Abstract
Dietary triglycerides are hydrolyzed in the small intestine principally by pancreatic lipase. Following uptake by enterocytes and secretion as chylomicrons, dietary lipids are cleared from the bloodstream via lipoprotein lipase. Whereas lipoprotein lipase is inhibited by several proteins including Angiopoietin-like 4 (Angptl4), no endogenous regulator of pancreatic lipase has yet been identified. Here we present evidence that Angptl4 is an endogenous inhibitor of dietary lipid digestion. Angptl4−/− mice were heavier compared to their wild-type counterparts without any difference in food intake, energy expenditure or locomotor activity. However, Angptl4−/− mice showed decreased lipid content in the stools and increased accumulation of dietary triglycerides in the small intestine, which coincided with elevated luminal lipase activity in Angptl4−/− mice. Furthermore, recombinant Angptl4 reduced the activity of pancreatic lipase as well as the lipase activity in human ileostomy output. In conclusion, our data suggest that Angptl4 is an endogenous inhibitor of intestinal lipase activity.
Keywords: Angptl4, Pancreatic lipase, Intestine, Obesity
1. Introduction
Dietary triglycerides (TGs) contribute a major portion of our daily energy requirement. TGs are digested in the gastrointestinal tract through the action of several lipases including lingual lipase and gastric lipase [1,2]. However, the far majority of dietary TGs are digested in the small intestine by pancreatic lipase (PL) assisted by pancreatic colipase, pancreatic lipase-related protein 2, and carboxyl ester lipase [3,4]. To increase the accessibility of PL to dietary TGs at the oil–water interphase, dietary lipids are emulsified by the action of bile acids, which are produced in the liver, stored in the gallbladder, and subsequently released upon ingestion of a high-fat meal [2,5]. The resulting mono-acylglycerol and free fatty acids are taken up by enterocytes and resynthesized into TGs, followed by the lipidation of apolipoproteins to yield chylomicrons that are subsequently secreted into the lacteals [6–8]. After traveling through the splanchnic lymphatic system and the thoracic duct, chylomicrons are released into the blood stream and ultimately reach the heart, skeletal muscle and adipose tissue as their primary target organs.
Clearance of circulating chylomicrons is mediated by lipoprotein lipase (LPL), which is anchored to the capillary endothelium via the protein GPIHBP1 [9–11]. LPL catalyzes the hydrolysis of TGs to free fatty acids, which are taken up by the parenchymal cells to serve as energy source, building blocks for membranes, or to be used for storage. LPL is thought to function as an active homodimer and its activity in tissues is profoundly influenced by various physiological stimuli, including nutritional status, exercise and cold [9]. In the last decade it has become evident that the activity of LPL is carefully controlled by three proteins belonging to the Angiopoietin-like protein family: Angptl3, Angptl4, and Angptl8 [12,13]. All three proteins have been shown to inhibit LPL activity in vitro and in vivo and accordingly raise circulating TG levels [12,14,15]. Angptl3 is produced primarily by the liver and its expression is regulated by the Liver X Receptor [16,17]. Angptl8, a paralog of Angptl3, was recently identified as the latest member of the Angptl family [13]. Deletion of Angptl8 results in decreased plasma TG levels due to decreased hepatic VLDL production and elevated LPL activity [14]. Angptl4 has previously been shown to potently inhibit LPL in several tissues and cells under a variety of circumstances. Indeed, we and others have identified Angptl4 as an important regulator of LPL in adipose tissue, heart, and macrophages [18–22]. Angptl4 forms higher order oligomers and is subject to proteolytic cleavage to yield C- as well as N-terminal Angptl4. The precise mechanism by which Angpl4 inhibits LPL as well as the localization of the inhibitory effect are not fully understood. It was reported that Angptl4 inhibits LPL by promoting the irreversible conversion of active homodimeric units into inactive monomers [23], whereas recent data suggest that Angptl4 functions as a reversible noncompetitive inhibitor of LPL [24]. The expression of Angptl4 is governed by a multitude of dietary and hormonal cues including fatty acids and glucocorticoids via peroxisome proliferator-activated receptors (PPARs) and glucocorticoid receptor (GR), respectively [25–27].
LPL is a part of the same family of extracellular lipases as PL, which also include endothelial lipase and hepatic lipase [28]. However, little is known about regulation of the activity of PL. Here we report that Angptl4 reduces intestinal lipase activity by inhibition of PL. As a result, Angptl4−/− animals harvest more lipids from ingested food resulting in increased fat mass and body weight.
2. Material and methods
2.1. Animal studies
Animals used for all experiments were pure-bred male wild-type and Angptl4−/− animals on a C57BL/6 background [29]. Body composition was determined by DEXA (Lunar, PIXImus) on chow-fed 24 weeks old wild-type and Angptl4−/− mice. Food intake was recorded over a 2-week period. Animals were sacrificed by cervical dislocation after being anesthetized using isoflurane and several organs were excised.
Eleven week old wild-type and Angptl4−/− animals were given access to HFD providing 45en% fat (D12451, Research Diets Services, Wijk bij Duurstede, The Netherlands). Food intake and body weight were recorded weekly for a period of 3 weeks.
Wild-type and Angptl4−/− animals (28 weeks old) were analyzed in an open-circuit LabMaster Metabolism Research Platform (TSE systems GmbH, Bad Homburg, Germany) as described previously [30], with minor adaptations. Animals were acclimatized for 24 h followed by 48 h measurements. Eight of the 12 cages were also equipped for locomotor activity measurements. Body composition analysis (Echo-MRI, Houston, USA) was performed immediately before and after the indirect calorimetric analyses, which showed similar lean mass between wild-type and Angptl4−/− mice. One week before the actual metabolic phenotyping, the animals were switched to HFD. During the analysis, mice were kept on the same HFD. Fecal fat was extracted from feces of 11-week old wild-type and Angptl4−/− animals after a 3-week HFD intervention using published methods [31]. Fatty acids were subsequently measured using an enzymatic assay (NEFA-HR(2), Wako Chemicals, Neuss, Germany).
Intestinal lipid absorption tests were performed using 3H-labeled triolein according to published methods [32]. For determination of intestinal TG content, wild-type and Angptl4−/− animals were fed a HFD for 24 h after a 1 week run in with LFD. Mice were killed by cervical dislocation and the small intestine was collected, washed with ice-cold PBS and snap frozen. Samples were homogenized in sucrose buffer (10 mM Tris, 2 mM EDTA, 0.25 M sucrose, pH 7.5) with a Qiagen TissueLyser II (Qiagen, Hilden, Germany) and subsequently analyzed for TGs using a Triglycerides liquicolormono kit (HUMAN, Wiesbaden, Germany).
qPCR analysis of PPARα target genes was performed in proximal small intestine of 10–15-week-old wild-type and Angptl4−/− mice fed LFD or a safflower oil-based HFD for 6 weeks. qPCR analysis of Pparα target genes was also performed on small intestine of 4-month-old wild-type and Angptl4−/− animals fasted for 4 h and subsequently intragastrically gavaged with 400 μL carboxymethyl cellulose (CMC) or synthetic trilinolenin. Proximal small intestines were excised, washed with ice-cold PBS, and subsequently snap frozen in liquid nitrogen.
To assess intestinal transit time, wild-type and Angptl4−/− mice (16-week old) were fed AIN-93W low fat semi-purified diet (Research Diets Services, Wijk bij Duurstede, Netherlands) for 3 days prior to the experiment. Next, the mice were fasted for 16 h and subsequently given access to AIN-93W diet which was colored with 60 mg/kg Fast Green FCF (Sigma-Aldrich, Schnelldorf, Germany). The time until the first green dropping appeared was recorded. Feces from 12–15-week-old wild-type and Angptl4−/− mice on standard chow were collected and analyzed for several bile acids as described previously [33].
Animal studies were performed according to protocols approved by the local animal ethics committee of Wageningen University.
2.2. Gene expression analysis
Proximal intestine samples from wild-type and Angplt4−/− animals after HFD or lipid gavage were homogenized in TRIzol® (Life Technologies Europe BV, Bleiswijk, The Netherlands) using a Qiagen TissueLyser II (Qiagen, Hilden, Germany) for RNA isolation. cDNA was synthesized with the First Strand cDNA synthesis kit (Thermo Scientific, Landsmeer, The Netherlands). qPCR was performed using SensiMix (Bioline, GC biotech, Alphen aan den Rijn, The Netherlands) and primers from the Harvard PrimerBank on a CFX384 platform (BioRad, Veenendaal, The Netherlands). For normalization 36b4 was used as a housekeeping gene. Affymetrix GeneChip analysis was performed on 10 longitudinal small intestine fragments collected along the total length of the small intestine of C57BL/6 mice fed LFD or HFD for 2 weeks. RNA was extracted as described above and subsequently purified using the RNeasy Minikit (Qiagen, Venlo, The Netherlands). RNA quality was assessed on RNA 6000 Nano chips with the Agilent 2100 Bioanalyzer (Agilent Technologies, Amsterdam, the Netherlands). Labeled RNA was hybridized to Affymetrix Mouse Gene 1.1 ST array plate on the Affymetrix GeneTitan platform, and scans were processed using Bioconductor followed by RMA normalization [34,35]. Probe sets were generated based on published methods [36] using chip definition file (CDF) version 15 derived from the Entrez gene database. Analysis of Villin and Angptl mRNA levels along the crypt-villus axis was performed according to published methods [37] using qPCR as described above.
2.3. Mouse luminal lipase activity
Luminal content collected from the small intestine was mixed with 19 volumes ice-cold PBS, vortexed vigorously and centrifuged at 15,000g/4 °C for 10 min. Supernatants were collected and diluted 1:100 in PBS for subsequent analysis. Lipase activity was measured using the Roar LPL activity assay kit (Roar Biomedical, Inc., New York, USA) according to the manufacturer's protocol. The LPL activity kit employs a substrate that becomes fluorescent upon hydrolysis of an ester and can be used to assess the activity of other lipases too. Lipase activity was calculated as µmol/h using a pre-hydrolyzed substrate and subsequently normalized for protein concentration of the fecal water as determined with BCA protein assay (Thermo Fisher Scientific, Landsmeer, The Netherlands).
2.4. Human luminal lipase activity
Three otherwise healthy volunteers with a distal ileostomy volunteered to provide stoma output. Volunteers were aged 50–67 years and included one male and two females. Collected material was transported on ice and centrifuged at 5000g/4 °C for 10 min. Supernatants were collected and stored at −80 °C until lipase activity assays. Samples were diluted 1:100 before determining lipase activity with the Roar LPL Activity Assay Kit. Recombinant mouse Angptl4 (R&D Systems, Abingdon United Kingdom) or Orlistat (Sigma-Aldrich, Schnelldorf, Germany) were allowed to pre-incubate with stoma output at 37 °C for 45 min after which the fluorescent substrate was added. Fluorescence was measured after an additional incubation period at 37 °C for 45 min. Fluorescent units were converted to µmol of hydrolyzed substrate per hour using the pre-hydrolyzed substrate, and normalized for protein content determined with a BCA protein assay.
2.5. Pancreatic lipase activity
Recombinant PL (Sigma-Aldrich, Schnelldorf, Germany) at an amount of 250 units was added to the fluorescent substrate of the Roar LPL Activity Assay Kit. Recombinant mouse Angptl4 or Orlistat were included to address the inhibitory capacity of these compounds on PL mediated hydrolysis. Fluorescence was measured after incubation at 37 °C for 45 min.
2.6. Statistical analysis
Unpaired two-tailed Student's t-tests and analysis of variances with corresponding post-hoc tests were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). The cutoff for significance level was set at p≤0.05.
3. Results
3.1. Angptl4 deletion leads to increased bodyweight independent of food intake, energy intake, or locomotor activity
We observed that chow-fed Angptl4−/− mice have a significantly higher bodyweight than age-matched wild-type mice (Figure 1A). Body composition analysis by DEXA indicated that the difference in body weight can be attributed to a higher amount of body fat (Figure 1B). In contrast, no difference in lean mass was observed between the two sets of mice, which was supported by the weight of major organs including liver, heart, and various muscles (Figure S1A–F). Also, no differences in food intake could be recorded (Figure 1C). Supporting a role of Angptl4 in dietary fat-induced weight gain, Angptl4−/− mice gained weight much faster compared to wild-type mice when switched to a high fat diet (Figure 1D), which again was independent of food intake (Figure 1E).
Figure 1.
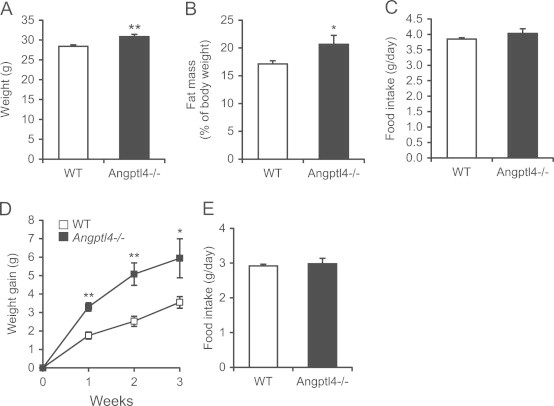
Angptl4−/− mice are heavier despite equal food intake. (A–D) Body weight (A), fat mass as determined with DEXA scan (B), average food intake per day over a 2-week period (C) of wild-type and Angptl4−/− mice on a standard chow diet (n=10–11 per group). (D and E) Body weight development (D) and food intake (E) of wild-type and Angptl4−/− mice after a switch to HFD (n=4–5 per group). Data are mean±SEM. Asterisks indicate significant differences according to Student's t-test; ⁎ p≤0.05, ⁎⁎ p≤0.01.
A difference in body weight can be explained by several mechanisms including differences in locomotor activity and/or energy expenditure. To explore these options we determined the total energy balance of wild-type and Angptl4−/− mice using indirect calorimetry. For 1 week prior to and during the measurements the mice were given access to HFD. The metabolic phenotyping revealed no differences in food intake (Figure 2A and B) and energy expenditure (Figure 2C and D), which was confirmed with ANCOVA analysis. Respiratory exchange ratios for wild-type and Angptl4−/− animals were equal at 0.85, indicative of the combustion of a mixture of fat and carbohydrates (Figure 2E). Moreover, locomotor activity did not differ between wild-type and Angptl4−/− animals (Figure 2F). These data indicate that the enhanced fat mass in Angptl4−/− mice is not due to increased food intake or decreased energy expenditure.
Figure 2.
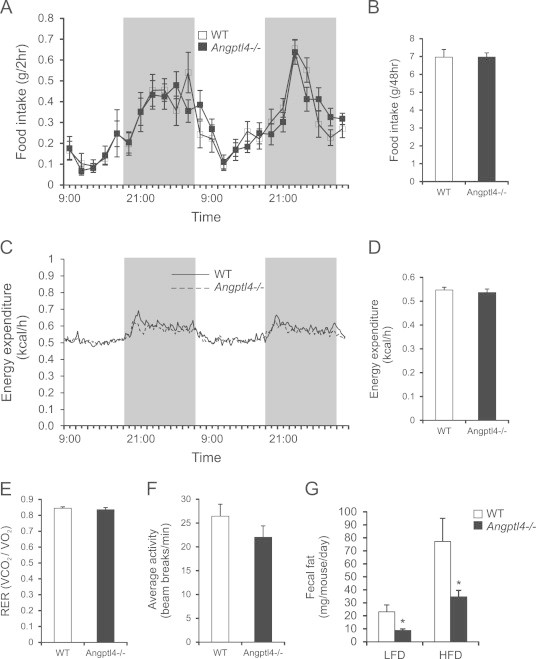
Metabolic phenotyping reveals no differences in energy expenditure, food intake or locomotor activity between wild-type and Angptl4−/− mice. (A and B) Food intake per 2 h (A) or accumulated over 2 days (B) of wild-type and Angptl4−/− mice on a 45en% HFD diet (n=11–12 per group). (C) Energy expenditure as determined every 13 min over the course of 2 days (n=11–12 per group). (D) Average energy expenditure over 2 days of wild-type and Angptl4−/− animals (n=11–12 per group). (E) RER of wild-type and Angptl4−/− animals (n=11–12 per group). (F) Locomotor activity of wild-type and Angptl4−/− animals (n=7–8 group). (G) Fecal fat content in wild-type and Angptl4−/− mice after a 3-week HFD intervention (n=4–5 group). Data are mean±SEM. Asterisk indicates significant differences according to two-way ANOVA with Bonferroni post-hoc test; ⁎ p≤0.05.
To examine if lipid absorption is elevated in Angptl4−/− mice, we determined the amount of fat in the feces in mice fed low fat diet or high fat diet. Strikingly, the amount of fat in the feces was significantly lower in Angptl4−/− mice as compared to wild-type mice (Figure 2G), indicating enhanced lipid absorption. Thus, the data suggest that Angptl4−/− mice harvest more lipids from the ingested food, pointing to the intestine as a key site of Angptl4 action.
Although Angptl4 is known to be produced by a number of intestinal cell lines [38,39], little is known about intestinal Angptl4 expression in vivo. In mouse small intestine, Angptl4 mRNA levels were highest at the top of the villi and lowest in the crypts (Figure 3A). Expression of Angptl4 did not vary much along the longitudinal axis of the murine small intestine (Figure 3B). Interestingly, except in the most proximal portion of the small intestine, Angptl4 mRNA levels were 2–3 fold elevated by high fat feeding (Figure 3B).
Figure 3.
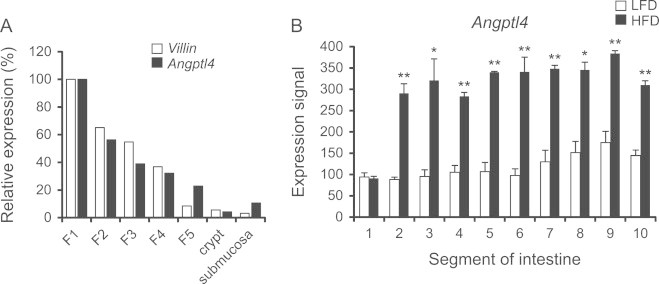
Increased intestinal Angptl4 mRNA expression after a HFD intervention. (A) Villin and Angptl4 expression in isolated epithelial fractions enriched for villi (n=2). (B) Angptl4 expression in 10 parts of the small intestine after 2 weeks HFD intervention, determined with Affymetrix Microarray (n=3). Data are mean±SEM. Asterisks indicate significant differences according to Student's t-test; ⁎ p≤0.05, ⁎⁎ p≤0.01.
To explore the possible cause for the increased harvesting of dietary fat we carried out a lipid absorption test via oral dosing of 3H-triolein. No difference was observed in the rate of appearance of 3H-tracer in the circulation between wild-type and Angptl4−/− mice (Figure 4A), indicating that chylomicron synthesis and secretion, which are rate-limiting for dietary TG entry into the blood, are not different between the two sets of mice. However, a significant increase in tracer abundance was observed in small intestinal tissue of Angptl4−/− mice (Figure 4B), suggesting a higher rate of fatty acid uptake and possibly digestion. This finding was confirmed by higher TG levels in small intestine of Angptl4−/− mice after a 1-day HFD intervention (Figure 4C). In accordance with activation of the lipid-sensitive transcription factor PPARα by dietary fat, HFD or lipid gavage caused marked induction of numerous PPARα target genes in the small intestine. Consistent with enhanced lipid uptake in Angptl4−/− mice, induction of PPARα targets by HFD or lipid gavage was markedly augmented in Angptl4−/− mice in comparison with wild-type mice (Figure 5A and B). These data suggest that Angptl4 regulates the amount of luminal TG entering the enterocytes while having no effect on the amount of TG released from the intestine into the circulation.
Figure 4.
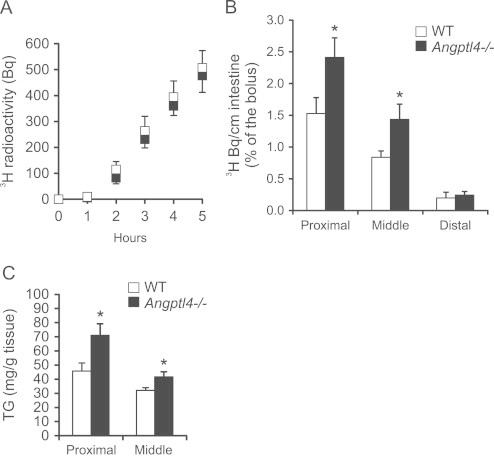
Angptl4−/− mice accumulate fat in their intestine. (A and B) 3H-triolein appearance in plasma (A) and intestine (B) after gavage with 3H-triolein in fasted wild-type and Angptl4−/− mice (n=8–10 per group). (C) Triglyceride content of wild-type and Angptl4−/− intestine after one day HFD intervention (n=6–7 per group). Data are mean±SEM. Asterisk indicates significant differences according to Student's t-test; ⁎ p≤0.05.
Figure 5.
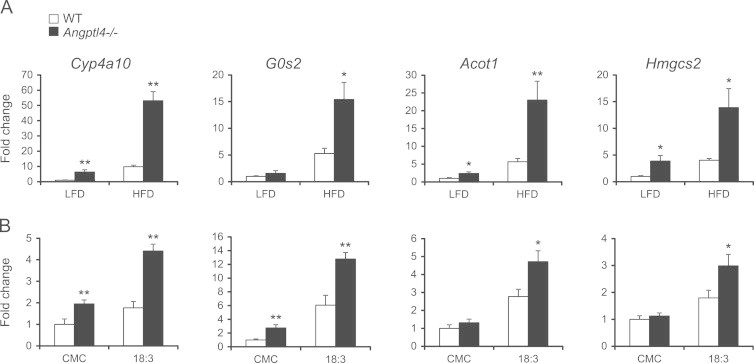
Expression of Pparα target genes is increased in Angptl4−/− mice intestine. (A and B) Expression of selected Pparα targets in the proximal part of the small intestine after a 6-week HFD intervention (A, n=6–7 per group) or oral gavage with 0.5% carboxymethyl cellulose (CMC) or C18:3 triglycerides (B, n=5–6 per group) in wild-type and Angptl4−/− mice. Data are mean±SEM. Asterisks indicate significant differences according to two-way ANOVA with Bonferroni post-hoc test; ⁎ p≤0.05, ⁎⁎ p≤0.01.
3.2. Increased lipid digestion in Angptl4−/− mice is independent of intestinal length, gastrointestinal transit time, and bile acid secretion or composition
An increase in intestinal lipid uptake might be explained by several factors, including a decrease in gastrointestinal (GI) transit time, allowing more time for lipases to digest dietary TG and causing a higher overall rate of lipid digestion. To explore this possibility we fed overnight fasted mice a semi-synthetic diet colored with Fast Green FCF and recorded the time until arrival of the first dropping. No difference was observed in GI transit time between wild-type and Angptl4−/− mice (Figure 6A). In addition, total length of the small intestine was unchanged between wild-type and Angptl4−/− mice (Figure 6B).
Figure 6.
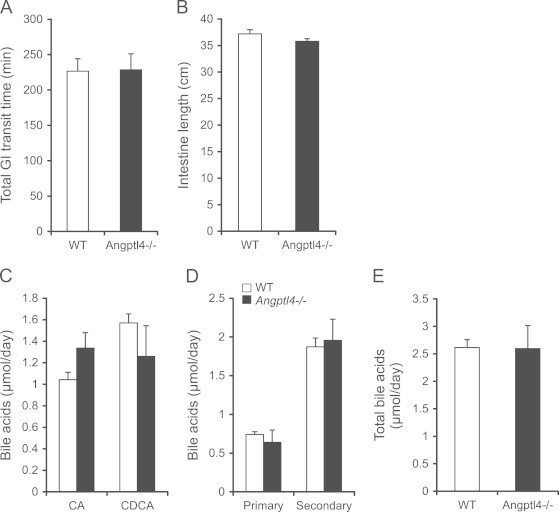
Increased lipid uptake in Angptl4−/− mice is independent of transit time or bile acid secretion. (A and B) GI transit time (A) and intestinal length (B) of wild-type and Angptl4−/− mice (n=8–9 per group). (C–E) Fecal bile acids in wild-type and Angptl4−/− mice expressed as CA vs. CDCA (C), primary vs. secondary (D), or total (E) bile acids (n=8–9 per group). Data are mean±SEM.
Bile acids play an important role in lipid digestion by emulsifying lipids and thereby allowing for increased efficiency of lipid harvesting. Hence, a difference in the composition or in the total amount of bile acids may contribute to increased lipid uptake. We found no difference in the fecal amount of cholic- or deoxycholic acid (Figure 6C), nor in the amount of primary-, secondary-, or total bile acids between wild-type and Angptl4−/− mice (Figure 6D and E). Together, these observations indicate that enhanced lipid uptake in intestine of Angptl4−/− mice is unrelated to changes in intestinal transit time, intestinal length or bile acid composition/secretion.
3.3. Angptl4 inhibits pancreatic lipase
Angptl4 serves as an endogenous inhibitor of LPL and there is evidence that it inhibits hepatic lipase as well [40]. Since PL, which serves as the principal intestinal lipase, belongs to the same family of extra-cellular lipases, we asked whether increased lipid uptake in Angptl4−/− mice may be explained by enhanced lipid digestion due to a lack of Angptl4-mediated inhibition of intestinal lipase activity. To test this hypothesis we collected the luminal content from (refed) wild-type and Angptl4−/− mice and assayed fecal water for lipase activity. Consistent with our hypothesis, Angptl4−/− mice had a significantly higher luminal lipase activity in comparison to wild-type mice (Figure 7A).
Figure 7.
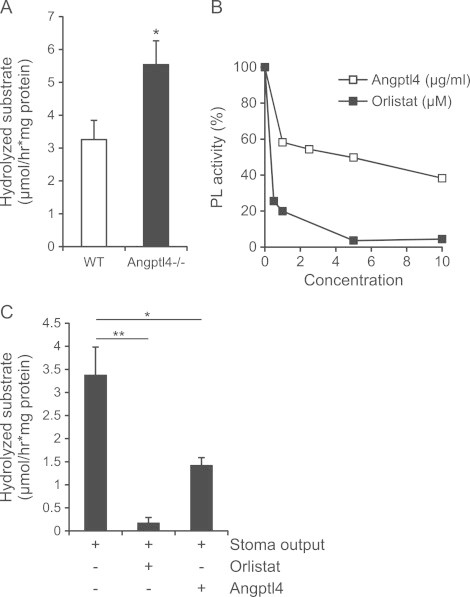
Angptl4 inhibits pancreatic lipase. (A) Luminal lipase activity in fecal water derived from wild-type and Angptl4−/− mice (n=9 per group). (B) Recombinant PL activity in the presence of increasing concentrations of Orlistat or recombinant Angptl4. (C) Lipase activity in stoma output of human volunteers (in the absence or presence of Orlistat 25 μM) or recombinant human Angptl4 (10 μg/ml) (n=3). Data are mean±SEM. Asterisks indicate significant differences according to Student's t-test (A) or one-way ANOVA with Tukey's post-hoc test (C); ⁎ p≤0.05, ⁎⁎ p≤0.01.
Inasmuch as PL is responsible for the major portion of intestinal lipase activity, we determined the direct effect of Angptl4 on PL by assaying the activity of recombinant PL in the presence of increasing amounts of recombinant Angptl4. As shown in Figure 7B, Angptl4 inhibited PL activity in a dose-dependent manner. To extend these findings we collected ileostomy output from three otherwise healthy humans. Similar to mouse intestinal content, human material contained high lipase activity, which was repressed by the PL inhibitor Orlistat (Figure 7C). Importantly, adding recombinant human Angptl4 significantly decreased the luminal lipase activity (Figure 7C). Together, these data indicate that Angptl4 inhibits intestinal lipase activity.
4. Discussion
Our results indicate that Angptl4 serves as an endogenous inhibitor of dietary lipid digestion via inhibition of PL, reducing the amount of energy that is harvested from dietary fat. As a result, mice lacking Angptl4 have less fat in their feces and gain more weight over time. Loss of Angptl4 does not appear to have any effect on energy expenditure or food intake. A key question elicited by our study is: what is the physiological relevance of an endogenous intestinal lipase inhibitor, production of which is increased by dietary fat? It is generally accepted that chylomicron secretion is rate limiting for lipid absorption, as evidenced by the constant rate of TG entry into the blood during an oral lipid loading test. Following consumption of a copious fat-rich meal, absence of any regulation on TG digestion may be expected to lead to excess fatty acid uptake into enterocytes, exceeding the capacity for re-esterification and chylomicron secretion and thus leading to lipid overload. To avoid such a scenario, production of Angptl4 is quickly stimulated upon entry of dietary fatty acids into enterocytes, putting a brake on dietary TG digestion. We propose that Angptl4 is an essential component of a feedback mechanism aimed at matching lipid uptake into enterocytes to the capacity for TG secretion. Previously, a similar role for Angptl4 in protection against lipid overload was demonstrated in cardiomyocytes and macrophages [19,20].
The mechanism by which Angptl4 inhibits PL at the biochemical level requires further investigation. While we present a compelling case for direct inhibition of PL by Angptl4 as the underlying mechanism accounting for enhanced fat uptake in Angptl4−/− mice, we cannot completely exclude a potential effect of Angptl4 deletion on total PL secretion. Indeed, an increase in PL secretion in Angptl4−/− mice would lead to increased lipid uptake and decreased fat secretion in the stools. However, recombinant Angptl4 was able to inhibit pancreatic lipase, pointing to a direct effect on PL. Since PL does not function as a homodimer, Angptl4 cannot inhibit PL by promoting the irreversible dimer to monomer conversion, as has been proposed for LPL [23]. Interestingly, recent data suggest that Angptl4 may reversibly inhibit LPL independent of dimer dissociation by forming an inhibitory complex with LPL that regains lipase activity upon dissociation [24]. It can be hypothesized that a similar mechanism may underlie inhibition of PL by Angptl4. It should be noted that Angptl4 was able to inhibit PL in the absence of colipase, and we therefore believe it is unlikely that the inhibitory effect is attributable to interference of the interaction between PL and colipase. Although the Angptl4 concentrations required for in vitro PL inhibition were relatively high, in vitro inhibition of LPL by Angptl4 under similar conditions also requires relatively high concentrations of Angptl4, yet there is unequivocal evidence that Angptl4 serves as physiological inhibitor of LPL in vivo [18,23,41]. It can be hypothesized that the high concentrations of Angptl4 required for in vitro LPL and PL inhibition may be related to absence of specific factors that shift the dose response curve.
Whereas numerous exogenous intestinal lipase inhibitors have been characterized, mainly representing plant derived components such as saponins, polyphenols, and terpenes [42,43], to our knowledge we have identified the first endogenous inhibitor of PL. Interestingly, several endogenous inhibitors of the major protein digestive enzymes including trypsin and chemotrypsin have been described [44,45]. Although the majority is thought to function in the pancreas to prevent premature activation of zymogens [44], expression of some of these inhibitors is found in the intestine [46,47], suggesting a role in controlling luminal protease activity.
In recent years, the gut microbiota has received much attention as alleged critical contributor to weight gain and obesity [48,49]. In this context, a role of Angptl4 in linking gut microbiota to fat storage has been proposed. Specifically, introducing microbiota into germ-free mice or zebrafish was found to markedly reduce intestinal Angptl4 expression [50,51]. Furthermore, whereas wild-type and Angptl4−/− mice have similar amounts of body fat on a germ-free background, introducing microbiota leads to a much smaller increase in body fat in Angptl4−/− mice compared to wild-type mice, suggesting that microbial suppression of Angptl4 contributes to increased fat deposition. Suppression of Angptl4 was suggested to promote fat storage by loss of inhibition of LPL activity in adipose tissue and reduction in expression of Pgc1α and fatty acid oxidation enzymes in liver and skeletal muscle [52]. Based on our data, it can be envisaged that microbial suppression of Angptl4 may also promote weight gain via loss of local action of Angptl4 in the gut, leading to elevated intestinal lipase activity and increased harvesting of dietary TGs. In apparent conflict with the decrease in Angptl4 expression upon colonization of germ-free animals, recent data suggest that short-chain fatty acids derived from microbial fermentation of dietary fibers induce Angptl4 expression in intestinal cells via PPARγ [38]. Although most of the short-chain fatty acids are produced in the colon, relatively high concentrations are also found in the small intestine [38]. It can be speculated that induction of Angptl4 may play a role in mediating the repressive effect of dietary fibers on lipid absorption [53,54].
Angptl4 undergoes cleavage to form N-terminal and C-terminal Angptl4 fragments. Recently, we found that C-terminal Angptl4, which is unable to inhibit lipase activity, is produced by entero-endocrine cells in human intestine, suggesting secretion towards the bloodstream [55]. We hypothesize that N-terminal Angptl4, which is able to inhibit lipase activity, is released selectively by enterocytes towards the lumen to inhibit PL. Future experiments will have to clarify both the exact site of N-terminal Angptl4 production as well as its presence in the lumen.
We have previously shown that Angptl4−/− mice develop a progressive and ultimately lethal inflammatory condition when chronically fed a HFD containing mainly saturated fatty acids [19]. However, it is highly unlikely that the enhanced intestinal lipid uptake in Angptl4−/− mice is related to underlying inflammatory processes, as enhanced weight gain is already apparent on a LFD and becomes more pronounced very quickly upon shifting to a HFD. Furthermore, the effect of Angptl4 on luminal lipase activity is apparent in mice fed a LFD or with tests that last only a few hours.
Recently, Kim and colleagues postulated that Angptl4 deletion leads to increased body weight due to an inhibitory effect of Angptl4 on hypothalamic AMPK and a subsequent increase in energy expenditure [56]. In our study we were unable to find a difference in energy expenditure when comparing wild-type and Angptl4−/− mice after a 1 week acclimatization to HFD, when the difference in weight gain between the two sets of mice is the most pronounced. Additionally, Kim and colleagues found that Angptl4−/− mice have increased food intake during fasting–refeeding, but not under ad libitum conditions [56]. Similarly, we have never observed any differences in food intake between ad libitum-fed wild-type and Angptl4−/− mice, either on chow diet, semi-synthetic LFD or a HFD. Interestingly, the Angptl4−/− mice employed in the studies by Kim et al. were resistant to diet induced obesity, while we have found several times that mice lacking Angptl4 gain more weight on a HFD, at least during the first 2 months. A potential explanation for the difference is that the development of the previously reported dietary saturated fat-induced inflammation in Angptl4−/− mice was greatly accelerated in the study by Kim and colleagues due to extremely high saturated fat content of the diet, leading to reduced food intake as described previously [19].
Collectively, we describe a novel function of Angptl4 in the regulation of lipid metabolism. By acting as a gatekeeper in the regulation of intestinal lipid uptake, Angptl4 decreases the amount of lipid being harvested from the ingested food and protects against enterocyte lipid overload.
Conflict of interest
The authors report no conflict of interest.
Acknowledgments
We thank Jvalini Dwarkasing for assistance with body composition analysis. This study was supported by the Netherlands Nutrigenomics Centre and the Netherlands Organization for Scientific Research (NWO) (TOP grant 40-00812-98-08030 to S.K.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2013.11.004.
Appendix A. Supplementary materials
Supplementary material
References
- 1.Armand M. Lipases and lipolysis in the human digestive tract: where do we stand? Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10:156–164. doi: 10.1097/MCO.0b013e3280177687. [DOI] [PubMed] [Google Scholar]
- 2.Wilde P.J., Chu B.S. Interfacial & colloidal aspects of lipid digestion. Advances in Colloid and Interface Science. 2011;165:14–22. doi: 10.1016/j.cis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Carriere F., Barrowman J.A., Verger R., Laugier R. Secretion and contribution to lipolysis of gastric and pancreatic lipases during a test meal in humans. Gastroenterology. 1993;105:876–888. doi: 10.1016/0016-5085(93)90908-u. [DOI] [PubMed] [Google Scholar]
- 4.Whitcomb D.C., Lowe M.E. Human pancreatic digestive enzymes. Digestive Diseases and Sciences. 2007;52:1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee I.A., Forster D.J., Wilcox M.D., Dettmar P.W., Seal C.J., Pearson J.P. Physiological parameters governing the action of pancreatic lipase. Nutrition Research Reviews. 2010;23:146–154. doi: 10.1017/S0954422410000028. [DOI] [PubMed] [Google Scholar]
- 6.Wang T.Y., Liu M., Portincasa P., Wang D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. European Journal of Clinical Investigation. 2013;43:1203–1223. doi: 10.1111/eci.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demignot, S., Beilstein, F., Morel, E., Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie. http://dx.doi.org/10.1016/j.biochi.2013.07.009, in press. [DOI] [PubMed]
- 8.Iqbal J., Hussain M.M. Intestinal lipid absorption. American Journal of Physiology – Endocrinology and Metabolism. 2009;296:E1183–E1194. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. American Journal of Physiology – Endocrinology and Metabolism. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 10.Beigneux A.P., Davies B.S., Gin P., Weinstein M.M., Farber E., Qiao X. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metabolism. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies B.S., Beigneux A.P., Barnes R.H., 2nd, Tu Y., Gin P., Weinstein M.M. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metabolism. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattijssen F., Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochimica et Biophysica Acta. 2012;1821:782–789. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E. Atypical angiopoietin-like protein that regulates ANGPTL3. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D.M., Cohen J.C. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16109–16114. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochemical and Biophysical Research Communications. 2012;424:786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan R., Zhang T., Hernandez M., Gan F.X., Wright S.D., Waters M.G. Regulation of the angiopoietin-like protein 3 gene by LXR. Journal of Lipid Research. 2003;44:136–143. doi: 10.1194/jlr.m200367-jlr200. [DOI] [PubMed] [Google Scholar]
- 17.Inaba T., Matsuda M., Shimamura M., Takei N., Terasaka N., Ando Y. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. Journal of Biological Chemistry. 2003;278:21344–21351. doi: 10.1074/jbc.M213202200. [DOI] [PubMed] [Google Scholar]
- 18.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. Journal of Biological Chemistry. 2006;281:934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein L., Mattijssen F., de Wit N.J., Georgiadi A., Hooiveld G.J., van der Meer R. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metabolism. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgiadi A., Lichtenstein L., Degenhardt T., Boekschoten M.V., van Bilsen M., Desvergne B. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circulation Research. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 21.Yu X., Burgess S.C., Ge H., Wong K.K., Nassem R.H., Garry D.J. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroupa O., Vorrsjo E., Stienstra R., Mattijssen F., Nilsson S.K., Sukonina V. Linking nutritional regulation of Angptl4, Gpihbp1, and Lmf1 to lipoprotein lipase activity in rodent adipose tissue. BMC Physiology. 2012;12:13. doi: 10.1186/1472-6793-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafferty M.J., Bradford K.C., Erie D.A., Neher S.B. Angiopoietin-like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. Journal of Biological Chemistry. 2013;288:28524–28534. doi: 10.1074/jbc.M113.497602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H.F., Hesselink M.K. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 26.Koliwad S.K., Kuo T., Shipp L.E., Gray N.E., Backhed F., So A.Y. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. Journal of Biological Chemistry. 2009;284:25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staiger H., Haas C., Machann J., Werner R., Weisser M., Schick F. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009;58:579–589. doi: 10.2337/db07-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong H., Schotz M.C. The lipase gene family. Journal of Lipid Research. 2002;43:993–999. doi: 10.1194/jlr.r200007-jlr200. [DOI] [PubMed] [Google Scholar]
- 29.Koster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 30.Hoevenaars F.P., Keijer J., Swarts H.J., Snaas-Alders S., Bekkenkamp-Grovenstein M., van Schothorst E.M. Effects of dietary history on energy metabolism and physiological parameters in C57BL/6J mice. Experimental Physiology. 2013;98:1053–1062. doi: 10.1113/expphysiol.2012.069518. [DOI] [PubMed] [Google Scholar]
- 31.Govers M.J., Van der Meet R. Effects of dietary calcium and phosphate on the intestinal interactions between calcium, phosphate, fatty acids, and bile acids. Gut. 1993;34:365–370. doi: 10.1136/gut.34.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goudriaan J.R., Dahlmans V.E., Febbraio M., Teusink B., Romijn J.A., Havekes L.M. Intestinal lipid absorption is not affected in CD36 deficient mice. Molecular and Cellular Biochemistry. 2002;239:199–202. [PubMed] [Google Scholar]
- 33.Plosch T., Kok T., Bloks V.W., Smit M.J., Havinga R., Chimini G. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. Journal of Biological Chemistry. 2002;277:33870–33877. doi: 10.1074/jbc.M206522200. [DOI] [PubMed] [Google Scholar]
- 34.Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 36.Dai M., Wang P., Boyd A.D., Kostov G., Athey B., Jones E.G. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Research. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunger M., van den Bosch H.M., van der Meijde J., Kersten S., Hooiveld G.J., Muller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiological Genomics. 2007;30:192–204. doi: 10.1152/physiolgenomics.00198.2006. [DOI] [PubMed] [Google Scholar]
- 38.Alex S., Lange K., Amolo T., Grinstead J.S., Haakonsson A.K., Szalowska E. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor gamma. Molecular and Cellular Biology. 2013;33:1303–1316. doi: 10.1128/MCB.00858-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korecka A., de Wouters T., Cultrone A., Lapaque N., Pettersson S., Dore J. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2013;304:G1025–G1037. doi: 10.1152/ajpgi.00293.2012. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenstein L., Berbee J.F., van Dijk S.J., van Dijk K.W., Bensadoun A., Kema I.P. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K., Shimizugawa T., Ono M., Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. Journal of Lipid Research. 2002;43:1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 42.de la Garza A.L., Milagro F.I., Boque N., Campion J., Martinez J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Medica. 2011;77:773–785. doi: 10.1055/s-0030-1270924. [DOI] [PubMed] [Google Scholar]
- 43.Birari R.B., Bhutani K.K. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discovery Today. 2007;12:879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Marchbank T., Freeman T.C., Playford R.J. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59:167–174. doi: 10.1159/000007485. [DOI] [PubMed] [Google Scholar]
- 45.Laskowski M., Jr., Kato I. Protein inhibitors of proteinases. Annual Review of Biochemistry. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- 46.Bohe H., Bohe M., Lundberg E., Polling A., Ohlsson K. Production and secretion of pancreatic secretory trypsin inhibitor in normal human small intestine. Journal of Gastroenterology. 1997;32:623–627. doi: 10.1007/BF02934111. [DOI] [PubMed] [Google Scholar]
- 47.Wang J., Ohmuraya M., Hirota M., Baba H., Zhao G., Takeya M. Expression pattern of serine protease inhibitor kazal type 3 (Spink3) during mouse embryonic development. Histochemistry and Cell Biology. 2008;130:387–397. doi: 10.1007/s00418-008-0425-8. [DOI] [PubMed] [Google Scholar]
- 48.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 49.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 50.Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camp J.G., Jazwa A.L., Trent C.M., Rawls J.F. Intronic cis-regulatory modules mediate tissue-specific and microbial control of angptl4/fiaf transcription. PLoS Genetics. 2012;8:e1002585. doi: 10.1371/journal.pgen.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Backhed F., Manchester J.K., Semenkovich C.F., Gordon J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santas J., Espadaler J., Cune J., Rafecas M. Partially hydrolyzed guar gums reduce dietary fatty acid and sterol absorption in guinea pigs independent of viscosity. Lipids. 2012;47:697–705. doi: 10.1007/s11745-012-3682-1. [DOI] [PubMed] [Google Scholar]
- 54.Vahouny G.V., Satchithanandam S., Chen I., Tepper S.A., Kritchevsky D., Lightfoot F.G. Dietary fiber and intestinal adaptation: effects on lipid absorption and lymphatic transport in the rat. American Journal of Clinical Nutrition. 1988;47:201–206. doi: 10.1093/ajcn/47.2.201. [DOI] [PubMed] [Google Scholar]
- 55.Alex, S., Lichtenstein, L., Dijk, W., Mensink, R.P., Tan, N.S., Kersten, S., ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochemistry and Cell Biology. http://dx.doi.org/10.1007/s00418-013-1157-y, in press. [DOI] [PubMed]
- 56.Kim H.K., Youn B.S., Shin M.S., Namkoong C., Park K.H., Baik J.H. Hypothalamic Angptl4/Fiaf is a novel regulator of food intake and body weight. Diabetes. 2010;59:2772–2780. doi: 10.2337/db10-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


