Highlights
-
•
Rice grain extract and digest inhibited BACE1 activity in vitro.
-
•
Rice digest inhibited amyloid-β peptide (Aβ) production in cultured neuroblastoma cells.
-
•
Rice extract and digest contained IP6 and IP4.
-
•
IP6 but not IP3, IP4 or IP5 inhibited BACE1 activity in vitro.
-
•
IP6 inhibited production of Aβ and β-CTF in cultured neuroblastoma cells.
Abbreviations: AD, Alzheimer’s disease; Aβ, amyloid-β peptide; APP, amyloid-β precursor protein; BACE1, β-secretase 1/beta-site APP cleaving enzyme 1; β-CTF, C-terminal fragment of β-cleaved APP; IP6, myo-inositol hexakisphosphate/phytic acid; IP6 (SS), phytic acid sodium salt; IP6 (PS), phytic acid dipotassium salt; IC50, half-maximal inhibitory concentration
Keywords: Alzheimer’s disease, Aβ, BACE1, Rice grain, IP6, SH-SY5Y cells
Abstract
Alzheimer’s disease (AD) is widely considered to be caused by amyloid-β peptide (Aβ) accumulation in the brain. Aβ is excised from amyloid-β precursor protein through sequential cleavage by β-secretase 1 (BACE1) and γ-secretase. Thus, BACE1 inhibition could prevent Aβ accumulation. Here, we identified myo-inositol hexakisphosphate (IP6) as a BACE1 inhibitory molecule in rice grain extract and digest. The rice digest and IP6 significantly inhibited Aβ production in neuroblastoma cells without cytotoxicity. These results suggested that rice components, including IP6, may be promising starting materials for the development of potent and safe drugs and/or food to prevent AD.
1. Introduction
Alzheimer’s disease (AD) is the most prevalent cause of senile dementia. Accumulation of amyloid-β peptide (Aβ) in the brain is widely considered to be critically involved in the pathogenesis of AD [1]. Aβ plaques emerge roughly 15 years before the symptoms of AD appear [2]. Once AD develops, cognitive decline that is caused by neuronal damage cannot be reversed, even after Aβ levels in the brain are lowered by immunotherapy [3]. Thus, the prevention of Aβ accumulation is important to prevent AD. Aβ is excised from amyloid-β precursor protein (APP) through sequential cleavage by aspartic protease β-secretase 1 (BACE1) and γ-secretase [1,4]. Because BACE1 initiates the processing of Aβ, inhibition of BACE1 activity could be an effective measure to prevent Aβ accumulation [5].
Many epidemiologic surveys have indicated that lifestyle, including diet habits, affect the incidence rate of AD [6–8]. Cohort studies have shown that when a group of Japanese citizens immigrated to Hawaii and then to the US mainland, their AD rate gradually increased from the Asian level to the Western level [9]. With respect to diet, the staple food in Western countries is wheat, whereas in Japan, it is rice. Thus, we hypothesized that rice grains might contain some components that prevent Aβ accumulation in the brain.
Here, we showed that rice extracts and digest indeed inhibit BACE1 activity in vitro and that the rice digest inhibited Aβ production in cultured human neuroblastoma cells. We further identified phytic acid (also called IP6, myo-inositol hexakisphosphate) as one of the potent components by ion chromatography. We demonstrated that IP6 inhibited BACE1 activity in vitro and Aβ production in cultured human neuroblastoma cells.
2. Materials and methods
2.1. Reagents
Rice-derived phytic acid sodium salt [IP6 (SS), C6H18O24P6·xNa + yH2O], phytic acid dipotassium salt [IP6 (PS), MR: 736.22], D-myo-inositol 1,4,5,6-tetrakisphosphate potassium salt (IP4), and D-myo-inositol 1,4,5-tris–phosphate trisodium salt (IP3) were purchased from Sigma–Aldrich Co. LLC (St. Louis, MO). D-myo-inositol 1,3,4,5,6-pentakisphosphate pentapotassium salt (IP5) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
2.2. Sample preparation
Unpolished or polished rice grains or bread flour was extracted in 10 volumes (w/v) of the BACE1 assay buffer (see below) for 1 h at room temperature. Undissolved components were removed by centrifugation at 11,000×g for 20 min at 4 °C. The supernatant was heated at 95 °C for 30 min to inactivate the rice or flour-derived proteases and then centrifuged at 11,000×g for 10 min at 4 °C. The supernatant was then subjected to a BACE1 activity assay.
For the in vitro digestion, unpolished rice grains were incubated in 10 volumes (w/v) of 0.26% pepsin (Sigma–Aldrich Co. LLC) in ultrapure water–HCl (pH 2.0) for 2.5 h at 37 °C. The pH of the pepsin digest was raised to 5.5 by dropwise addition of 1 M NaHCO3. After addition of pancreatin (Sigma–Aldrich Co. LLC) at a final concentration of 0.7%, the digest was further incubated for 2 h at 37 °C, and then heated at 100 °C for 20 min to inactivate the enzymes. The digest was centrifuged at 12,000×g for 20 min at 4 °C, and the supernatant was collected and separated using a 5K MWCO spin filter (catalog No. UFC900524, Merck KGaA, Darmstadt, Germany). The flow-through fraction (termed 5k-lower) was collected and subjected to a BACE1 activity assay, a cell-based Aβ production activity assay, and a cytotoxicity assay.
2.3. In vitro BACE1 activity assay
BACE1 activity was determined using recombinant human BACE1 (Sigma–Aldrich Co., LLC) and a fluorogenic H-RE(EDANS)EVNLDAEFK(DABCYL)R-OH substrate (catalog No. 565758, Merck KGaA). The assays were performed in the BACE1 assay buffer [20 mM acetate buffer (pH 4.5)/0.1% Triton X-100/0.05% CHAPS] in black 96-well microplates (PerkinElmer Inc., Waltham, MA). Each well contained 10 μM of the substrate, 0.2 μg/ml of BACE1 and the test samples or β-secretase inhibitor III (catalog No. 565780, Merck KGaA) in a final volume of 60 μl. The mixture was incubated for 5 h at 37 °C or for 17 h at 25 °C, and the substrate cleavage was determined by measuring fluorescence (excitation at 355 nm, emission at 490 nm) using a Wallac 1420 plate reader (PerkinElmer Inc.). The data that were obtained were assessed for statistical significance using the Student’s t-test.
2.4. Cell-based Aβ production activity and cytotoxicity assays
SH-SY5Y human neuroblastoma cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 medium supplemented with 10% fetal bovine serum at 37 °C in a 5% CO2 atmosphere. The cells were seeded in a 96-well ViewPlate (catalog No. 6005182, PerkinElmer Inc.) at a density of 8 × 105 cells/ml with 150 μl of the culture medium in each well and then incubated for 2 h. After removing 15 μl of the culture medium, the cells were treated with 15 μl of the test samples [rice digest or IP6 (SS) dissolved in phosphate-buffered saline (PBS)] for 24 h. Aβ1−x that was secreted into the medium was detected by an enzyme-linked immunosorbent assay (ELISA) according to the method of Yamakawa et al. [10] with slight modifications. In brief, the conditioned medium (100 μl) was transferred to a Maxisorp plate (catalog No. 436110, Thermo Fisher Scientific Inc., Waltham, MA) coated with an 82E1 antibody specific to the N-terminal end of Aβ (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan). Aβ that was captured on the plate was detected using a biotinylated 4G8 antibody (Covance Inc., Princeton, NJ), horseradish peroxidase (HRP)-conjugated streptavidin (Thermo Fisher Scientific Inc.), and SuperSignal ELISA Pico chemiluminescent substrate (Thermo Fisher Scientific Inc.). The luminescent signals were measured using the plate reader.
To check the cytotoxicity of the test samples, MultiTox-Fluor Multiplex reagent (Promega Corporation, Madison, WI) was added to the cells in the culture medium (50 μl) that remained in the ViewPlate. After incubation for 30 min, the signals of the live-cell marker glycyl-phenylalanyl-amino-fluorocoumarin (GF-AFC) (excitation at 400 nm, emission at 505 nm) and the dead-cell marker bis-alanyl-alanyl-phenylalanyl-rhodamine-110 (bis-AAF-R110) (excitation at 485 nm, emission at 520 nm) were sequentially measured using the plate reader.
Aβ production (shown in Figs. 2A and 6A, and Supplementary Fig. 1) was expressed as the SuperSignal ELISA Pico chemiluminescent count/GF-AFC count.
Fig. 2.
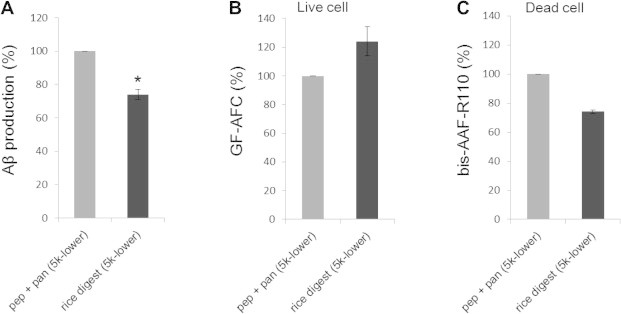
Reduction of amyloid-β peptide (Aβ) production by the unpolished rice digest in cultured SH-SY5Y cells. The cells were treated with the components that were smaller than 5 kDa (5k-lower) of the mixture of pepsin and pancreatin (pep + pan) or unpolished rice digest that was treated with pepsin and pancreatin (rice digest) for 24 h, as described in Section 2. (A) Aβ1−x that was secreted into the medium was detected by enzyme-linked immunosorbent assay. The cytotoxicity of the rice digest was assessed using a MultiTox-Fluor Multiplex reagent kit with the (B) live-cell marker glycyl-phenylalanyl-amino-fluorocoumarin (GF-AFC) and (C) the dead-cell marker bis-alanyl-alanyl-phenylalanyl-rhodamine-110 (bis-AAF-R110). The data (mean ± SEM, n = 3) are expressed as percentages of the pep + pan treatment. ∗P = 0.001.
Fig. 6.
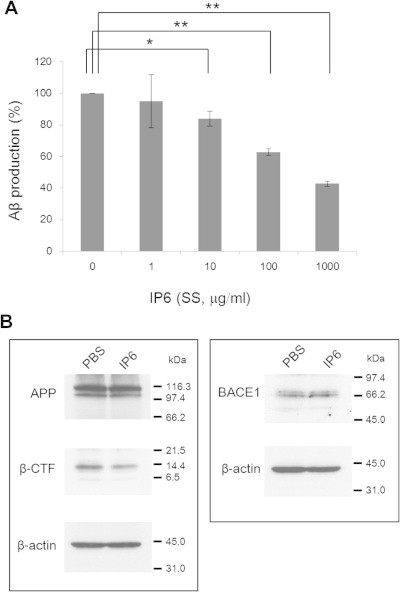
Reduced production of Aβ and C-terminal fragment of β-cleaved APP (β-CTF) by IP6 (SS) in cultured SH-SY5Y cells. (A) The cells were treated with IP6 (SS) (0–1,000 μg/ml) dissolved in phosphate-buffered saline (PBS) for 24 h. Note that Aβ1−x secreted into the culture medium significantly decreased in an IP6 dose-dependent manner. The data (mean ± SEM, n = 3) are expressed as percentages of the sample without IP6 (0 μg/ml). ∗P < 0.05, ∗∗P < 0.001. (B) The cells were treated with PBS (control) or IP6 (SS) (1,000 μg/ml) for 24 h, harvested, and lysed with SDS-sample buffer. Samples (40 μg protein/lane) were fractionated by SDS–polyacrylamide gel electrophoresis, and then subjected to immunoblotting (see Section 2 for details). Note that IP6 treatment decreased β-CTF but not APP or BACE1.
2.5. Analyses of IP3, IP4, IP5, and IP6 in the rice extract and digest by ion chromatography
Ion chromatography was performed by Japan Food Research Laboratories (Tokyo, Japan). In brief, the unpolished rice extract or digest, or bread flour extract was mixed with trichloroacetic acid according to the method of Matsunaga et al. [11]. The mixture was filtered and passed through a Na+-type cation exchange column (OnGuard II Na 1-cc cartridge, Thermo Fisher Scientific Inc.). Then, the flow-through was fractionated using a Dionex ICS-2000 ion chromatography system with an IonPac AS11 column (Thermo Fisher Scientific Inc.) and an electric conductivity detector.
2.6. Western blotting
Cells treated with or without IP6 (SS) for 24 h were harvested with a cell scraper and lysed with SDS-sample buffer [62.5 mM Tris–HCl (pH 6.8)/2.3% SDS/10% glycerol]. Protein concentration of the cell lysate was determined using a BCA protein assay kit (Thermo Fisher Scientific Inc.). The proteins were denatured by boiling for 3 min with 5% β-mercaptoethanol, separated by SDS–PAGE on an 8–16% polyacrylamide gradient gel, and then blotted onto a nitrocellulose membrane. The blot was blocked with 5% non-fat powdered milk in TBST [20 mM Tris–HCl (pH7.6)/150 mM NaCl/0.1% Tween 20], and then incubated with the following antibodies: for the C-terminal fragment of β-cleaved APP (β-CTF), monoclonal 82E1 antibody; for APP full length protein, monoclonal APP(N) (10D1) antibody (Immuno-Biological Laboratories Co., Ltd.); for BACE1, anti-BACE1 N-terminus (46–62) antibody (Sigma–Aldrich Co. LLC); for β-actin as an internal control, polyclonal beta Actin antibody (GeneTex, Inc., Irvine, CA). The antibodies were then detected with HRP-conjugated protein A/G and enhanced chemiluminescence (Thermo Fisher Scientific Inc.).
3. Results
3.1. Rice extracts and digest inhibited BACE1 activity
We first investigated whether the rice grain components had BACE1 inhibitory activity by an in vitro BACE1 activity assay. Polished and unpolished rice extracts markedly inhibited BACE1 activity compared to the control, which contained only recombinant BACE1 and the fluorogenic substrate in the assay buffer (Fig. 1). Unpolished rice was more potent than polished rice, and its potency was comparable to that of 1 μM of β-secretase inhibitor III, which is a BACE1 substrate analog. Bread flour extract did not exert significant BACE1 inhibitory activity.
Fig. 1.
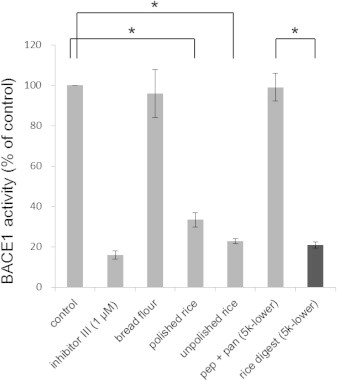
Comparison of the β-secretase 1 (BACE1) inhibitory activities of flour, rice, and rice digest. Five microliters of extracts from bread flour, polished rice, and unpolished rice were subjected to an in vitro BACE1 activity assay, as described in Section 2. Five microliters of the fractions that contained components smaller than 5 kDa (5k-lower) of the mixture of pepsin and pancreatin (pep + pan) and unpolished rice digest with pepsin and pancreatin (rice digest) were also assayed. The control samples contained BACE1 and the fluorogenic substrate in the BACE1 assay buffer without the rice or flour extract. Beta-secretase inhibitor III (inhibitor III) served as a positive control. Note that the unpolished rice extract and digest exhibited the highest inhibition among the tested samples. The data are represented as mean ± standard error of the mean (SEM; n = 3–4). ∗P < 0.001.
We next digested the unpolished rice grains with pepsin and pancreatin in vitro to mimic the gastrointestinal digestion that occurs in the body. The fraction containing components smaller than 5 kDa of the digest (5k-lower) exhibited a potency that was similar to that of the unpolished rice extract (Fig. 1). The mixture of pepsin and pancreatin without rice grains, which was incubated in a similar way as the rice digest and then passed through a 5K MWCO spin filter, did not affect BACE1 activity [pep + pan (5k-lower) in Fig. 1]. This result excluded the possibility that the observed BACE1 inhibitory activity in the rice digest (5k-lower) sample did not derive from the small components (molecular mass <5 kDa) in the pepsin or the pancreatin preparation that was used.
3.2. The rice digest inhibited Aβ production in cultured human neuroblastoma cells
To examine whether the rice components actually inhibit Aβ production in cells, we treated human neuroblastoma (SH-SY5Y) cells with the 5k-lower fraction of the unpolished rice digest. As shown in Fig. 2A, the rice digest significantly inhibited the Aβ production compared to that of the control [pepsin and pancreatin (pep + pan/5k-lower)]. When the rice digest was applied to the cells, the live cell marker GF-AFC signals tended to increase and the dead cell marker bis-AAF-R110 signals tended to decrease (Fig. 2B and C). These results indicated that the rice digest was not toxic to the cells. Rather, it tended to enhance cell survival.
Decrease of Aβ production by the rice digest was also observed in the cells overexpressing wild-type human APP (APPwt) as shown in Supplementary Fig. 1A.
3.3. Identification of IP6 as a BACE1 inhibitory molecule in the unpolished rice extract and digest
IP6 is abundant in water-soluble rice bran [12]. Thus, we analyzed whether IP6 and its degradation products, IP3, IP4, and IP5, existed in the unpolished rice extract and digest. As shown in Fig. 3, both the rice extract and digest contained IP6 and IP4, but IP3 or IP5 were not detected in these samples. We next tested whether IP3–6 inhibited BACE1 activity by an in vitro BACE1 activity assay. As shown in Fig. 4, IP6 (SS) strongly inhibited BACE1 activity at a final concentration of 1 μg/ml, and this potency was comparable to that of 1 μM of β-secretase inhibitor III. However, IP3–5 did not inhibit BACE1 activity at a final concentration of 1 μg/ml.
Fig. 3.
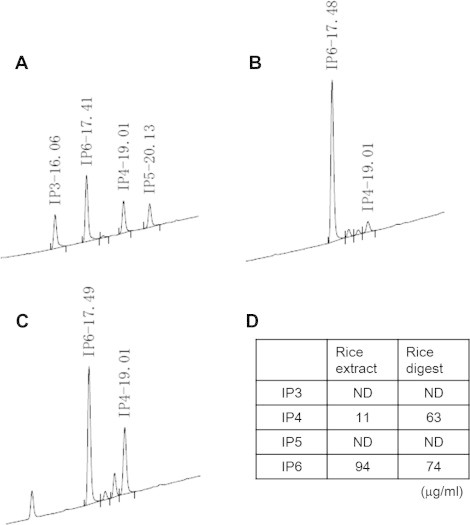
Identification of myo-inositol hexakisphosphate (IP6) and myo-inositol tetrakisphosphate (IP4) in the unpolished rice extract and digest. (A) A chromatogram of the standard solution containing IP3, IP4, IP5, and IP6 [dipotassium salt (PS)] that was obtained by ion chromatography. The value at each peak represents the retention time (in minutes) for each component. (B) A chromatogram of the unpolished rice extract. (C) A chromatogram of the unpolished rice digest. (D) Amounts of IP3, IP4, IP5, and IP6 in the unpolished rice extract and digest that were determined using the standard solution. ND, not detected.
Fig. 4.
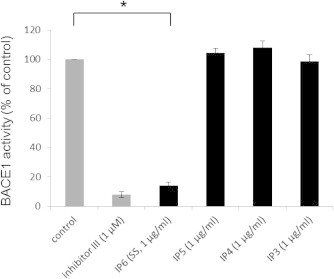
IP6 [sodium salt (SS)] inhibits BACE1 activity. IP6 (SS), IP5, IP4, and IP3 were subjected to an in vitro BACE1 activity assay. The control represents a sample containing only BACE1 and the fluorogenic substrate in the BACE1 assay buffer. Beta-secretase inhibitor III (inhibitor III) served as a positive control. Note that IP6 (SS) significantly inhibited BACE1 activity at 1 μg/ml, while IP3–5 did not show significant inhibition at the same concentration. The data are represented as mean ± SEM (n = 3). ∗P < 0.001.
To determine the IC50 value of IP6, we performed an in vitro BACE1 activity assay using various concentrations of IP6 (PS). IP6 (PS) inhibited BACE1 in a dose-dependent manner, as shown in Fig. 5, and the IC50 value was estimated to be approximately 0.18 μg/ml (0.25 μM).
Fig. 5.
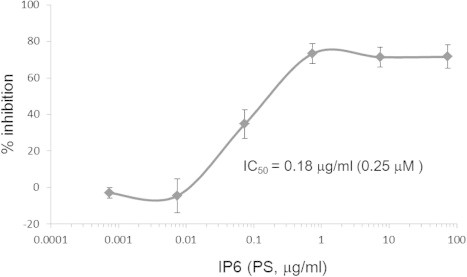
Dose-dependent inhibition of BACE1 activity by IP6 (PS). BACE1 activity was determined in the presence of various concentrations of IP6 (PS). The half-maximal inhibitory concentration (IC50) was 0.18 μg/ml (0.25 μM). The data are represented as mean ± SEM (n = 3).
We also analyzed IP6 content in the bread flour extract by ion chromatography. IP6 was undetectable, consistent with the finding that the bread flour extract did not inhibit BACE1 activity (see Fig. 1).
3.4. IP6 inhibited production of Aβ and β-CTF in cultured human neuroblastoma cells
We investigated the effects of IP6 on production of Aβ and β-CTF in cultured SH-SY5Y cells. IP6 (SS) significantly reduced the Aβ amounts in the culture media in a dose-dependent manner at 10–1000 μg/ml (Fig. 6A). The treatment of the cells with IP6 (SS) increased the live cell marker GF-AFC signals and decreased the dead cell marker bis-AAF-R110 signals compared to that of the control (data not shown), suggesting that IP6 (SS) was not toxic to the cells. These results were consistent with those of the rice digest shown in Fig. 2. Decrease of Aβ production by IP6 (SS) was also observed in the cells overexpressing human APPwt as illustrated in Supplementary Fig. 1B.
Treatment with IP6 (SS) also reduced the amount of β-CTF in the cultured native SH-SY5Y cells (Fig. 6B), consistent with the reduction of Aβ induced by IP6 (SS) treatment. The expression levels of neither APP nor BACE1 changed by IP6 (SS) treatment (Fig. 6B).
4. Discussion
In this study, we demonstrated that rice grains contained components that inhibited BACE1 activity in vitro, as well as Aβ production in cultured cells (Figs. 1 and 2). Furthermore, we identified IP6 as one of these potent components (Figs. 3–6). To the best of our knowledge, this is the first report of BACE1 inhibition by rice components and IP6. Existing γ-secretase inhibitors are known to produce undesirable side effects in clinical trials due to their low specificity toward the APP processing pathway [13,14]. In addition, the BACE1 inhibitor LY2811376 has been shown to cause adverse effects in preclinical animal models, although it reduces Aβ in the lumbar cerebrospinal fluid of healthy volunteers [15]. In contrast, the safety of rice-derived components to the human body has been warranted by the long history of rice as a staple food in many countries. Indeed, our findings indicated that the rice digest was not toxic to SH-SY5Y cells (Fig. 2). BACE1 inhibitory activity of rice components remained even after treatment with pepsin and pancreatin (Figs. 1 and 2). This suggested that potent activity of the rice components remains active after gastrointestinal digestion in the body. Thus, rice components are promising starting materials for designing drugs to prevent AD by their oral administration.
We identified IP6 in the unpolished rice extract and digest. IP6 strongly inhibited BACE1 activity in vitro (Figs. 4 and 5). In SH-SY5Y cells, IP6 (SS) significantly inhibited Aβ production in a dose-dependent manner, as described above. This observation was consistent with the results that have been reported by Anekonda et al., in which the treatment of Tg2576 AD model mice with IP6 (SS) slightly reduces the amounts of soluble Aβ1–40 and insoluble Aβ1–42 in the frontal cortex of the brain [16]. This consistency of findings suggests that one mechanism of Aβ reduction in these mice is through BACE1 inhibition by IP6 (SS), although this was not concluded by Anekonda et al. Although IP6 may chelate metals and reduce their absorption by the body [17,18], Anekonda et al. have reported that the IP6 (SS) treatment of mice that were fed a nutritionally balanced diet does not affect the status of brain metal ions (Cu, Fe, and Zn) [16]. In our study, IP6 (SS) was not toxic to SH-SY5Y cells, as described above. These observations suggested that IP6 is safe to the body.
Although we detected IP4 in the unpolished rice extract and digest, it did not inhibit BACE1 activity (Figs. 3 and 4). Likewise, IP5 did not significantly inhibit BACE1 activity (Fig. 4). These results indicated that myo-inositol needs to bear six phosphates to exert its BACE1 inhibitory activity.
Several BACE1 substrates other than APP are known, and these include neuregulin 1/heregulin-beta-2, β-galactoside α2,6-sialyltransferase, P-selectin glycoprotein ligand-1, IL-1 receptor-2, and the voltage-gated sodium channel beta 2 subunit [19]. IP6 did not inhibit the cleavage of neuregulin 1/heregulin-beta-2 (between aa237 and aa238) by BACE1 (Supplementary Fig. 2), suggesting that IP6 did not block the catalytic domain of BACE1. In addition, IP6 does not bind to a peptide coding the BACE1 cleavage site of APP, as determined by surface plasmon resonance analyses using a BiacoreX system (our unpublished observations). These results suggest that IP6 specifically inhibits APP digestion by modulating BACE1 activity. This characteristic of IP6 is preferable for developing drugs/foods to prevent AD because adverse effects that possibly result from the inhibition of the cleavage of BACE1 substrates other than APP will likely be avoided. Also, because the APP expression level in the SH-SY5Y cells did not increase by IP6 treatment despite the decrease of β-CTF (Fig. 6B), IP6 not only inhibits BACE1 but perhaps also activates α-secretases which cleave APP between β- and γ-site (within the Aβ-domain). Taken together, rice components, including IP6, are promising starting materials for the development of potent and safe drugs and/or food to prevent AD.
Acknowledgments
We thank Dr. Shoichi Ishiura for the kind gift of the APP expression vector, Drs. Takaaki Tanaka and Akihito Ochiai for their helpful discussions and technical suggestions, and Enago (www.enago.jp) for the English language review. This work was partly supported by grants from Niigata University Research Projects, Japan Science and Technology Agency and Japan Society for the Promotion of Science (KAKENHI, No. 24614005).
Appendix A. Supplementary data
Reduction of amyloid-β peptide (Aβ) production by the unpolished rice digest and IP6 [sodium salt, (SS)] in cultured SH-SY5Y cells overexpressing wild-type human APP (APPwt).
Digestion of neuregulin-1 (Nrg-1) peptide by BACE1 in the presence or absence of IP6.
References
- 1.Querfurth H., LaFerla F. Mechanisms of disease: Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D. Preventing Alzheimer’s disease. Science. 2012;337:1488–1492. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- 3.Gandy S. Perspective: prevention is better than cure. Nature. 2011;475:S15. doi: 10.1038/475S15a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X., Jin L., Peixue L. Review of drugs for Alzheimer’s disease. Drug Discov. Ther. 2012;6:285–290. [PubMed] [Google Scholar]
- 5.Vassar R., Kandalepas P. The beta-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res. Ther. 2011;3:20. doi: 10.1186/alzrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sofi F., Macchi C., Abbate R., Gensini G.F., Casini A. Effectiveness of the mediterranean diet: can it help delay or prevent Alzheimer’s disease? J. Alzheimers Dis. 2010;20:795–801. doi: 10.3233/JAD-2010-1418. [DOI] [PubMed] [Google Scholar]
- 7.Deweerdt S. Prevention: activity is the best medicine. Nature. 2011;475:S16–S17. doi: 10.1038/475S16a. [DOI] [PubMed] [Google Scholar]
- 8.Solfrizzi V., Panza F., Frisardi V., Seripa D., Logroscino G., Imbimbo B.P., Pilotto A. Diet and Alzheimer’s disease risk factors or prevention: the currrent evidence. Expert Rev. Neurother. 2011;11:677–708. doi: 10.1586/ern.11.56. [DOI] [PubMed] [Google Scholar]
- 9.Farrer L. Intercontinental epidemiology of Alzheimer disease – a global approach to bad gene hunting. JAMA. 2001;285:796–798. doi: 10.1001/jama.285.6.796. [DOI] [PubMed] [Google Scholar]
- 10.Yamakawa H., Yagishita S., Futai E., Ishiura S. Beta-secretase inhibitor potency is decreased by aberrant beta-cleavage location of the “Swedish mutant” amyloid precursor protein. J. Biol. Chem. 2010;285:1634–1642. doi: 10.1074/jbc.M109.066753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsunaga A., Yamamoto A., Kurokawa H., Sekiguchi Y. Determination of condensed phosphates in food stuffs by ion chromatography. Food Hyg. Saf. Sci. 1998;39:1–6. [Google Scholar]
- 12.Okazaki Y., Katayama T. Effects of dietary water-soluble rice bran, phytic acid and myo-inositol on prevention of fatty liver in rats fed DDT. Trace Nutrients Res. 2005;22:81–86. [Google Scholar]
- 13.Mangialasche F., Solomon A., Winblad B., Mecocci P., Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 14.Imbimbo B., Giardina G. Gamma-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr. Top. Med. Chem. 2011;11:1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 15.May P., Dean R., Lowe S., Martenyi F., Sheehan S., Boggs L., Monk S., Mathes B., Mergott D., Watson B., Stout S., Timm D., LaBell E., Gonzales C., Nakano M., Jhee S., Yen M., Ereshefsky L., Lindstrom T., Calligaro D., Cocke P., Hall D., Friedrich S., Citron M., Audia J. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J. Neurosci. 2011;31:16507–16516. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anekonda T., Wadsworth T., Sabin R., Frahler K., Harris C., Petriko B., Ralle M., Woltjer R., Quinn J. Phytic acid as a potential treatment for Alzheimer’s pathology: evidence from animal and in vitro models. J. Alzheimers Dis. 2011;23:21–35. doi: 10.3233/JAD-2010-101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf E., Eaton J. Antioxidant functions of phytic acid. Free Radical Biol. Med. 1990;8:61–69. doi: 10.1016/0891-5849(90)90146-a. [DOI] [PubMed] [Google Scholar]
- 18.Reddy M., Hurrell R., Juillerat M., Cook J. The influence of different protein sources on phytate inhibition of nonheme-iron absorption in humans. Am. J. Clin. Nutr. 1996;63:203–207. doi: 10.1093/ajcn/63.2.203. [DOI] [PubMed] [Google Scholar]
- 19.Luo X., Prior M., He W., Hu X., Tang X., Shen W., Yadav S., Kiryu-Seo S., Miller R., Trapp B., Yan R. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J. Biol. Chem. 2011;286:23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reduction of amyloid-β peptide (Aβ) production by the unpolished rice digest and IP6 [sodium salt, (SS)] in cultured SH-SY5Y cells overexpressing wild-type human APP (APPwt).
Digestion of neuregulin-1 (Nrg-1) peptide by BACE1 in the presence or absence of IP6.


