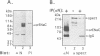Abstract
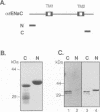
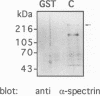
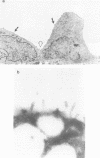
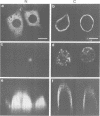
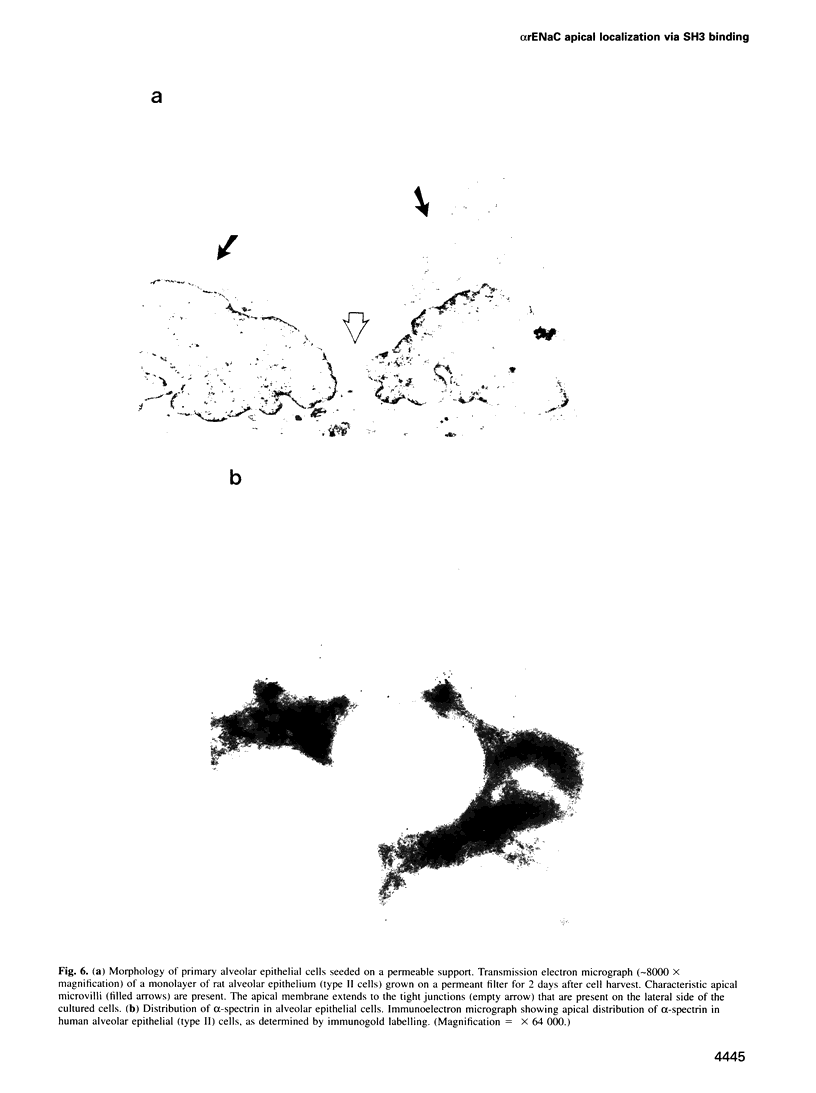
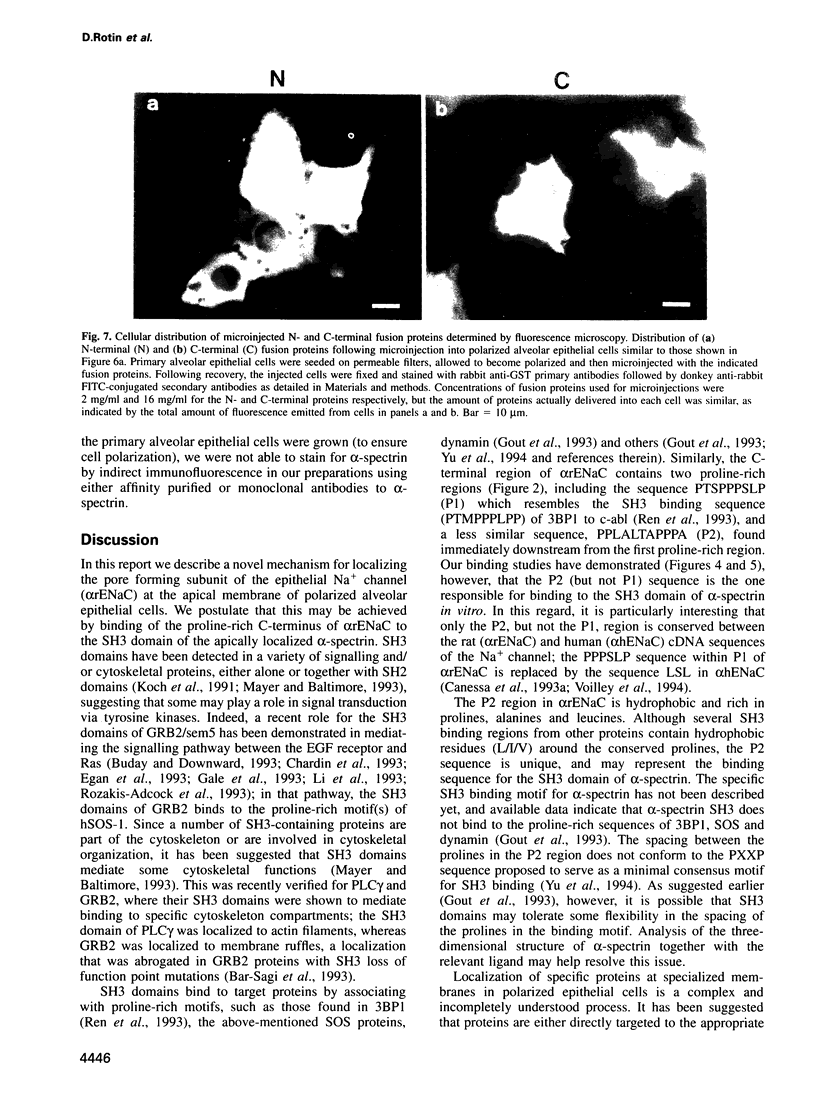
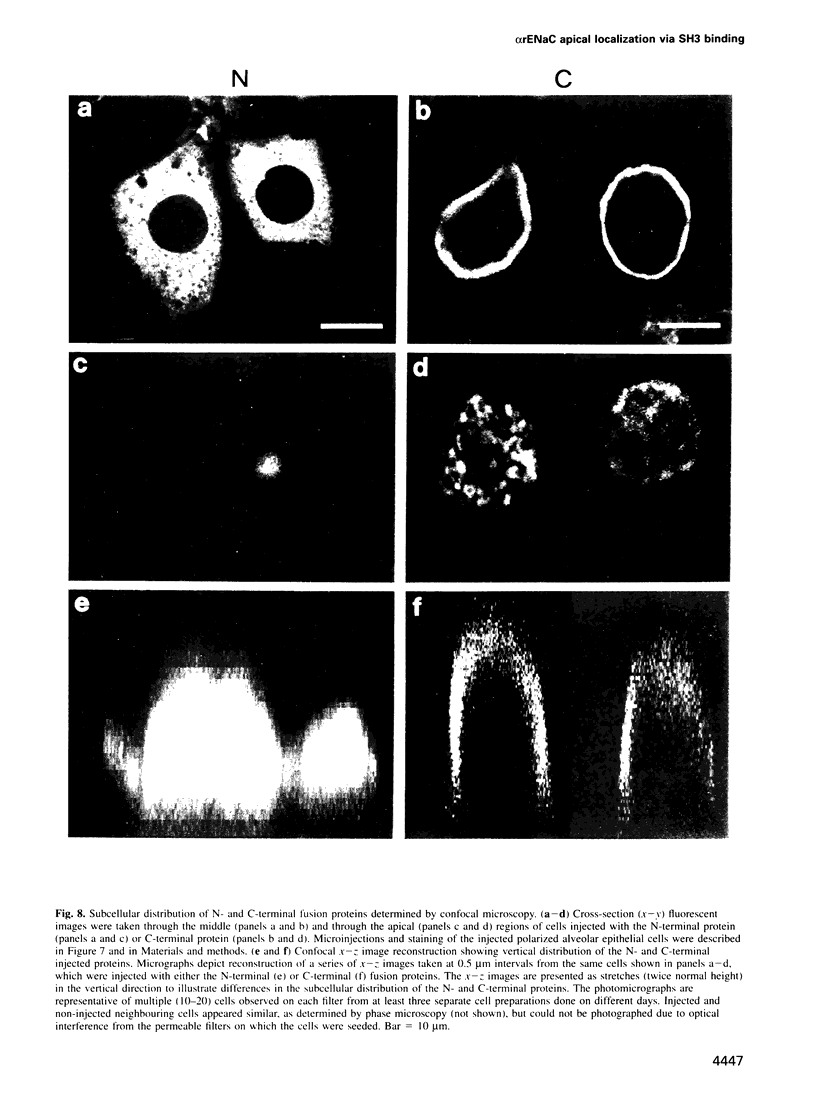
The amiloride-sensitive Na+ channel constitutes the rate-limiting step for Na+ transport in epithelia. Immunolocalization and electrophysiological studies have demonstrated that this channel is localized at the apical membrane of polarized epithelial cells. This localization is essential for proper channel function in Na+ transporting epithelia. In addition, the channel has been shown to associate with the cytoskeletal proteins ankyrin and alpha-spectrin in renal epithelia. However, the molecular mechanisms underlying the cytoskeletal interactions and apical membrane localization of this channel are largely unknown. In this study we show that the putative pore forming subunit of the rat epithelial (amiloride-sensitive) Na+ channel (alpha ENaC) binds to alpha-spectrin in vivo, as determined by co-immunoprecipitation. This binding is mediated by the SH3 domain of alpha-spectrin which binds to a unique proline-rich sequence within the C-terminal region of alpha rENaC. Accordingly, the C-terminal region is sufficient to mediate binding to intact alpha-spectrin from alveolar epithelial cell lysate. When microinjected into the cytoplasm of polarized primary rat alveolar epithelial cells, a recombinant fusion protein containing the C-terminal proline-rich region of alpha rENaC localized exclusively to the apical area of the plasma membrane, as determined by confocal microscopy. This localization paralleled that of alpha-spectrin. In contrast, microinjected fusion protein containing the N-terminal (control) protein of alpha rENaC remained diffuse within the cytoplasm. These results suggest that an SH3 binding region in alpha rENaC mediates the apical localization of the Na+ channel. Thus, cytoskeletal interactions via SH3 domains may provide a novel mechanism for retaining proteins in specific membranes of polarized epithelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Sagi D., Rotin D., Batzer A., Mandiyan V., Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993 Jul 16;74(1):83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- Bowtell D., Fu P., Simon M., Senior P. Identification of murine homologues of the Drosophila son of sevenless gene: potential activators of ras. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6511–6515. doi: 10.1073/pnas.89.14.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Horisberger J. D., Rossier B. C. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993 Feb 4;361(6411):467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa C. M., Schild L., Buell G., Thorens B., Gautschi I., Horisberger J. D., Rossier B. C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994 Feb 3;367(6462):463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Stow J. L., Prat A. G., Ausiello D. A. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991 Nov;261(5 Pt 1):C882–C888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Chardin P., Camonis J. H., Gale N. W., van Aelst L., Schlessinger J., Wigler M. H., Bar-Sagi D. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993 May 28;260(5112):1338–1343. doi: 10.1126/science.8493579. [DOI] [PubMed] [Google Scholar]
- Compeau C. G., Rotstein O. D., Tohda H., Marunaka Y., Rafii B., Slutsky A. S., O'Brodovich H. Endotoxin-stimulated alveolar macrophages impair lung epithelial Na+ transport by an L-Arg-dependent mechanism. Am J Physiol. 1994 May;266(5 Pt 1):C1330–C1341. doi: 10.1152/ajpcell.1994.266.5.C1330. [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Sillman A. L., Bar-Zvi D., Goldstein L. S., Branton D. The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989 Nov;109(5):2197–2205. doi: 10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature. 1993 May 6;363(6424):45–51. doi: 10.1038/363045a0. [DOI] [PubMed] [Google Scholar]
- Els W. J., Chou K. Y. Sodium-dependent regulation of epithelial sodium channel densities in frog skin; a role for the cytoskeleton. J Physiol. 1993 Mar;462:447–464. doi: 10.1113/jphysiol.1993.sp019563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993 May 6;363(6424):88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I. D., Fry M. J., Panayotou G., Das P., Truong O., Totty N. F., Hsuan J., Booker G. W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25–36. [PubMed] [Google Scholar]
- Ishii T., Moriyoshi K., Sugihara H., Sakurada K., Kadotani H., Yokoi M., Akazawa C., Shigemoto R., Mizuno N., Masu M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993 Feb 5;268(4):2836–2843. [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Wasenius V. M., Salvén P., Saraste M. Transforming and membrane proteins. Nature. 1988 Aug 4;334(6181):388–388. doi: 10.1038/334388a0. [DOI] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Lingueglia E., Voilley N., Waldmann R., Lazdunski M., Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993 Feb 22;318(1):95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Liu X., Marengere L. E., Koch C. A., Pawson T. The v-Src SH3 domain binds phosphatidylinositol 3'-kinase. Mol Cell Biol. 1993 Sep;13(9):5225–5232. doi: 10.1128/mcb.13.9.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon S., Bauer M. L., Benos D. J., Kleyman T. R., Lin C., Cragoe E. J., Jr, O'Brodovich H. Fetal lung epithelial cells contain two populations of amiloride-sensitive Na+ channels. Am J Physiol. 1993 Apr;264(4 Pt 1):L357–L364. doi: 10.1152/ajplung.1993.264.4.L357. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Baltimore D. Signalling through SH2 and SH3 domains. Trends Cell Biol. 1993 Jan;3(1):8–13. doi: 10.1016/0962-8924(93)90194-6. [DOI] [PubMed] [Google Scholar]
- Meriläinen J., Palovuori R., Sormunen R., Wasenius V. M., Lehto V. P. Binding of the alpha-fodrin SH3 domain to the leading lamellae of locomoting chicken fibroblasts. J Cell Sci. 1993 Jul;105(Pt 3):647–654. doi: 10.1242/jcs.105.3.647. [DOI] [PubMed] [Google Scholar]
- Nelson W. J. Regulation of cell surface polarity from bacteria to mammals. Science. 1992 Nov 6;258(5084):948–955. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H., Canessa C., Ueda J., Rafii B., Rossier B. C., Edelson J. Expression of the epithelial Na+ channel in the developing rat lung. Am J Physiol. 1993 Aug;265(2 Pt 1):C491–C496. doi: 10.1152/ajpcell.1993.265.2.C491. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H. Epithelial ion transport in the fetal and perinatal lung. Am J Physiol. 1991 Oct;261(4 Pt 1):C555–C564. doi: 10.1152/ajpcell.1991.261.4.C555. [DOI] [PubMed] [Google Scholar]
- O'Brodovich H., Hannam V., Rafii B. Sodium channel but neither Na(+)-H+ nor Na-glucose symport inhibitors slow neonatal lung water clearance. Am J Respir Cell Mol Biol. 1991 Oct;5(4):377–384. doi: 10.1165/ajrcmb/5.4.377. [DOI] [PubMed] [Google Scholar]
- Orser B. A., Bertlik M., Fedorko L., O'Brodovich H. Cation selective channel in fetal alveolar type II epithelium. Biochim Biophys Acta. 1991 Aug 13;1094(1):19–26. doi: 10.1016/0167-4889(91)90021-o. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Saumon G., Basset G. Electrolyte and fluid transport across the mature alveolar epithelium. J Appl Physiol (1985) 1993 Jan;74(1):1–15. doi: 10.1152/jappl.1993.74.1.1. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Benos D. J. Epithelial Na+ channels. Annu Rev Physiol. 1991;53:509–530. doi: 10.1146/annurev.ph.53.030191.002453. [DOI] [PubMed] [Google Scholar]
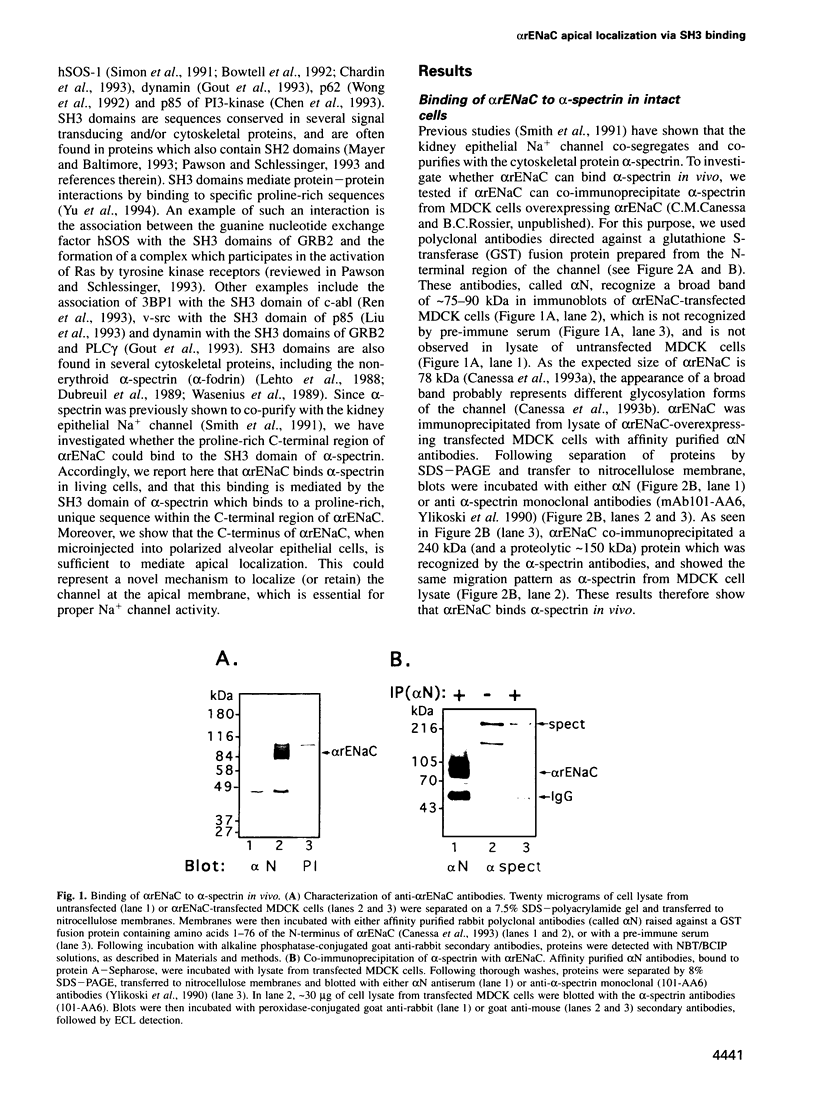
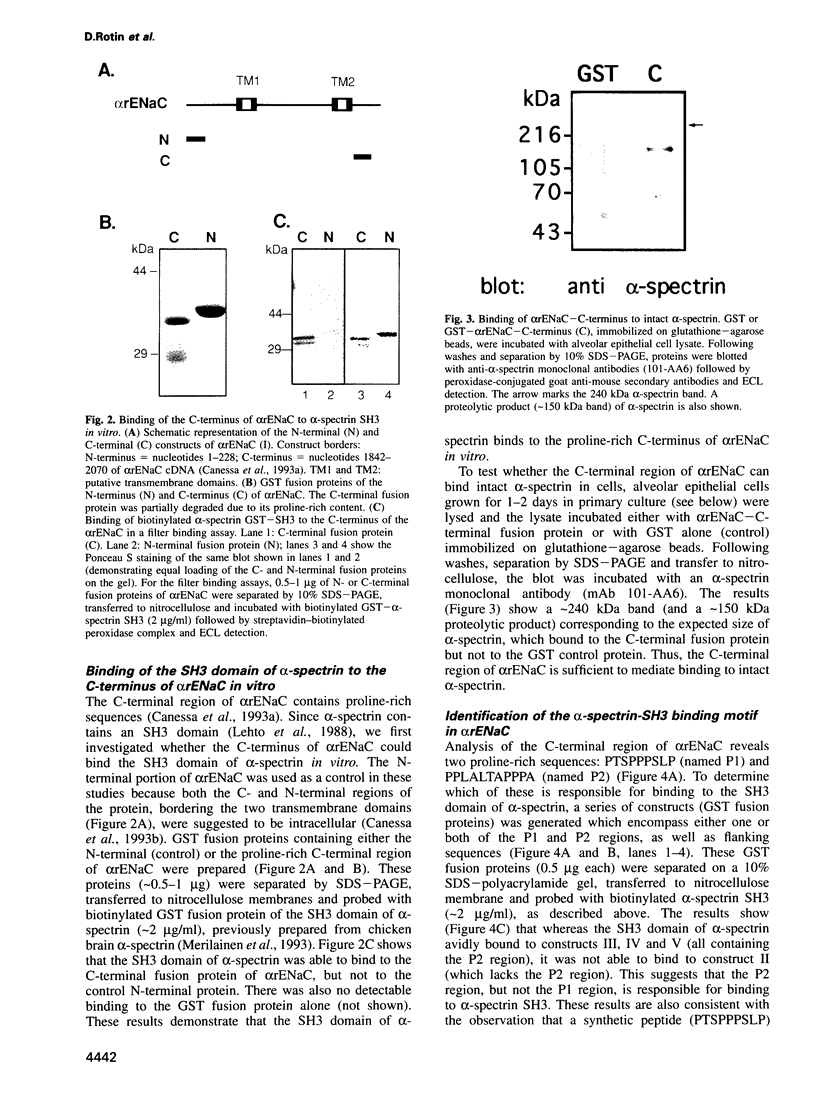
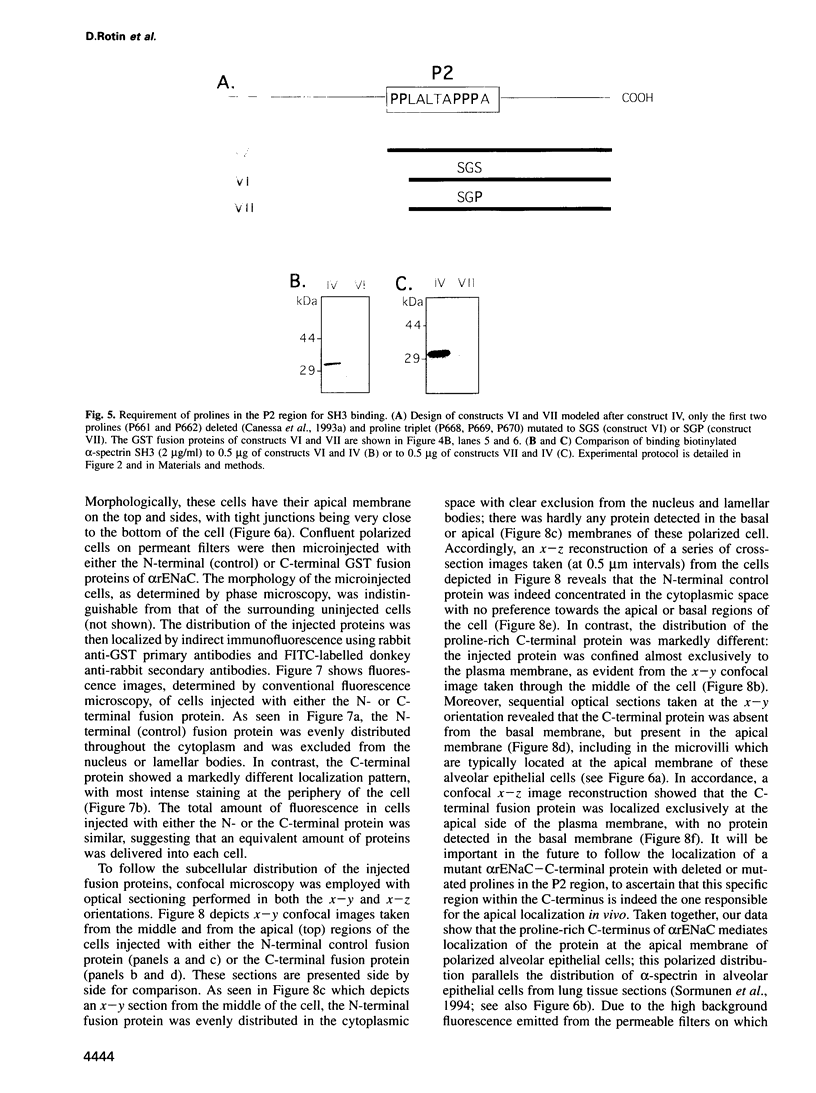
- Smith P. R., Saccomani G., Joe E. H., Angelides K. J., Benos D. J. Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6971–6975. doi: 10.1073/pnas.88.16.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormunen R., Päkkö P., Palovuori R., Soini Y., Lehto V. P. Fodrin and actin in the normal, metaplastic, and dysplastic respiratory epithelium and in lung carcinoma. Am J Respir Cell Mol Biol. 1994 Jul;11(1):75–84. doi: 10.1165/ajrcmb.11.1.8018340. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., Jentsch T. J. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Tousson A., Alley C. D., Sorscher E. J., Brinkley B. R., Benos D. J. Immunochemical localization of amiloride-sensitive sodium channels in sodium-transporting epithelia. J Cell Sci. 1989 Jun;93(Pt 2):349–362. doi: 10.1242/jcs.93.2.349. [DOI] [PubMed] [Google Scholar]
- Voilley N., Lingueglia E., Champigny G., Mattéi M. G., Waldmann R., Lazdunski M., Barbry P. The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]
- Wang X., Kleyman T. R., Tohda H., Marunaka Y., O'Brodovich H. 5-(N-Ethyl-N-isopropyl)amiloride sensitive Na+ currents in intact fetal distal lung epithelial cells. Can J Physiol Pharmacol. 1993 Jan;71(1):58–62. doi: 10.1139/y93-009. [DOI] [PubMed] [Google Scholar]
- Wang Z., Orlowski J., Shull G. E. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na/H exchanger. J Biol Chem. 1993 Jun 5;268(16):11925–11928. [PubMed] [Google Scholar]
- Wasenius V. M., Saraste M., Salvén P., Erämaa M., Holm L., Lehto V. P. Primary structure of the brain alpha-spectrin. J Cell Biol. 1989 Jan;108(1):79–93. doi: 10.1083/jcb.108.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Müller O., Clark R., Conroy L., Moran M. F., Polakis P., McCormick F. Molecular cloning and nucleic acid binding properties of the GAP-associated tyrosine phosphoprotein p62. Cell. 1992 May 1;69(3):551–558. doi: 10.1016/0092-8674(92)90455-l. [DOI] [PubMed] [Google Scholar]
- Xu J. C., Lytle C., Zhu T. T., Payne J. A., Benz E., Jr, Forbush B., 3rd Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J., Pirvola U., Närvänen O., Virtanen I. Nonerythroid spectrin (fodrin) is a prominent component of the cochlear hair cells. Hear Res. 1990 Jan;43(2-3):199–203. doi: 10.1016/0378-5955(90)90228-h. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]