Abstract
We are reporting the first case of spontaneous endocarditis caused by rapid grower non-tuberculous Mycobacterium chelonae in a case of rheumatic heart disease. The diagnosis was confirmed as there was repeated isolation of rapidly growing atypical Mycobacterium from blood culture which was identified as M. chelonae by Reverse line probe assay. The patient presented with pyrexia of unknown origin. Later she was found to have rheumatic heart disease with severe aortic regurgitation & large vegetation was seen attached to the aortic valve. She was treated with rifampicin, clarithromycin, amikacin & levofloxacin based on culture & sensitivity. She succumbed to her illness after development of large cerebral infarction due to embolization of vegetation from aortic valve.
Keywords: Non-tuberculous mycobacteria, Aortic valve, Endocarditis, Mycobacterium chelonae, Rheumatic heart disease
1. Introduction
Clinical syndromes caused by non-tuberculous mycobacteria (NTM) have gained attention since the 1980s due to the increase in disseminated infections in association with the human immunodeficiency virus (HIV) epidemic.1 Non-tuberculous mycobacterial disease occurs infrequently in immunocompetent individuals.1 Based on their growth rates, NTM have been classified into slowly growing or rapidly growing mycobacteria.2 The most commonly implicated mycobacterial species belonging to the rapid grower group (Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium abscessus) of NTM.2 Endocarditis caused by these organisms is rare and usually involves prosthetic valve infections with M. chelonae.3 We are reporting a case of spontaneous endocarditis caused by rapid grower M. chelonae. Even after giving appropriate treatment according to the culture & sensitivity report, she succumbed to her illness due to embolic stroke.
2. Case report
A 41-year-old female patient was admitted to our hospital with fever with chills, rigor & night sweats of one-month duration. She was not a known case of rheumatic heart disease and there was no history of recent surgery & skin infection. Initial examination revealed a temperature of 101 °F. General physical examination revealed peripheral features of severe aortic regurgitation (AR) with pulse rate of 110/min & blood pressure of 130/40 mm of Hg. Cardiovascular examination revealed a long diastolic murmur in the aortic and neo-aortic area along with pansystolic & mid diastolic murmur at the apex. Laboratory evaluation showed the following values: haemoglobin- 10.9 g/dL (normal – 12–15 gm/dl); leukocytes – 14000/cmm (normal – 4500–11,000/cmm); and platelets – 4.5 lacs/cmm (1.5–4.5 lacs/cmm). Initial renal & liver function test and serum electrolytes were within normal range. She was non-reactive for human immuno-virus antibodies. Transthoracic echocardiography (TTE) at admission showed presence of large vegetation attached to the aortic valve with severe AR & thickening of the mitral valve with mild mitral regurgitation & stenosis with normal LV function. The patient was treated initially with injection Penicillin (24 lacs units/day) and Gentamicin (75 mg/12 hourly). But even after 7 days of treatment, she showed no signs of improvement. So, she underwent transesophageal echocardiography (TEE) which showed large vegetation attached to left coronary cusp along with severe AR (Figs 1 and 2). Blood culture report came after 7 days of incubation which showed growth of rapidly growing NTM which was susceptible to Amikacin, Ciprofloxacin, Levofloxacin & Clarithromycin. Repeat blood cultures also yielded rapidly growing NTM. The isolate was identified as M. chelonae by Line Probe assay at National Institute for Research in Tuberculosis. The antibiotics were changed to Amikacin (250 mg/12 hourly), Levofloxacin (750 mg/day), Clarithromycin & Rifampicin (600 mg/day) on 7th day after admission. Though she was planned for emergency surgery, but as her renal function deteriorated and fever was persisted, so surgeons deferred the emergency surgery. Suddenly, she developed left sided hemiparesis & became deeply comatose. Finally after 15 days of treatment, she succumbed to her illness.
Fig. 1.
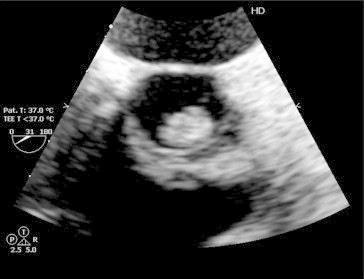
Aortic valve short axis in TEE showed large vegetation attached with left coronary cusp.
Fig. 2.
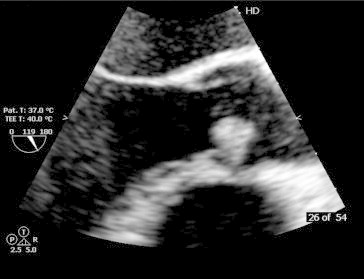
TEE long axis view showed large freely mobile vegetation attached with aortic valve.
3. Discussion
Endocarditis caused by NTM in immune competent HIV negative patients is exceedingly rare.2 The most reported cases involve diseased valves or prosthetic heart valves3 and very rarely native valve.4 Most of the non-HIV cases have other immunocompromised conditions like haematological malignancy or comorbid condition like chronic kidney disease on hemodialysis.5 Although widespread in the environment, they only rarely cause a wide array of infections in immunocompetent and immunocompromised hosts; typically include skin and soft-tissue infections following puncture wounds or inoculations as well as pulmonary infections, infections of foreign material (porcine and prosthetic cardiac grafts, tympanotomy tubes, intravenous and dialysis catheters).5 These infections are difficult to diagnose because blood cultures are often negative. Clinically, it is important to recognize the possibility of NTM endocarditis in the differential diagnosis of culture-negative patients who develop signs and symptoms of endocarditis, whether they present early or late in onset after the surgical procedure. Though there are several reported cases of NTM endocarditis in rheumatic heart valves which occur after valve replacement. Previously, one case of NTM endocarditis and meningitis developed after balloon mitral valvotomy has been reported.6
M. chelonae is being recognized as a cause of an increasing spectrum of illnesses, including soft-tissue infections, pulmonary disease, and postoperative infections. However, they have very less propensity to cause infections on native cardiac valves.7 Our case represents the first report of spontaneous M. chelonae endocarditis of rheumatic heart valve in an immunocompetent non-HIV patient and interestingly, it was the first presentation of rheumatic heart disease. Prognosis of M. chelonae endocarditis is usually grave due to high mortality with report of little survival cases after medical & surgical treatment.6 Most isolates are resistant to numerous antibiotics but with sufficient variability to require susceptibility testing in each case. Most isolates are resistant to cephamycins and show variable susceptibility to aminoglycosides, doxycycline, imipenem, and ciprofloxacin. Trimethoprim-sulfamethoxazole and the newer beta-lactam antibiotics have little or no activity against M. chelonae.7 Surgical removal is recommended in non-responder or cases with prosthetic valve endocarditis.8 Combination therapy for infections due to the rapidly growing NTM seems advisable, as monotherapy has been shown to select for the emergence of resistance.7 We also had used combination of antimicrobial drugs for the treatment of our patient. There have been no controlled trials of therapy, the results of which could help direct the length of treatment, although prolonged courses of antibiotic therapy have been advocated for most infections.7 In our case, we had started antimicrobial drugs which showed sensitivity towards rapid grower NTM but prognosis of our patient was poor.
So, in any case of prolonged fever, endocarditis has to be ruled out and even if there is no predisposing factor present for non-tuberculous mycobacterial endocarditis; NTM organism has to be ruled out if patient is not responding to usual treatment by sending repeated blood cultures. Early treatment for NTM has to be started and if needed surgical removal of infected tissue & valves has to be done otherwise it will be ended fatally.
Contributors
Naveena J was involved in the microbiological diagnosis of this case. Soumya Patra, Ajit Pal Singh, Nagesh C.M, Babu Reddy & Srinivas B.C diagnosed the cases, reviewed the literature and drafted the manuscript. Srinivas B.C & Manjunath C. N corrected the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
All authors have none to declare.
Acknowledgement
We are thankful to Dr Sowmya Swaminathan, Director, National Institute for Research in Tuberculosis and Dr. Gomathi, Bacteriologist, National Institute for Research in Tuberculosis, Chennai, India for their involvement in the mycobacterial analysis in our case.
References
- 1.Lohr D.C., Goeken J.A., Doty D.B., Donta S.T. Mycobacterium gordonae infection of a prosthetic aortic valve. JAMA. 1978;239:1528–1530. [PubMed] [Google Scholar]
- 2.van Duin D., Goldfarb J., Schmitt S.K., Tomford J.W., Tuohy M.J., Hall G.S. Nontuberculous mycobacterial blood stream and cardiac infections in patients without HIV infection. Diagn Microbiol Infect Dis. 2010;67:286–290. doi: 10.1016/j.diagmicrobio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Bush L.M., Paturi A., Chaparro-Rojas F., Perez M.T. Mycobacterial prosthetic valve endocarditis. Curr Infect Dis Rep. 2010;12:257–265. doi: 10.1007/s11908-010-0108-1. [DOI] [PubMed] [Google Scholar]
- 4.Collison S.P., Trehan N. Native double-valve endocarditis by Mycobacterium fortuitum following percutaneous coronary intervention. J Heart Valve Dis. 2006;15:836–838. [PubMed] [Google Scholar]
- 5.Strabelli T.M., Siciliano R.F., Castelli J.B. Mycobacterium chelonae valve endocarditis resulting from contaminated biological prostheses. J Infect. 2010;60:467–473. doi: 10.1016/j.jinf.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Kuruvila M.T., Mathews P., Jesudason M., Ganesh A. Mycobacterium fortuitum endocarditis and meningitis after balloon mitral valvotomy. J Assoc Physicians India. 1999;47:1022–1023. [PubMed] [Google Scholar]
- 7.Wallace R.J., Jr., Brown B.A., Onyi G.O. Skin, soft tissue, and bone infections due to Mycobacterium chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405–412. doi: 10.1093/infdis/166.2.405. [DOI] [PubMed] [Google Scholar]
- 8.Kunin M., Salamon F., Weinberger M., Genkin I., Sagie A., Tur-Kaspa R. Conservative treatment of prosthetic valve endocarditis due to Mycobacterium fortuitum. Eur J Clin Microbiol Infect Dis. 2002;21:539–541. doi: 10.1007/s10096-002-0763-8. [DOI] [PubMed] [Google Scholar]


