Abstract
Brain lipid sensing is necessary to regulate energy balance. Lipoprotein lipase (LPL) may play a role in this process. We tested if hippocampal LPL regulated energy homeostasis in rodents by specifically attenuating LPL activity in the hippocampus of rats and mice, either by infusing a pharmacological inhibitor (tyloxapol), or using a genetic approach (adeno-associated virus expressing Cre-GFP injected into Lpllox/lox mice). Decreased LPL activity by either method led to increased body weight gain due to decreased locomotor activity and energy expenditure, concomitant with increased parasympathetic tone (unchanged food intake). Decreased LPL activity in both models was associated with increased de novo ceramide synthesis and neurogenesis in the hippocampus, while intrahippocampal infusion of de novo ceramide synthesis inhibitor myriocin completely prevented body weight gain. We conclude that hippocampal lipid sensing might represent a core mechanism for energy homeostasis regulation through de novo ceramide synthesis.
Abbreviations: LPL, lipoprotein lipase; CNS, central nervous system; TG, triglycerides; AAV, adeno-associated virus; GFP, green fluorescent protein; ANS, autonomic nervous system; RQ, respiratory quotient; SPT, serine palmitoyltransferase; CERS, ceramide synthase; SMPD1, acid sphingomyelin phosphodiesterase 1; SPHK1, sphingosine kinase 1
Keywords: Lipid sensing, Obesity, Ceramides, Parasympathetic nervous system, Energy expenditure
Graphical abstract
Hippocampal LPL contributes to body weight regulation by controlling locomotor activity and energy expenditure. Such LPL activity involves local de novo ceramide synthesis and parasympathetic nervous system output. LPL: lipoprotein lipase, PNS: parasympathetic nervous system.
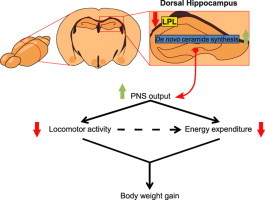
1. Introduction
The central nervous system (CNS) is a key player in the regulation of energy balance in mammals [1,2]. This process involves a combination of signals arising from the periphery, including hormones and nutrients, which are detected by specialized areas like the hypothalamus and brainstem [3–5]. Since the work of Oomura et al. [6], there is a growing amount of evidence to suggest that hypothalamic fatty acid sensing plays a role in the regulation of energy balance, including insulin secretion and action, hepatic glucose production and food intake [7–10]. However the molecular mechanisms involved in this fatty acid sensing are still a matter of debate [8]. Postprandial triglycerides (TG)-enriched particles are abundant lipid species hydrolyzed by the lipoprotein lipase (LPL), and recent studies have highlighted a role for neuronal LPL-mediated hydrolysis of TG particles in the regulation energy balance [11,12].
Otherwise, other areas beside the hypothalamus have been shown to be involved in the regulation of energy homeostasis. Among them, the hippocampus has also been described as a potential site for the regulation of feeding behavior and body weight homeostasis [13,14]. For example, hippocampal lesions are associated with increased body weight and food intake in ad libitum fed rats [15]. Hunger and satiety circulating signals such as leptin or ghrelin were also shown to bind to hippocampus cells to regulate food intake in rats [16]. Moreover the orexigenic peptide ghrelin was shown to modulate hippocampal dendritic spine and memory acquisition [17]. Studies in both rodents and humans have reported that high fat/refined sugar diets impair hippocampal function [18]. Interestingly, LPL is highly expressed in the hippocampus [19,20] suggesting a potential role for this enzyme in body weight regulation.
The present work was aimed at studying whether TG hydrolysis by LPL specifically in the dorsal hippocampus could represent a physiologically relevant mechanism in energy homeostasis and body weight regulation. To this end, two different species and two experimental approaches were used to locally decrease LPL activity in the hippocampus: (1) the infusion of tyloxapol, an inhibitor of LPL activity, into the hippocampus of rats and (2) the bilateral injection of an adeno-associated viral vector expressing a Cre-GFP fusion protein (AAV Cre-GFP) into the hippocampus of Lpllox/lox mice, leading to the specific deletion of the Lpl gene in the hippocampus of these mice (“LPL Hip−/−” ). We observed using both techniques that hippocampal LPL inhibition led to both decreased locomotor activity and energy expenditure and ultimately induced body weight gain. Importantly, food intake remained unchanged while the parasympathetic nervous system activity was increased suggesting a change in autonomic nervous system (ANS)-mediated peripheral energy handling. Inhibition of hippocampal LPL activity led to increased de novo ceramide biosynthesis, and a concomitant increase in neurogenesis, while, conversely, local infusion of myriocin – a potent inhibitor of de novo ceramide biosynthesis – completely prevented the metabolic changes in both mice and rats. Taken collectively these results highlight for the first time to our knowledge that nutritional lipid detection by LPL within hippocampus controls energy balance through de novo ceramide synthesis pathway.
2. Material and methods
The experimental protocol was approved by the institutional animal care and use committee of the Paris Diderot University (CEEA40).
2.1. Animal models
Two-month-old male Wistar rats (225–250 g, Charles River, l'Arbresle, France) and two-month-old Lpllox/lox mice (Jackson laboratory, strain B6.129S4-Lpltm1Ijg/J, no. 006503) were used. Littermates Lpl+/+ mice were used as controls. They were housed individually in stainless steel cages in a room maintained at 22±1 °C with lights on from 7 a.m. to 7 p.m. They were given a standard laboratory diet (proteins 19.4%; carbohydrates 59.5%; lipids 4.6%; vitamins and minerals 16.5%) and water ad libitum.
2.2. Solutions
Osmotic minipumps (Alzet® model 2004) were used to chronically infuse different solutions specifically into the hippocampus, through a catheter connected to a depth-adjustable cannula (Alzet® brain infusion kit 1 for rats and Alzet® brain infusion kit 3 for mice). The vehicle solution consisted of saline (Lavoisier). Tyloxapol solution (10 µg/day) was prepared by diluting 25 µL of tyloxapol (Sigma Aldrich # T8761) with 16.6 ml of saline. Myriocin solution (100 nM) was prepared by diluting a myriocin mother solution (Sigma Aldrich # M117; 2 mg/ml in methanol) 50,000 times in saline.
2.3. Viral production
An adeno-associated virus, AAV Cre-GFP, was used in order to induce genetic recombination within the hippocampus in Lpllox/lox mice. The plasmid CBA.nls myc Cre.eGFP expressing the myc-nls-Cre-GFP fusion protein was kindly provided by Richard Palmiter (University of Washington, Seattle, USA). Adeno-associated viruses of the serotype 2/9 (AAV2/9) (6×1011 vg/ml and 1.7×108 pi/µl) were produced by the viral production facility of the UMR INSERM 1089 (Nantes, France).
2.4. Surgical procedures
Both rats and mice were anesthetized with isoflurane and received a 10 µg/kg i.p. administration of xylazine. They were then placed on a stereotaxic frame. In rats, osmotic minipumps containing either vehicle, tyloxapol, myriocin, or both tyloxapol and myriocin were inserted subcutaneously and connected to cannula implanted within the hippocampus (X : −3.5 mm; Y: −4.5 mm; Z: −3.5 mm; [21]). In Lpl+/+ and Lpllox/lox littermate mice, 1 µl of 2/9 AAV Cre-GFP was injected per side (∼6×108 particles/µl at a rate of 0.20 µL/min for 5 min) bilaterally into the hippocampus (X : ±1 mm; Y: −2.06 mm; Z: −1.55 mm; [22]) to induce genetic recombination in floxed animals. After viral injection, mice were implanted with osmotic minipumps containing myriocin or vehicle.
2.5. Measurement of body weight and body composition
In rats, body weight was measured daily between 9 and 10 a.m. In mice, body weight was measured weekly at the same time. For both, body mass composition (lean tissue mass, fat mass, free water and total water content) was analyzed before the indirect calorimetry studies using an Echo Medical Systems EchoMRI 100 (Whole Body Composition Analyzers, EchoMRI, Houston, USA) according to manufacturer's instructions [23]. Briefly, awake animals were weighed before they were placed in a mouse holder and inserted in the MRI analyzer. Readings of body composition were obtained within 1 min. Body composition was expressed as a percentage of body weight.
2.6. Spontaneous alternation plus-maze task
Both vehicle and tyloxapol-infused rats were used for memory testing. Animals were extensively handled for one week prior to testing. Animals were placed into the center the center of the maze and allowed to explore freely for 20 min while arm entries were recorded. Performance scores were calculated as described previously [24].
2.7. Indirect calorimetry
Indirect calorimetry studies were performed in both rats and mice at day 14 during 5 days; this period corresponded to the beginning of increased body weight gain compared to controls. Animals were analyzed for whole energy expenditure (kcal/h), oxygen consumption and carbon dioxide production (VO2 and VCO2, where V is the volume), respiratory quotient (RQ=VCO2/VO2), food intake (g) and locomotor activity (counts/hour) using calorimetric cages with bedding, food and water (Labmaster, TSE Systems GmbH, Bad Homburg, Germany). Gas ratio was determined using an indirect open-circuit calorimeter [25,26], which monitored O2 and CO2 concentrations by volume at the inlet ports of a tide cage with an airflow of 0.4 L/min, with regular comparisons to an empty reference cage. Whole energy expenditure was calculated according to the Weir equation, using respiratory gas exchange measurements [27]. The flow was previously calibrated with a O2 and CO2 mixture of known concentrations (Air Liquide, S.A. France). Animals were individually housed in a cage with lights on from 7 a.m. to 7 p.m. and an ambient temperature of 22±±1 °C. All animals were acclimated to their cages for 48 h before experimental measurements. Data regarding food and water consumption were collected every 40 min, and all ambulatory movements recorded during the entire experiment, with the aid of an automated online measurement system combining highly sensitive feeding and drinking sensors and an infrared beam-based activity monitoring system. Gas and movement detection sensors were operational during both light and dark phases, allowing for continuous recording. Animals were monitored for body weight and composition at the beginning and end of the experiment. Data analysis was carried out with Excel XP using extracted raw values of VO2 consumption, VCO2 production (in ml/h), and energy expenditure (kcal/h). Subsequently, each value was expressed either by whole lean tissue mass extracted from the EchoMRI analysis.
2.8. Tissue collection
Brain tissues were dissected following the Glowinski and Iversen technique [28] at day 28; total hippocampus, adjacent cortex and hypothalamus were immediately frozen.
2.9. LPL activity assay
Heparin-releasable LPL activity was assayed in brain regions using a Roar LPL activity assay kit (RB-LPL, Roar Biomedical, Inc.). Briefly, tissues at day 28, then were lysed in 500 µL of assay buffer (150 mM NaCl, 10 mM Tris, 2 mM EDTA, pH 7.4) and incubated for 45 min at 37 °C with an equivalent volume of heparin (100 U/ml). After incubation, samples were centrifuged for 10 min at 3000g, and aliquots (50 µL for rats and 10 µL for mice) of the aqueous phase deposited on LPL substrate emulsion in 96-well black microplates (VWR International, # 25227-304) and incubated for 1 h at 37 °C. Finally, fluorescence was read using a fluorimeter (370 nm excitation/450 nm emission) and compared to a standard curve made using known concentrations of pre-hydrolyzed LPL substrate. LPL activity was expressed as nanomoles of free fatty acid produced per minute per gram of tissue.
2.10. Real-time quantitative PCR
Total RNA was isolated from the hippocampus dissected at day 28 at the end of the experimentation using RNeasy Lipid Tissue mini kit (Qiagen, Courtaboeuf, France). Real-time quantitative PCR was carried out in a LightCycler 1.5 detection system (Roche, Meylan, France) using the Light-Cycler FastStart DNA Master plus SYBR Green I kit (Roche). The mRNA transcript level for each gene was normalized to levels of the housekeeping gene TBP, which we have previously shown to be unaffected by LPL inhibition.
2.11. Measurement of ceramide levels
Ceramide levels in tissues extracts collected at day 28 were measured by the diacylglycerol kinase enzymatic method as previously described [29,30]. Briefly, aliquots of the chloroform phases from cellular lipid extracts were re-suspended in 7.5% (w/v) octyl-α-d-glucopyranoside/5 mM cardiolipin in 1 mM DETPAC/10 mM imidazole (pH 6.6). The enzymatic reaction was started by the addition of 20 mM DTT, 0.88 U/ml Escherichia coli diacylglycerol kinase, 5 µCi/10 mM [γ-32P] ATP and the reaction buffer (100 mM imidazole (pH 6.6), 100 mM NaCl, 25 mM MgCl2, and 2 mM EGTA). After incubation for 1 h at room temperature, lipids were extracted with chloroform/methanol/HCl (100:100:1, v/v) and 1 M KCl. [γ-32P]-ceramide-1-phosphate was resolved by TLC with chloroform/acetone/methanol/acetic acid/water (10:4:3:2:1, v/v) and quantified with a FLA700 phosphorimager (Fuji, Japan). Known amounts of bovine ceramide standards were included with each assay. Each measurement was done in duplicate. Ceramide levels were expressed as nanomoles per gram of tissue.
2.12. Parasympathetic firing-rate recordings
The firing rate of the thoracic branch of vagus nerve along the carotid artery was recorded at the end of the experimentation at day 28 as previously described [31]. Recordings were carried out as follows: animals were anesthetized with isoflurane (Sanofi, Libourne, France). The vagus nerve, which lies close to the carotid artery, was dissected free of underlying tissues to a distance of approximately 5 mm. The nerve was then covered with paraffin oil to prevent dehydration and carefully placed on a pair of silver-wire recording electrodes (0.6-mm diameter). The electrodes were connected to a high-impedance probe and action potentials were displayed and saved on a computer after initial amplification through a low-noise amplifier (BIO amplifier, AD Instrument, Rabalot, France). Unipolar nerve activity was recorded continuously for 15 min. Data were digitized with PowerLab/4sp digitizer. Signals were amplified 105 times and filtered using low- and high-frequency cut-offs of 100 and 1000 Hz, and monitored using the Chart 4 computer program [32].
2.13. Statistical analysis
Effects of treatments were assessed by either two-way ANOVA with Bonferoni post hoc test or Student t-test (Figure 1A and B and Figure 2B). A p-value lower than 0.05 was defined to be statistically significant. Statistical analysis were performed using GraphPad Prism 5®.
Figure 1.
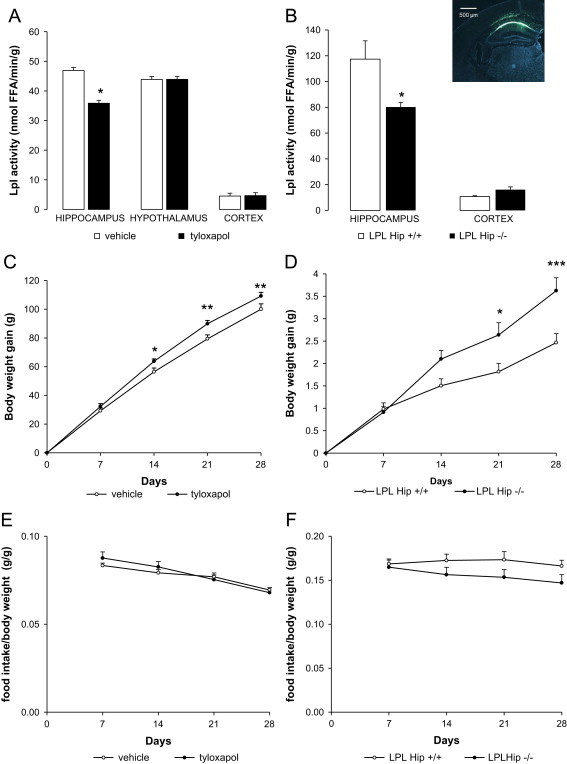
Decreased LPL activity in the hippocampus increases body weight gain in mice and rats without affecting food intake. (A) LPL activity in the hippocampus, hypothalamus and cortex of rats infused with vehicle or tyloxapol for 28 days, through an osmotic minipump connected to a cannula implanted in hippocampus; n≥6. FFA: free fatty acids. (B) LPL activity in the hippocampus and cortex of mice with a specific deletion of the Lpl gene in the hippocampus after AAV Cre-GFP injection (LPL Hip−/− mice) and their control littermates (LPL Hip+/+); n≥3. Inset in (B): Cre-GFP fusion protein fluorescence in right hippocampus (coordinates X: +1 mm; Y: −2.06 mm; Z: −1.55 mm, 20× magnification) 4 weeks after injection shows specific expression of the protein within hippocampus with high fluorescence in CA1 pyramidal layer. (C and D) Body weight gain after the beginning of intrahippocampal tyloxapol infusion in rats (C) or AAV Cre-GFP injection in Lpllox/lox mice (D); n≥6. (E and F) Time course of food intake reported to body weight in rats (E) and mice (F) during the 28 days of treatment; n≥6 for both rats and mice.
Figure 2.
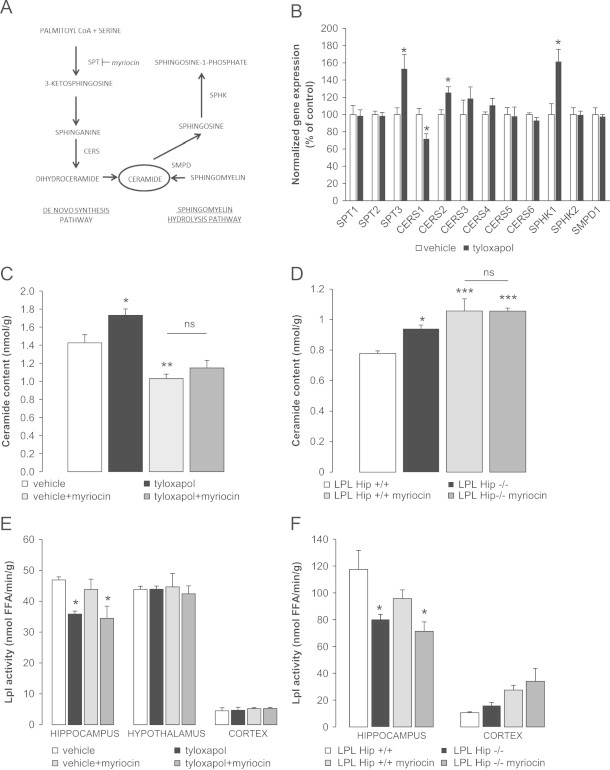
Decreased LPL activity is associated with increased hippocampal ceramide content through the activation of the de novo synthesis pathway. (A) Ceramide synthesis pathways: the de novo synthesis pathway that is inhibited by myriocin (left) and the sphingomyelin hydrolysis pathway (right). (B) Changes in gene expression of enzymes involved in ceramide synthesis pathways in tyloxapol-treated rats vs. controls; n≥5. (C and D) Hippocampal ceramide content in tyloxapol-treated and control rats co-infused with myriocin or vehicle (C), and hippocampal ceramide content in LPL Hip+/+ and LPL Hip−/− mice with or without myriocin treatment (D); n≥6 in rats and n≥3 in mice. (E and F) LPL activity in rats and mice in the four groups above; n≥6 and n≥3, respectively. *: p<0.05, **: p<0.01 vs. controls.
3. Results
3.1. Decreased LPL activity in the hippocampus increases body weight gain in mice and rats without any change in food intake
LPL activity was specifically decreased in the hippocampus using both pharmacological and genetic approaches. In rats, tyloxapol, an inhibitor of LPL activity, or vehicle was infused into the hippocampus for 28 days using a depth adjustable cannula (X: −3.5 mm; Y: −4.5 mm; Z: −3.5 mm [21]) connected to an osmotic minipump (Alzet). In this model, LPL activity was significantly decreased by approximately 25% in the hippocampus of tyloxapol-infused animals compared to controls, while the hypothalamic and cortical LPL activity were unchanged (Figure 1A). Importantly, there was no change in performance on a spontaneous alternation task in tyloxapol-infused rats compared to controls, indicating that there were no untoward effects of drug infusion such as a hippocampal lesion (percent alternation: 51%±9 vs. 65%±14 in vehicle group, N.S.). In the genetic approach, Lpl+/+ and Lpllox/lox mice received a single injection of Cre-GFP AAV into the hippocampus (X: ±1 mm; Y: −2.06 mm; Z: −1.55 mm [22]), yielding LPL Hip+/+ and LPL Hip−/− mice, respectively. In this model, we found a 33% decrease in LPL activity (Figure 1B). In either of the paradigm, a decrease in LPL activity selectively in the hippocampus led to enhanced body weight gain as compared to controls (Figure 1C and D). The time course of body weight gain was similar in both species, i.e. there was a significant increase compared to controls beginning on day 14. Interestingly, this increase in body weight gain was not related to a concomitant increase in food intake (Figure 1E and F).
3.2. Selective decreased in hippocampal LPL activity induces de novo ceramide biosynthesis
In the rat model, using real-time quantitative PCR, we observed changes in the expression of key enzymes involved in de novo ceramide synthesis specifically in the hippocampus, following tyloxapol treatment (Figure 2A and B). The mRNA levels of both serine palmitoyltransferase 3 (SPT3) and ceramide synthase 2 (CERS2) were increased, whereas the expression of acid sphingomyelin phosphodiesterase (SMPD1) – a key enzyme in the sphingomyelin hydrolysis pathway – was not affected by the reduction in LPL activity, thus suggesting an activation of the de novo ceramide biosynthesis (Figure 2B). The expression of sphingosine kinase 1 (SPHK1) was also increased in tyloxapol-infused rats (Figure 2B). The decrease of hippocampal LPL activity induced a concomitant increase in hippocampal ceramide content in tyloxapol-treated rats (Figure 2C) whereas cortex ceramide content was unchanged (tyloxapol 1.55±0.12 nmol/g vs. vehicle 1.68±0.24 nmol, NS). Hippocampal ceramide content was also increased in LPL Hip−/− mice (Figure 2D).
In rats, the infusion of the specific inhibitor of the SPTs, myriocin, completely prevented the tyloxapol-induced increase in the ceramide content through the inhibition of de novo ceramide synthesis in the hippocampus and had no effect on ceramide content in the cortex (vehicle+myriocin 1.33±0.18 nmol/g, tyloxapol+myriocin 1.46±0.19 nmol/l, NS vs. vehicle and NS vs. tyloxapol). These changes did not restore LPL activity (Figure 2C–F) therefore pointing at ceramide synthesis as a downstream mechanism. In mice, myriocin infusion led to increased total ceramide content in LPL Hip+/+ and LPL Hip−/− mice (Figure 2D), likely due to the compensatory stimulation of the sphingomyelin hydrolysis pathway as it has been already described [33]. However, myriocin also prevented any additional ceramide accumulation in LPL Hip−/− vs. LPL Hip+/+ mice (Figure 2D), again without any significant impact on LPL activity (Figure 2F).
3.3. Hippocampus de novo ceramide synthesis is required to mediate LPL action on energy balance
Blockade of de novo ceramide synthesis through myriocin infusion into the hippocampus selectively prevented in both rats and mice the gain in body weight associated with decreased LPL activity (Figure 3A and B). This was not due to changes in food intake (Figure 3C and D) or alterations in body composition that remained unchanged (data not shown). As depicted in Figure 3E, energy expenditure was significantly decreased in tyloxapol-treated rats during light phase (p=0.06 at night), while, in LPL Hip−/− mice, energy expenditure was significantly decreased during both light and dark phase (Figure 3F). The respiratory quotient (RQ), i.e. the ratio of VCO2/VO2 that provides an indication of the nature of the substrate being used by an organism, remained unchanged after the decreasing of LPL activity in both rats and mice (figure not shown). This decreased energy expenditure in LPL-attenuated animals was correlated with a reduction in locomotor activity in both rats (Figure 3G) and mice (Figure 3H) during dark phase. Importantly, myriocin treatment blocked the effects of LPL attenuation on energy expenditure as well as locomotor activity in both rats (Figure 3E and G) and mice (Figure 3F and H). Taken together, these results support the concept that de novo ceramide synthesis is a core molecular mechanism relaying the action of hippocampal TG sensing onto body weight regulation.
Figure 3.
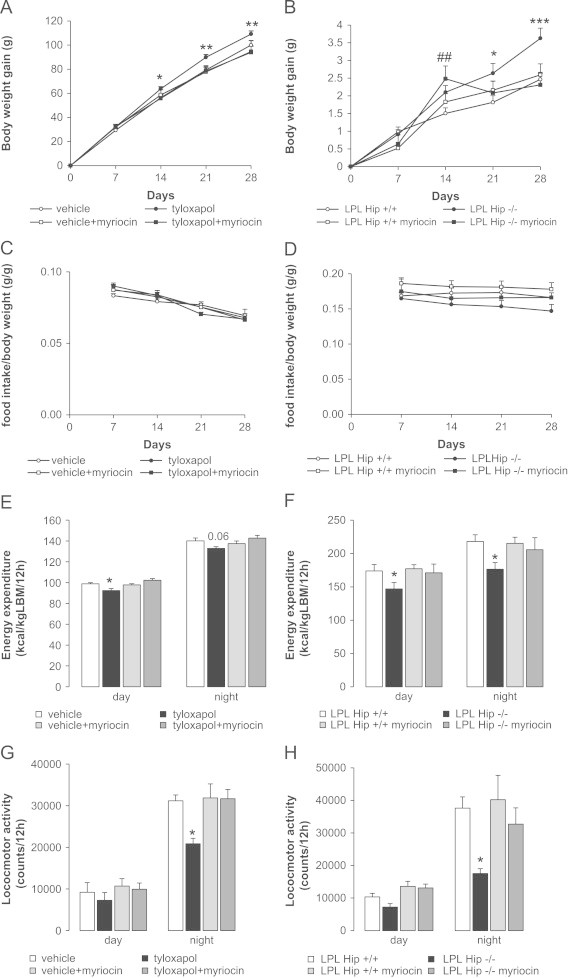
Myriocin infusion into the hippocampus completely prevents increased body weight gain in both models. (A and B) Body weight gain over 28 days in tyloxapol-treated and control rats with or without co-infusion with myriocin (A) and LPL Hip+/+ and LPL Hip−/− mice with or without myriocin treatment (B); n≥6 each. (A and D) Time course of food intake reported to body weight in rats (E) and mice (F) during the 28 days of treatment; n≥6 for both rats and mice. (E and F) Energy expenditure in tyloxapol-treated and control rats with or without co-infusion with myriocin (C) and LPL Hip+/+ and LPL Hip−/− mice with or without myriocin treatment (D); n≥6 each. (G and H) Locomotor activity in the 4 groups of rats (E) and mice (F); n≥6 for both. *: p<0.05, ***: p<0.001; tyloxapol or LPL Hip+/+ vs. controls. ##: p<0.01; LPL Hip−/− myriocin vs. LPL Hip+/+.
3.4. Hippocampal attenuation of TG hydrolysis increases parasympathetic nervous system activity in a ceramide dependent mechanism
The change in body weight was independent from feeding, we therefore investigated to what extend non-food related modification in ANS output could be affected by hippocampus LPL knockdown.
The activity of parasympathetic nervous system was recorded in vagus nerve along the carotid artery under basal conditions. Parasympathetic nervous system activity was significantly increased in tyloxapol-treated rats (Figure 4A and C) and LPL Hip−/− mice (Figure 4B and D) when compared to their respective controls. Again we found that myriocin treatment normalized parasympathetic nervous system activity in both models of LPL activity inhibition the hippocampus.
Figure 4.
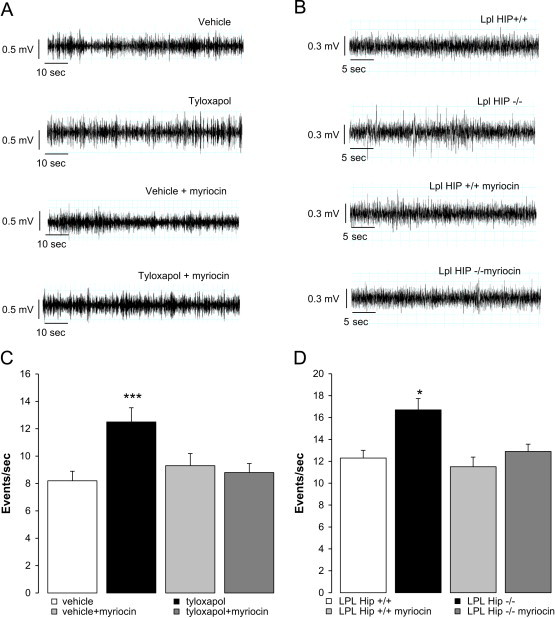
Increased body weight gain is related to increased parasympathetic nervous system activity and is prevented by myriocin treatment. (A and B) Parasympathetic nervous system activity recorded in tyloxapol-treated and control rats with or without co-infusion with myriocin (A) and LPL Hip+/+ and LPL Hip−/− mice with or without myriocin treatment (B); n≥4 each. (C and D) Quantification of parasympathetic nervous system activity in the 4 groups of rats (C) and mice (D); n≥4 each. *: p<0.05, ***: p<0.001 vs. controls.
3.5. Selective decrease in hippocampal LPL activity increases hippocampal neurogenesis in both mice and rats through ceramide signaling
As shown in Supplementary Figures S1 and S2, the inhibition of hippocampal LPL activity led to an increase in neurogenesis, assessed by BrdU and neuronal marker co-labeling. Myriocin administration fully prevented the increased neurogenesis in tyloxapol-treated rats (Figure S1) and LPL Hip−/− mice (Figure S2), while it had no effect on neurogenesis in controls.
4. Discussion
Fatty acid sensing by the brain plays a key role in the regulation of energy homeostasis by the central nervous system (CNS) [8,34]. Within the last decade, there is a growing evidence to demonstrate that hypothalamic fatty-acid-sensing is critical for the regulation of food intake as well as of insulin secretion, hepatic glucose production and lipogenesis [8]. The intracerebroventricular infusion of oleic acid has been shown to decrease both food intake and hepatic glucose production [7,9]. However, the idea that an increase in brain fatty acid levels could act as a satiety signal to inhibit feeding appears counterintuitive, given that plasma fatty acid levels do not rise substantially after food ingestion, but do rise significantly during fasting [35], a situation in which food intake would be expected to increase. On the other hand, plasma levels of triglyceride-enriched lipoproteins do rise after food ingestion and could represent physiologically relevant signals to modulate energy balance.
The TG-rich particles hydrolyzing enzyme LPL has recently been demonstrated to play a role in the regulation of energy balance by neurons [11]. The role of LPL in the brain is to convert TG-rich lipoproteins into fatty acids locally, thus providing a signal of the metabolic state to fatty-acid-sensitive neurons [12]. Mice lacking LPL in neurons (NEXLPL−/− mice) were shown to develop transient hyperphagia and obesity further confirming a role for central TG hydrolysis in body weight regulation [11]. However the precise structures that relay the action of TG detection onto body weight remain elusive. In particular, the hippocampus exhibits Lpl mRNA levels and activity that are at least 2.5 times higher than in the cortex, cerebellum or remaining brain [19] pointing at hippocampal TG hydrolysis as a potential mechanism linking daily variation in TG particles and energy homeostasis.
In order to specifically address this hypothesis we used several approaches and rodent models to selectively alter hippocampal TG hydrolysis ability. The first approach relied on local infusion of the non-ionic detergent tyloxapol (oxyethylated t-octylphenol polymethylene polymer, also named Triton WR 1339), the second approach relied on virus-mediated genetic knock out of Lpl gene through bilateral injection of AAV Cre-GFP into the hippocampus of Lpllox/lox mice.
In both models, we demonstrated that dampening hippocampal LPL activity in the hippocampus led to body weight gain. The body weight changes were not due to change in food intake but rather involved a decreased locomotor activity and decreased energy expenditure as well as an increased parasympathetic tone. The reduction of hippocampal LPL activity increases de novo ceramide synthesis, while the administration of myriocin, a de novo ceramide synthesis inhibitor, completely prevented metabolic disorders.
Among the physiological determinants that account for body weight gain, we predict that increased parasympathetic nervous system activity, which is known to facilitate energy storage by reducing energy expenditure [36] come in addition to decreased locomotor-dependent energy expenditure to achieve body weight gain in a feeding-independent manner.
Hippocampal lesion in rats leads to body weight gain when fed ad libitum [14]. It has recently been reported that leptin signaling in the hippocampus contributes to the inhibition of food-related memories elicited by contextual stimuli [16]. In mice fed a high-fat diet, NMDA receptors in the hippocampus are desensitized, possibly accounting for the cognitive deficits associated with obesity [37]. Furthermore, in obese individuals, functional magnetic resonance imaging (fMRI) studies have documented a discrepancy in homeostatic and non-homeostatic integration of hunger related signals that results in the opposite stimulation profile of hypothalamus vs. sensorimotor, emotional and cognitive areas including the amygdala, hippocampus, insula and precentral gyrus [38]. These observations suggest a putative link between high fat diet, hippocampal dysfunction and altered energy balance. Our data support this hypothesis and provide a physiological and molecular mechanism by which daily variation of nutritional TG rich particles directly alters hippocampus activity and body weight homeostasis. As a major excitatory input to nucleus accumbens interneurons, the hippocampus is thought to participate in the striatal encoding of goal directed behavior [39]. It is therefore formally possible that the reduction of nocturnal locomotor activity, following intra-hippocampal knock down of TG hydrolysis, is an indirect consequence of decreased motivational output. In that line, Ben Zeev et al. have observed a 60% increase in hippocampal LPL activity in rats deprived of food for 12 h when compared to fed rats, suggesting that hippocampal LPL activity during fasting could contribute to the enhanced locomotor activity necessary for animals to search for food [19]. In the fed state, in which animals did not have to move to find food, hippocampal LPL activity was decreased. In addition, exercise and hippocampal function have also been previously shown to be linked [40–42].
Brain areas controlling parasympathetic nervous system activity are located in the lateral hypothalamus, and the number of c-fos/orexin-immunoreactive neurons in the perifornical lateral hypothalamus is positively correlated with the amount of locomotion [43]. There are important reciprocal connections between the hippocampus and the hypothalamus. Projections between the lateral hypothalamus and the hippocampal dentate gyrus, have been documented by Wayner et al., who showed that long-term potentiation in hippocampal granule cells was inhibited by lateral hypothalamic afferents [44]. A recent study has demonstrated that hippocampal BDNF levels were enhanced by hypothalamic stimulation, in proportion to the metabolic rate [45]. Thus, any changes in hippocampal activity may have an impact on specific lateral hypothalamic neurons, in turn modifying parasympathetic activity and thus both locomotor activity and energy expenditure, and eventually, body weight.
We identified de novo ceramide synthesis as a molecular mechanism by which altered hippocampal TG hydrolysis affected energy balance. Ceramide content was increased by hippocampal LPL inhibition whereas blockade of de novo ceramide synthesis completely rescue the physiological output of LPL knock down. By definition, a decrease in LPL activity would limit the supply of fatty acids from outside the cell, likely triggering de novo fatty acid synthesis in order to provide acyl-CoA to the ceramide synthesis pathway. Consistently with this hypothesis, normal fat deposition in adipose tissue can occur in the complete absence of LPL, and conversely, if LPL activity is increased by pharmacological means, increased fat storage does not necessarily follow [46]. For example, fat mass is preserved by the endogenous synthesis of lipids in mice lacking LPL in adipose tissue [47], a process that involves an increase in glucose uptake and the concomitant induction of endogenous fatty acid and triglyceride production, probably through a mechanism involving SREBP-1 (sterol regulatory element-binding protein 1) [48]. Such a mechanism could also be involved in our study, with decreased LPL activity leading to increased de novo synthesis of palmitoyl-CoA, in the hippocampus. This could in turn be incorporated into the de novo ceramide synthesis pathway, as described in other cells such as monocytes [49]. The ceramides are known to act as an important cellular signaling molecule, and recent literature pointed out that ceramides with distinct acyls chains have different cellular functions [50]. In that view, it is formally possible that TG rich particles, which under normal condition enter the brain only during postprandial period, would not provide the main substrate for ceramide synthesis but rather act as signaling modulator of hippocampal cell activity.
The stimulation of de novo ceramide biosynthesis in the hippocampus could affect its function through changes in neuronal plasticity and neurogenesis. Hence, it has been reported that ceramide levels and/or composition are important for proper dendritic spine maturation in hippocampal neurons [51] and several studies have highlighted a role for lipids and dietary regulation in the control of neurogenesis in the hippocampus [52,53]. The relationship between global metabolic efficiency and neuroplasticity in the hippocampus has also been demonstrated [54]. In our study, we observed that the increased ceramide content associated with LPL activity knock down correlated positively with neurogenesis as evidenced by BrdU labeling (Supplementary Figures S1A and B and S2A and B). BrdU positive cells were mainly putative neurons in rats (Figure S1C and D) and both putative neurons and GFAP-positive astrocytes or neuronal precursors in mice (Figure S2C and D). Ceramide synthesis inhibition resulted in the opposite consequences. TG-mediated modulation of hippocampal neurogenesis might provide a cellular basis for long-term adaptive mechanism in response to nutritional changes.
To conclude, our study highlights for the first time on the role of hippocampal LPL in the regulation of energy balance depending on de novo ceramide biosynthesis pathway and the modulation of the parasympathetic nervous system activity.
Conflict of interest
None declared.
Acknowledgments
This work was supported by grants from the ANR (French National Agency for Research): Lipobrain, Grant number: 11-BSV1-021 01; and other Grant sponsors: CORDDIM Ile-de-France and European Foundation for the Study of Diabetes (EFSD).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary materials
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2013.11.002.
Appendix A. Supplementary materials
Supplementary material
Supplementary material
Supplementary material
References
- 1.Luquet S., Magnan C. The central nervous system at the core of the regulation of energy homeostasis. Frontiers in Bioscience (Scholar Edition) 2009;1:448–465. doi: 10.2741/s37. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Lasheras C., Konner A.C., Bruning J.C. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Frontiers in Neuroendocrinology. 2010;31(1):4–15. doi: 10.1016/j.yfrne.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Blouet C., Schwartz G.J. Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural Brain Research. 2010;209(1):1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Cowley M.A. Hypothalamic melanocortin neurons integrate signals of energy state. European Journal of Pharmacology. 2003;480(1–3):3–11. doi: 10.1016/j.ejphar.2003.08.087. [DOI] [PubMed] [Google Scholar]
- 5.Levin B.E., Magnan C., Dunn-Meynell A., Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152(7):2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oomura Y., Nakamura T., Sugimori M., Yamada Y. Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiology and Behavior. 1975;14(04):483–486. doi: 10.1016/0031-9384(75)90015-3. [DOI] [PubMed] [Google Scholar]
- 7.Lam T.K., Pocai A., Gutierrez-Juarez R., Obici S., Bryan J., Aguilar-Bryan L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nature Medicine. 2005;11(3):320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 8.Migrenne S., Le Foll C., Levin B.E., Magnan C. Brain lipid sensing and nervous control of energy balance. Diabetes and Metabolism. 2011;37(2):83–88. doi: 10.1016/j.diabet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Obici S., Feng Z., Morgan K., Stein D., Karkanias G., Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51(2):271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Cruciani-Guglielmacci C., Hervalet A., Douared L., Sanders N.M., Levin B.E., Ktorza A. Beta oxidation in the brain is required for the effects of non-esterified fatty acids on glucose-induced insulin secretion in rats. Diabetologia. 2004;47(11):2032–2038. doi: 10.1007/s00125-004-1569-2. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Astarita G., Taussig M.D., Bharadwaj K.G., DiPatrizio N.V., Nave K.A. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metabolism. 2011;13(1):105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H., Eckel R.H. Lipoprotein lipase in the brain and nervous system. Annual Review of Nutrition. 2012 doi: 10.1146/annurev-nutr-071811-150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson T.L., Jarrard L.E. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology. 1993;59(2):167–171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 14.Davidson T.L., Chan K., Jarrard L.E., Kanoski S.E., Clegg D.J., Benoit S.C. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19(3):235–252. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson T.L., Kanoski S.E., Chan K., Clegg D.J., Benoit S.C., Jarrard L.E. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behavioral Neuroscience. 2010;124(1):97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanoski S.E., Hayes M.R., Greenwald H.S., Fortin S.M., Gianessi C.A., Gilbert J.R. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36(9):1859–1870. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diano S., Farr S.A., Benoit S.C., McNay E.C., da Silva I., Horvath B. Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neuroscience. 2006;9(3):381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 18.Francis H.M., Stevenson R.J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behavioral Neuroscience. 2011;125(6):943–955. doi: 10.1037/a0025998. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Zeev O., Doolittle M.H., Singh N., Chang C.H., Schotz M.C. Synthesis and regulation of lipoprotein lipase in the hippocampus. Journal of Lipid Research. 1990;31(7):1307–1313. [PubMed] [Google Scholar]
- 20.Paradis E., Clavel S., Julien P., Murthy M.R., de Bilbao F., Arsenijevic D. Lipoprotein lipase and endothelial lipase expression in mouse brain: regional distribution and selective induction following kainic acid-induced lesion and focal cerebral ischemia. Neurobiology of Disease. 2004;15(2):312–325. doi: 10.1016/j.nbd.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G., Watson C., editors. The rat brain in stereotaxis coordinates. Academic Press; San Diego, USA: 2005. [Google Scholar]
- 22.Paxinos G., Franklin K., editors. The mouse brain in stereotaxic coordinates: compact. second edition. Gulf Professional Publishing; San Diego, USA: 2003. [Google Scholar]
- 23.Taicher G.Z., Tinsley F.C., Reiderman A., Heiman M.L. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Analytical and Bioanalytical Chemistry. 2003;377(6):990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 24.McNay E.C., Gold P.E. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. Journal of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(2):B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 25.Arch J.R., Hislop D., Wang S.J., Speakman J.R. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. International Journal of Obesity (London) 2006;30(9):1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- 26.Even P.C., Mokhtarian A., Pele. A. Practical aspects of indirect calorimetry in laboratory animals. Neuroscience and Biobehavioral Reviews. 1994;18(3):435–447. doi: 10.1016/0149-7634(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. Journal of Physiology. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glowinski J., Iversen L.L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. Journal of Neurochemistry. 1966;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 29.Le Stunff H., Galve-Roperh I., Peterson C., Milstien S., Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. Journal of Cell Biology. 2002;158(6):1039–1049. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veret J., Coant N., Berdyshev E.V., Skobeleva A., Therville N., Bailbe D. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 beta-cells. Biochemical Journal. 2011;438(1):177–189. doi: 10.1042/BJ20101386. [DOI] [PubMed] [Google Scholar]
- 31.Magnan C., Collins S., Berthault M.F., Kassis N., Vincent M., Gilbert M. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. Journal of Clinical Investigation. 1999;103(3):413–419. doi: 10.1172/JCI3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R., Cruciani-Guglielmacci C., Migrenne S., Magnan C., Cotero V.E., Routh V.H. Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. Journal of Neurophysiology. 2006;95(3):1491–1498. doi: 10.1152/jn.00697.2005. [DOI] [PubMed] [Google Scholar]
- 33.Car H., Zendzian-Piotrowska M., Prokopiuk S., Fiedorowicz A., Sadowska A., Kurek K. Ceramide profiles in the brain of rats with diabetes induced by streptozotocin. FEBS Journal. 2012;279(11):1943–1952. doi: 10.1111/j.1742-4658.2012.08575.x. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen B.A., Breen D.M., Lam T.K. Lipid sensing in the gut, brain and liver. Trends in Endocrinology and Metabolism. 2012;23(2):49–55. doi: 10.1016/j.tem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Ruge T., Hodson L., Cheeseman J., Dennis A.L., Fielding B.A., Humphreys S.M. Fasted to fed trafficking of Fatty acids in human adipose tissue reveals a novel regulatory step for enhanced fat storage. Journal of Clinical Endocrinology and Metabolism. 2009;94(5):1781–1788. doi: 10.1210/jc.2008-2090. [DOI] [PubMed] [Google Scholar]
- 36.Peterson H.R., Rothschild M., Weinberg C.R., Fell R.D., McLeish K.R., Pfeifer. M.A. Body fat and the activity of the autonomic nervous system. New England Journal of Medicine. 1988;318(17):1077–1083. doi: 10.1056/NEJM198804283181701. [DOI] [PubMed] [Google Scholar]
- 37.Valladolid-Acebes I., Merino B., Principato A., Fole A., Barbas C., Lorenzo M.P. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. American Journal of Physiology – Endocrinology and Metabolism. 2012;302(4):E396–E402. doi: 10.1152/ajpendo.00343.2011. [DOI] [PubMed] [Google Scholar]
- 38.Martin L.E., Holsen L.M., Chambers R.J., Bruce A.S., Brooks W.M., Zarcone J.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 39.Fidalgo C., Conejo N.M., Gonzalez-Pardo H., Lazo P.S., Arias J.L. A role for dorsal and ventral hippocampus in response learning. Neurosciences Research. 2012;73(3):218–223. doi: 10.1016/j.neures.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich M.O., Andrews Z.B., Horvath T.L. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. Journal of Neuroscience. 2008;28(42):10766–10771. doi: 10.1523/JNEUROSCI.2744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gobeske K.T., Das S., Bonaguidi M.A., Weiss C., Radulovic J., Disterhoft J.F. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4(10):e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santin K., da Rocha R.F., Cechetti F., Quincozes-Santos A., de Souza D.F., Nardin P. Moderate exercise training and chronic caloric restriction modulate redox status in rat hippocampus. Brain Research. 2011;1421:1–10. doi: 10.1016/j.brainres.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Li F.W., Deurveilher S., Semba K. Behavioural and neuronal activation after microinjections of AMPA and NMDA into the perifornical lateral hypothalamus in rats. Behavioural Brain Research. 2011;224(2):376–386. doi: 10.1016/j.bbr.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Wayner M.J., Phelix C.F., Armstrong D.L. Lateral hypothalamic stimulation inhibits dentate granule cell LTP: direct connections. Brain Research Bulletin. 1997;43(1):5–15. doi: 10.1016/s0361-9230(96)00425-x. [DOI] [PubMed] [Google Scholar]
- 45.Ying Z., Covalin A., Judy J., Gomez-Pinilla F. Hypothalamic stimulation enhances hippocampal BDNF plasticity in proportion to metabolic rate. Brain Stimulation. 2012;5(4):642–646. doi: 10.1016/j.brs.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fielding B.A., Frayn K.N. Lipoprotein lipase and the disposition of dietary fatty acids. British Journal of Nutrition. 1998;80(6):495–502. doi: 10.1017/s0007114598001585. [DOI] [PubMed] [Google Scholar]
- 47.Weinstock P.H., Levak-Frank S., Hudgins L.C., Radner H., Friedman J.M., Zechner R. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(19):10261–10266. doi: 10.1073/pnas.94.19.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner E.M., Kratky D., Haemmerle G., Hrzenjak A., Kostner G.M., Steyrer E. Defective uptake of triglyceride-associated fatty acids in adipose tissue causes the SREBP-1c-mediated induction of lipogenesis. Journal of Lipid Research. 2004;45(2):356–365. doi: 10.1194/jlr.M300293-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Gao D., Pararasa C., Dunston C.R., Bailey C.J., Griffiths H.R. Palmitate promotes monocyte atherogenicity via de novo ceramide synthesis. Free Radical Biology and Medicine. 2012;53(4):796–806. doi: 10.1016/j.freeradbiomed.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 50.Park J.W., Park W.J., Futerman A.H. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochimica et Biophysica Acta. 2013 doi: 10.1016/j.bbalip.2013.08.019. , Epub 2013/09/12. [DOI] [PubMed] [Google Scholar]
- 51.Carrasco P., Sahun I., McDonald J., Ramirez S., Jacas J., Gratacos E. Ceramide levels regulated by carnitine palmitoyl transferase 1C control dendritic spine maturation and cognition. Journal of Biological Chemistry. 2004;287(25):21224–21232. doi: 10.1074/jbc.M111.337493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung J.U., Ko K., Lee D.H., Chang K.T., Choo Y.K. The roles of glycosphingolipids in the proliferation and neural differentiation of mouse embryonic stem cells. Experimental and Molecular Medicine. 2009;41(12):935–945. doi: 10.3858/emm.2009.41.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindqvist A., Mohapel P., Bouter B., Frielingsdorf H., Pizzo D., Brundin P. High-fat diet impairs hippocampal neurogenesis in male rats. European Journal of Neurology. 2006;13(12):1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 54.Stranahan A.M., Mattson M.P. Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Medicine. 2008;10(4):209–218. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material


