 doi: 10.1152/ajpendo.00376.2013. [DOI] [PubMed] [Google Scholar]
- 23.Tomas E., Habener J.F. Insulin-like actions of glucagon-like peptide-1: a dual receptor hypothesis. Trends in Endocrinology and Metabolism. 2010;21:59–67. doi: 10.1016/j.tem.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young R.L. Sensing via intestinal sweet taste pathways. Frontiers in Neuroscience. 2011;5:23. doi: 10.3389/fnins.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang H.J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.J., Zhou J., Kim H.H., Xu X., Chan S.L., Juhaszova M., Bernier M., Mosinger B., Margolskee R.F., Egan J.M. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Kokrashvili Z., Mosinger B., Margolskee R.F. Gustducin couples fatty acid receptors to GLP-1 release in colon. American Journal of Physiology- Endocrinology and Metabolism. 2013;304:E651–E660. doi: 10.1152/ajpendo.00471.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruiz-Avila L., Wong G.T., Damak S., Margolskee R.F. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha-gustducin. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8868–8873. doi: 10.1073/pnas.151235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen S., Laermans J., Iwakura H., Tack J., Depoortere I. Sensing of fatty acids for octanoylation of ghrelin involves a gustatory G-protein. PLoS One. 2012;7:e40168. doi: 10.1371/journal.pone.0040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen S., Laermans J., Verhulst P.J., Thijs T., Tack J., Depoortere I. Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefater M.A., Wilson-Perez H.E., Chambers A.P., Sandoval D.A., Seeley R.J. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocrine Reviews. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirksen C., Jorgensen N.B., Bojsen-Moller K.N., Jacobsen S.H., Hansen D.L., Worm D., Holst J.J., Madsbad S. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890–1901. doi: 10.1007/s00125-012-2556-7. [DOI] [PubMed] [Google Scholar]
- 32.Scrocchi L.A., Brown T.J., MaClusky N., Brubaker P.L., Auerbach A.B., Joyner A.L., Drucker D.J. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Medicine. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 33.Wong G.T., Gannon K.S., Margolskee R.F. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 34.Zechner J.F., Mirshahi U.L., Satapati S., Berglund E.D., Rossi J., Scott M.M., Still C.D., Gerhard G.S., Burgess S.C., Mirshahi T., Aguirre V. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144:580–590. doi: 10.1053/j.gastro.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravussin Y., Gutman R., LeDuc C.A., Leibel R.L. Estimating energy expenditure in mice using an energy balance technique. International Journal of Obesity (London) 2013;37:399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pullar J.D., Webster A.J. The energy cost of fat and protein deposition in the rat. British Journal of Nutrition. 1977;37:355–363. doi: 10.1079/bjn19770039. [DOI] [PubMed] [Google Scholar]
- 37.Almeida N.G., Levitsky D.A., Strupp B. Enhanced thermogenesis during recovery from diet-induced weight gain in the rat. American Journal of Physiology. 1996;271:R1380–1387. doi: 10.1152/ajpregu.1996.271.5.R1380. [DOI] [PubMed] [Google Scholar]
- 38.Mercer S.W., Trayhurn P. Effect of high fat diets on energy balance and thermogenesis in brown adipose tissue of lean and genetically obese ob/ob mice. Journal of Nutrition. 1987;117:2147–2153. doi: 10.1093/jn/117.12.2147. [DOI] [PubMed] [Google Scholar]
- 39.Eggert D.L., Nielsen M.K. Comparison of feed energy costs of maintenance, lean deposition, and fat deposition in three lines of mice selected for heat loss. Journal of Animal Science. 2006;84:276–282. doi: 10.2527/2006.842276x. [DOI] [PubMed] [Google Scholar]
- 40.Lee S., Muniyappa R., Yan X., Chen H., Yue L.Q., Hong E.G., Kim J.K., Quon M.J. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. American Journal of Physiology- Endocrinology and Metabolism. 2008;294:E261–E270. doi: 10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ali S., Lamont B.J., Charron M.J., Drucker D.J. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. Journal of Clinical Investigation. 2011;121:1917–1929. doi: 10.1172/JCI43615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knauf C., Cani P.D., Perrin C., Iglesias M.A., Maury J.F., Bernard E., Benhamed F., Gremeaux T., Drucker D.J., Kahn C.R., Girard J., Tanti J.F., Delzenne N.M., Postic C., Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. Journal of Clinical Investigation. 2005;115:3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandoval D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiology and Behavior. 2008;94:670–674. doi: 10.1016/j.physbeh.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousseif A., Emmanuel J., Karra E., Millet Q., Elkalaawy M., Jenkinson A.D., Hashemi M., Adamo M., Finer N., Fiennes A.G., Withers D.J., Batterham R.L. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 Levels in non-diabetic humans. Obesity Surgery. 2013 doi: 10.1007/s11695-013-1066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saeidi N., Nestoridi E., Kucharczyk J., Uygun M.K., Yarmush M.L., Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. International Journal of Obesity. 2012;36:1396–1402. doi: 10.1038/ijo.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grayson B.E., Fitzgerald M.F., Hakala-Finch A.P., Ferris V.M., Begg D.P., Tong J., Woods S.C., Seeley R.J., Davidson T.L., Benoit S.C. Improvements in hippocampal-dependent memory and microglial infiltration with calorie restriction and gastric bypass surgery, but not with vertical sleeve gastrectomy. International Journal of Obesity (London) 2013 doi: 10.1038/ijo.2013.100. [DOI] [PubMed] [Google Scholar]
- 47.Scott W.R., Batterham R.L. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. American Journal of Physiology. Regulatory, Integrative and Comparative physiology. 2011;301:R15–R27. doi: 10.1152/ajpregu.00038.2011. [DOI] [PubMed] [Google Scholar]
- 48.Lingvay I., Guth E., Islam A., Livingston E. Rapid improvement in diabetes after gastric bypass surgery: is it the diet or surgery? Diabetes Care. 2013;36:2741–2747. doi: 10.2337/dc12-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackness C., Karmally W., Febres G., Conwell I.M., Ahmed L., Bessler M., McMahon D.J., Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isbell J.M., Tamboli R.A., Hansen E.N., Saliba J., Dunn J.P., Phillips S.E., Marks-Shulman P.A., Abumrad N.N. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campos G.M., Rabl C., Peeva S., Ciovica R., Rao M., Schwarz J.M., Havel P., Schambelan M., Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. Journal of Gastrointestinal Surgery. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarwer D.B., Wadden T.A., Moore R.H., Baker A.W., Gibbons L.M., Raper S.E., Williams N.N. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surgery for Obesity and Related Diseases. 2008;4:640–646. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


