Abstract
Variation in FTO is the strongest genetic determinant of body weight and has recently been linked with impaired neural processing of food stimuli. However, whether this brain-expressed gene affects neuronal processing of food-related stimuli after ingestion is still poorly understood.
In this study, twenty-four participants were examined before, 30 and 120 min after ingesting 75 g of glucose solution or water on two separate days. Functional magnetic resonance imaging (fMRI) during visual food presentation was performed. All participants were genotyped for FTO SNP rs8050136.
We detected significant differences between FTO genotypes in the prefrontal cortex 30 min post-glucose load in BOLD-response to food pictures (p=0.0017), while no differences were detected in response to water ingestion or 120 min post-glucose load.
Since the prefrontal cortex plays a major role in the inhibitory control of eating, we propose that reduced postprandial activity in FTO risk allele carriers contributes to overeating and obesity.
Keywords: FTO, Brain, Human, fMRI, Obesity
1. Introduction
Obesity is a major risk factor for type 2 diabetes and other diseases like hypertension, stroke, and coronary heart disease. Unfortunately, prevalence of overweight and obesity is dramatically increasing worldwide, mainly due to changes in eating behavior and physical activity. Besides environmental factors, body weight is strongly determined genetically [1], with heritability estimated to explain more than 50% of the variance in body mass index (BMI) [1]. The strongest genetic determinant, with the highest population-attributable risk of increased body weight known today, is variation in the FTO (fat mass- and obesity-associated) gene. Single nucleotide polymorphisms (SNPs) within this gene explain up to 3 kg of weight differences [2,3].
The FTO gene encodes an enzyme that demethylates nucleic acids [4–6] and is involved in amino acid sensing [6,7]. While it is expressed in most human tissues [2,8], the highest expression levels were detected in the brain [2,4,8]. Interestingly, expression levels of FTO were found to be strongly regulated by the feeding state in rodents [9,10] and seem to be involved in dopaminergic signaling [11].
In humans, recent neuroimaging studies have shown anatomical and functional brain differences between FTO genotypes. Anatomical brain differences revealed volume deficits [12] in frontal and occipital brain regions in risk allele carriers, comparable to obese persons [12,13]. In addition, Karra et al. [14] reported significant differences between homozygote carriers of the non-risk and risk allele in reward and homeostatic areas elicited by food versus non-food pictures. Regarding obesity-prone behaviors, genetic variation in the FTO locus is associated with food intake but not with energy expenditure [15–17].
Taken together, these findings suggest that the association of FTO SNPs with human obesity might be related to processes in the central nervous system (CNS) regulating eating behavior.
Given that the regulation of FTO expression is affected by the nutritional state [10] and the associated neuronal response [14], we hypothesize that FTO effects on human brain activity are affected by food intake. We reported recently that a standard oral glucose tolerance test (OGTT) significantly alters the central processing of food cues in areas important for eating behavior [18]. We therefore investigated the neural processing of food-related stimuli between FTO SNP rs8050136 genotypes not only in the fasting state but also during an OGTT.
2. Material and methods
2.1. Participants
Twenty-four participants participated in the imaging study. Their mean age was 24±2 years and their mean BMI was 25.8±5.0 kg/m2. All participants were normal glucose tolerant as tested by OGTT. Details on the metabolic and anthropometric characteristics are reported in Table 1 and supplementary Figure 1. All participants were healthy as ascertained by a physician. Any volunteer with diabetes or with a family history of diabetes was excluded, as well as those with chronic disease or taking any kind of medication other than oral contraceptives.
Table 1.
Characteristics of the participants.
| FTO SNP rs8050136 | CC | CA | AA | punadjusted | padjusted |
|---|---|---|---|---|---|
| Gender (female/male) | 6/4 | 5/5 | 1/3 | 0.5 | – |
| Age (yr) | 24±3 | 24±3 | 23±1 | 0.5 | – |
| BMI (kg/m2) | 26.8±4.9 | 24.9±5.3 | 25.6±5.2 | 0.6 | – |
| Fasting glucose (mmol/l) | 4.8±0.4 | 5.0±0.3 | 5.2±0.3 | 0.0419 | 0.0229 |
| Glucose120 min (mmol/l) | 5.7±1.1 | 6.1±1.8 | 4.1±0.8 | 0.1 | 0.3 |
| AUC Glucose (mmol/l) | 12.8±2.2 | 14.5±2.6 | 11.8±1.6 | 0.9 | 0.9 |
| Fasting insulin (pmol/l) | 64±36 | 54±31 | 49±32 | 0.4 | 0.8 |
| Insulin30 min (pmol/l) | 582±222 | 719±338 | 615±256 | 0.7 | 0.4 |
| Insulin120 min (pmol/l) | 326±182 | 482±349 | 168±62 | 0.5 | 0.8 |
| AUC Insulin (pmol/l) | 805±260 | 1177±713 | 676±165 | 0.9 | 0.6 |
| Insulin sensitivity index, OGTT-derived (AU) | 15.4±8.3 | 14.1±8.4 | 17.9±6.7 | 0.7 | 0.5 |
| HOMA-IR (mmol/l×µU/ml) | 1.90±1.15 | 1.68±0.10 | 1.59±1.10 | 0.6 | 0.9 |
| HbA1c (%) | 5.4±0.2 | 5.4±0.3 | 5.1±0.1 | 0.1 | 0.6 |
Data are given as means±SD. punadjusted=p-values of the comparison of unadjusted loge-transformed data by ANOVA. Distribution of gender was compared by chi-square test. padjusted=p-values of the comparison of loge-transformed data adjusted for gender, age, BMI by multiple linear regression analyses. AUC – area under the curve; FTO – fat mass- and obesity-associated, HOMA-IR – homeostatic model assessment of insulin resistance, OGTT – oral glucose tolerance test.
Informed written consent was obtained from all participants and the local Ethics Committee approved the protocol.
2.2. Genotyping
DNA from whole blood was isolated using a commercial DNA isolation kit (NucleoSpin, Macherey&Nagel, Düren, Germany). Genotyping for FTO SNP rs8050136 was performed using the TaqMan assay (Applied Biosystems, Forster City, CA, USA). The TaqMan genotyping reaction was amplified on a GeneAmp PCR system 7000 and fluorescence was detected on an ABI PRISM7000 sequence detector (Applied Biosystems).
2.3. OGTT
All participants participated in a standard OGTT after an overnight fast. The experiment started at 7.00a.m. with an fMRI measurement under basal conditions (fMRI basal). After this basal measurement, participants ingested the 75 g glucose solution. After 30 min, the second fMRI measurement was performed (fMRI 30), and after 120 min, the third measurement was carried out (fMRI 120). Venous blood samples were obtained every half hour and both plasma glucose and insulin concentrations were determined (Figure 1).
Figure 1.
Course of the experiment. The experiment started at 7.00a.m. with an fMRI measurement under basal conditions (fMRI basal). After this basal measurement, participants ingested the 75 g glucose solution or water on two different days in randomized order. After 30 min, the second fMRI measurement was performed (fMRI 30), and after 120 min, the third measurement was carried out (fMRI 120).
2.4. Analytical procedures
Blood glucose was determined using the glucose oxidase method by a bedside glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma insulin was measured by commercial chemiluminescence assays for ADVIA Centaur (Siemens, Fernwald, Germany).
2.5. Experimental protocol
We used the same experimental protocol as previously described [18]. In short, we used a block design with food and non-food pictures, presented either during a one-back visual recognition task or a null-back control task separated by baseline blocks (fixation cross). For the one-back task, participants had to press a button at each picture depending on whether the presented picture was the same or different as the one before (yes=left button, no=right button). For the control task (food and non-food pictures combined), participants had to press only the left button as soon as they saw the picture.
2.6. fMRI blood oxygen level dependency (BOLD) imaging procedures
Whole-brain fMRI data was obtained using a 3.0T scanner (Siemens TimTrio, Erlangen, Germany). Each of the 6 sessions consisted of 226 scans (TR=2 s, TE=30 ms, matrix 64×64, flip angle 90°, voxel size 3×3×3.75 mm3, slice thickness 3 mm, 0.75 mm gap, 30 slices) lasting for approximately 8min; the images were acquired in ascending order. In addition, high-resolution T1-weighted anatomical images (MPRage: 176 slices, matrix: 256×224, 1×1×1 mm3) of the brain were obtained.
Pre-processing and statistical analysis of the fMRI data were conducted using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were realigned and resliced to the first image after unwarping in the phase encoding direction. A mean image was created and co-registered to the T1 structural image. The anatomical image was normalized in MNI space, and the resulting parameter file was used to normalize the functional images (voxel size: 3×3×3 mm3). Finally the normalized images were smoothed with a three-dimensional isotropic Gaussian kernel (FWHM: 6 mm). fMRI data were highpass (cutoff period 128 s) filtered and global AR(1) auto-correlation correction was performed.
2.7. Image processing
For the first level of analysis, a design matrix was created individually for glucose and water day (as described in [18]). For each participant, the blocks of high- and low-caloric food pictures (in relation to the implicit baseline) were modeled separately for the three different time points (fMRI basal, fMRI 30 and fMRI 120) using a fixed effect analysis. Food pictures were selected for the analysis based on our recent finding of a specific insulin modulation on brain activation elicited by food pictures [19]. Individual contrast images were computed to estimate activation changes to food cues after oral glucose and water ingestion compared to the basal measurement.
These differential contrasts were used for a multiple regression analysis to evaluate the association between FTO genotypes (additive model) and the postprandial response to food stimuli 30 and 120 min after glucose and water ingestion separately (p<0.05, corrected for multiple comparisons).
2.8. Statistical analyses of anthropometric and metabolic data
Data was logarithmically transformed prior to statistical analysis. Unless otherwise stated, data is given as mean±SD. For those statistical analyses, the software package JMP10 (SAS Institute, Cary, NC, USA) was used. Associations with FTO genotype were studied under an additive inheritance model.
3. Results
3.1. fMRI results
Since FTO variants showed an additive effect on BMI in previous studies (e.g. [2,20]), we investigated the differences between genotypes under an additive inheritance model. The 24 participants showed no significant differences between FTO genotypes for BMI, which allowed us to study the effect of FTO independent of FTO-induced BMI changes (all p≥0.5, Table 1). Fasting glucose levels were slightly higher in carriers of the A-risk allele (p=0.0419, Table 1), whereas fasting insulin levels did not differ between genotypes (p=0.4, Table 1).
The fMRI measurements showed no significant whole-brain effect between FTO genotypes on the cerebral processing of food-stimuli in the fasting state and 120 min after glucose intake (both p>0.05, corrected for multiple comparisons). However, 30 min after glucose ingestion, we detected significant differences between FTO genotypes in the prefrontal cortex (peak coordinate: x=21, y=27, z=45, Brodmann area 9; z=4.41; p<0.05, corrected for multiple comparisons). Food-elicited activity in this area was only markedly reduced in obesity risk allele carriers (Figure 2, p=0.0017). These differences remained significant even after adjustment for the possible confounders of gender, age, and BMI (p=0.0002).
Figure 2.
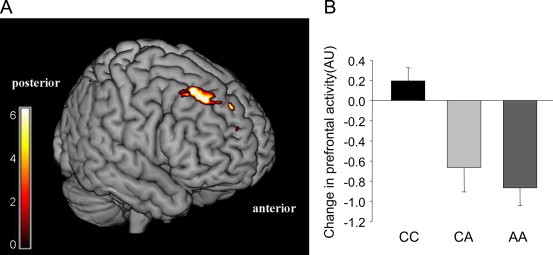
Differences between FTO genotypes in the processing of food stimuli 30 min after glucose intake. (A) Color map represents significant (p<0.05, FWE-corrected) differential activation in the prefrontal cortex between FTO rs8050136 genotypes, overlaid on a normalized brain. Color bar represents T-values. (B) Plot shows post-load response of the prefrontal cortex in participants carrying two non-risk alleles (CC), with one copy of the obesity risk allele (CA), and participants with two copies of the obesity risk allele (AA). Data is expressed as parameter estimates±SE.
Because we previously found this specific area to be insulin responsive [18], we analyzed possible interactions between FTO genotype and changes in insulin levels after glucose intake. However, no such interactions were present (ANCOVA p=0.6). Accordingly, the differential prefrontal response between FTO genotypes (p=0.0004) remained significant after additional adjustment for insulin levels. Furthermore, the prefrontal activity was associated with changes in plasma insulin independent of FTO genotype (p=0.0004). Both associations remained significant after further adjustment for gender, age, and BMI (p<0.0001 and p=0.0034, respectively).
4. Discussion
In this study, we investigated the neural processing of food-related stimuli before and after a 75 g OGTT in FTO risk and non-risk allele carriers. Since the studied polymorphism is associated with obesity [20] and obesity is known to markedly alter brain response to food [21,22], participants were matched for BMI, gender, and age. Additionally, insulin levels were comparable between FTO genotypes. Thus, our study allowed us to investigate the effect of the FTO genotype on brain function without the influence of these important metabolic determinants, which have been shown to considerably influence cerebral processes [21,23].
In the fasting state, we found no significant differences between FTO genotypes in response to food stimuli. Interestingly, marked genotype differences became visible in the prefrontal cortex 30 min after glucose ingestion. Hereby, FTO risk allele carriers showed an attenuated prefrontal food-cue response after glucose ingestion.
This is well in line with expression profiles showing that the prefrontal cortex has the strongest FTO expression of the whole body in human tissue ([8] accessed via BioGPS [24]). By integrating sensory information, the prefrontal cortex in humans is evolutionarily one of the newest areas and it is largely increased in humans compared to primates and other animals [25]. The primary function of the prefrontal cortex is top-down control of human behavior, which is important in diverse cognitive functions, e.g. working memory, inhibitory control, and executive function [25]. Regarding eating behavior, the prefrontal cortex contributes to the inhibitory control of eating [26,27]. Accordingly, higher activity in the prefrontal cortex was associated with the selection of healthy rather than tasty food in persons on a diet [28]. Furthermore, it is well established that activity in this area is regulated by feeding state with lower activity when hungry and higher activity when satiated [27,29]. Our present imaging results indicate that the postprandial response of the prefrontal cortex is modulated by FTO genotype with lower activity in obesity risk allele carriers.
Of interest, we recently identified insulin as one modulator of activity in the prefrontal cortex [18,30,31]. Given recent data on potential interactions between ghrelin and FTO [14], indirect insulin effects via ghrelin could also possibly be a contributing factor. We therefore investigated whether FTO genotype determines the insulin responsiveness of the prefrontal cortex. However, FTO genotype and plasma insulin seem to be two independent mechanisms influencing the activity of this brain area. This is especially interesting since the variation in FTO is a novel factor besides insulin that is associated with prefrontal activity in the postprandial situation in humans. Clearly, studies on the molecular level are needed to dissect the underlying molecular pathways and to define the type of neurons involved in either insulin or FTO effects. A recent study by Karra et al. [14] suggested that FTO influences ghrelin, and that this hormone might be the molecular mediator of FTO on ingestive behavior. This study reported widespread differences in brain networks associated with homeostatic and reward processing between 10 homozygote risk- and 10 homozygote non-risk allele carriers. They found differences in the fasted state for food versus non-food pictures and also interactions for high-caloric versus low-caloric food pictures and nutritional status (fasted versus satiated) 60 min after standardized meal ingestion. In our study the major effect was observed 30 min after glucose ingestion, indicating also to a highly dynamic effect of FTO on the interaction of brain activity and nutritional status.
Our metabolic and neuroimaging study is relatively small for genetic associations and some conclusions are speculative. Therefore, our analyses could not detect small effect sizes that might be present at baseline or in other brain regions. Our findings, nevertheless, need replication in larger cohorts.
In conclusion, we detected postprandial differences between FTO genotypes in the prefrontal cortex, a major region for the inhibitory control of eating. Thus, reduced prefrontal activity in postprandial risk allele carriers could contribute to their overeating and promote obesity risk. Our findings help understand why FTO variants are associated with human obesity. They provide novel insights into how genetic background influences body weight via the brain.
Conflict of interest
All authors declare that they have no relevant conflict of interest.
Acknowledgments
We thank all study participants for their cooperation. We gratefully acknowledge the excellent technical assistance of Dr. Martina Guthoff, Maike Borutta, Margarete Bayer, Anna Bury, Alke Guirguis and Roman Werner (all University of Tuebingen).
The study was supported by grants from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V., 01GI0925) and by the Helmholtz Alliance ICEMED – Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Network Fund of the Helmholtz Association.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at 10.1016/j.molmet.2013.11.009.
Appendix A. Supplementary materials
Supplementary Data
References
- 1.Walley A.J., Asher J.E., Froguel P. The genetic contribution to non-syndromic human obesity. Nature Reviews Genetics. 2009;10:431–442. doi: 10.1038/nrg2594. [DOI] [PubMed] [Google Scholar]
- 2.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dina C., Meyre D., Gallina S., Durand E., Körner A., Jacobson P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nature Genetics. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 4.Gerken T., Girard C.A., Tung Y.C.L, Webby C.J., Saudek V., Hewitson K.S. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung Y.C.L., Yeo G.S.H. From GWAS to biology: lessons from FTO. Annals of the New York Academy of Sciences. 2011;1220:162–171. doi: 10.1111/j.1749-6632.2010.05903.x. [DOI] [PubMed] [Google Scholar]
- 6.Gulati P., Yeo G.S.H. The biology of FTO: from nucleic acid demethylase to amino acid sensor. Diabetologia. 2013 doi: 10.1007/s00125-013-2999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulati P., Cheung M.K., Antrobus R., Church C.D., Harding H.P., Tung Y.C.L. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2557–2562. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su A.I., Wiltshire T., Batalov S., Lapp H., Ching K.A., Block D. A gene atlas of the mouse and human protein-encoding transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredriksson R., Hägglund M., Olszewski P.K., Stephansson O., Jacobsson J.A., Olszewska A.M. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149:2062–2071. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 10.Boender A.J., van Rozen A.J., Adan R.A.H. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity (Silver Spring) 2012 doi: 10.1038/oby.2012.128. [DOI] [PubMed] [Google Scholar]
- 11.Hess M.E., Hess S., Meyer K.D., Verhagen L.A.W., Koch L., Brönneke H.S. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nature Neuroscience. 2013;16:1042–1048. doi: 10.1038/nn.3449. [DOI] [PubMed] [Google Scholar]
- 12.Ho A.J., Stein J.L., Hua X., Lee S., Hibar D.P., Leow A.D. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8404–8409. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raji C.A., Ho A.J., Parikshak N.N., Becker J.T., Lopez O.L., Kuller L.H. Brain structure and obesity. Human Brain Mapping. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karra E., O’Daly O.G., Choudhury A.I., Yousseif A., Millership S., Neary M.T. A link between FTO, ghrelin, and impaired brain food-cue responsivity. Journal of Clinical Investigation. 2013;123:3539–3551. doi: 10.1172/JCI44403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haupt A., Thamer C., Staiger H., Tschritter O., Kirchhoff K., Machicao F. Variation in the FTO gene influences food intake but not energy expenditure. Experimental and Clinical Endocrinology & Diabetes. 2009;117:194–197. doi: 10.1055/s-0028-1087176. [DOI] [PubMed] [Google Scholar]
- 16.Berentzen T., Kring S.I.I., Holst C., Zimmermann E., Jess T., Hansen T. Lack of association of fatness-related FTO gene variants with energy expenditure or physical activity. Journal of Clinical Endocrinology and Metabolism. 2008;93:2904–2908. doi: 10.1210/jc.2008-0007. [DOI] [PubMed] [Google Scholar]
- 17.Müller T.D., Tschöp M.H., Hofmann S. Emerging function of fat mass and obesity-associated protein (fto) PLoS Genetics. 2013;9:e1003223. doi: 10.1371/journal.pgen.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heni M., Kullmann S., Ketterer C., Guthoff M., Bayer M., Staiger H. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Human Brain Mapping. 2013 doi: 10.1002/hbm.22223. (n/a–n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthoff M., Grichisch Y., Canova C., Tschritter O., Veit R., Hallschmid M. Insulin modulates food-related activity in the central nervous system. Journal of Clinical Endocrinology and Metabolism. 2010;95:748–755. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- 20.Haupt A., Thamer C., Machann J., Kirchhoff K., Stefan N., Tschritter O. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008;16:1969–1972. doi: 10.1038/oby.2008.283. [DOI] [PubMed] [Google Scholar]
- 21.Guthoff M., Stingl K.T., Tschritter O., Rogic M., Heni M., Stingl K. The insulin-mediated modulation of visually evoked magnetic fields is reduced in obese subjects. PLoS ONE. 2011;6:e19482. doi: 10.1371/journal.pone.0019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin L.E., Holsen L.M., Chambers R.J., Bruce A.S., Brooks W.M., Zarcone J.R. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 23.Kullmann S., Heni M., Veit R., Ketterer C., Schick F., Häring H.U. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Human Brain Mapping. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Orozco C., Boyer J., Leglise M., Goodale J., Batalov S. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biology. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuster J.M. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 26.Carnell S., Gibson C., Benson L., Ochner C.N., Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obesity Reviews. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tataranni P.A., Gautier J.F., Chen K., Uecker A., Bandy D., Salbe A.D. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hare T.A., Camerer C.F., Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 29.Del Parigi A., Gautier J.F., Chen K., Salbe A.D., Ravussin E., Reiman E. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Annals of the New York Academy of Sciences. 2002;967:389–397. [PubMed] [Google Scholar]
- 30.Kullmann S., Frank S., Heni M., Ketterer C., Veit R., Häring H.U. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology. 2012 doi: 10.1159/000341406. [DOI] [PubMed] [Google Scholar]
- 31.Heni M., Kullmann S., Ketterer C., Guthoff M., Linder K., Wagner R. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia. 2012;55:1773–1782. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data


