Abstract
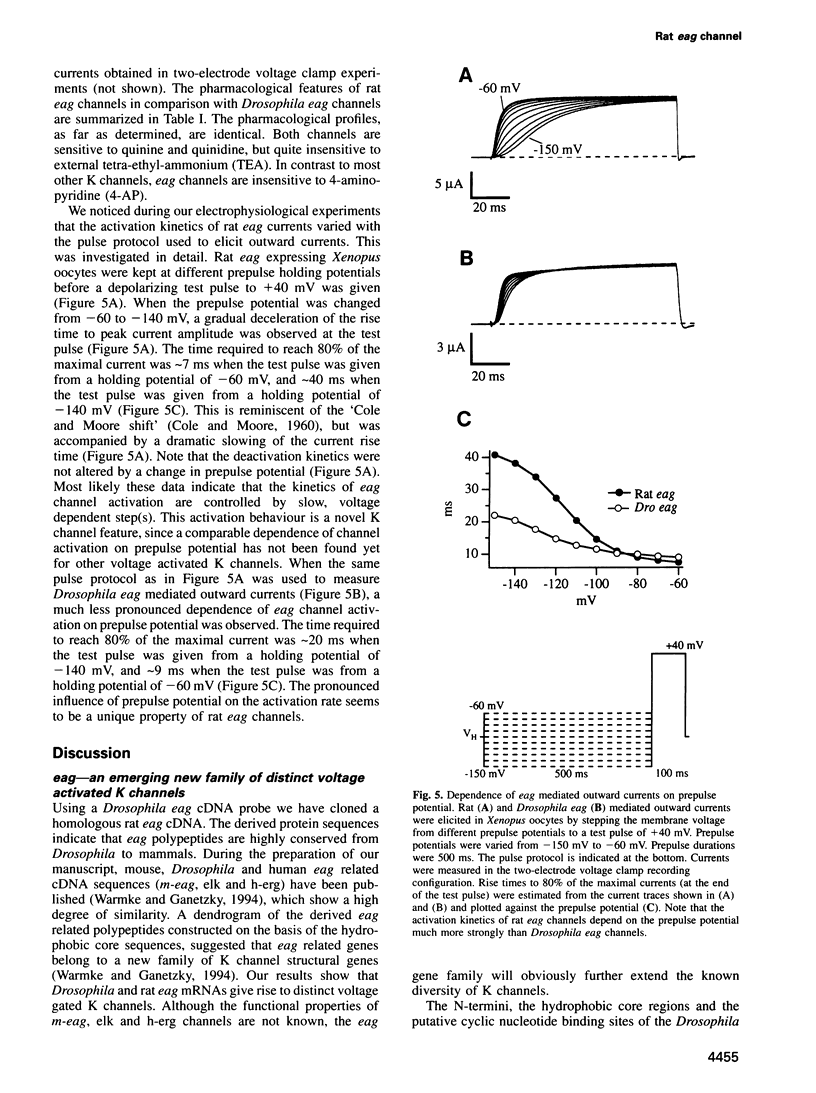
We have cloned a mammalian (rat) homologue of Drosophila ether á go-go (eag) cDNA, which encodes a distinct type of voltage activated potassium (K) channel. The derived Drosophila and rat eag polypeptides share > 670 amino acids, with a sequence identity of 61%, exhibiting a high degree of similarity at the N-terminus, the hydrophobic core including the pore forming P region and a potential cyclic nucleotide binding site. Rat eag mRNA is specifically expressed in the central nervous system. In the Xenopus oocyte expression system rat eag mRNA gives rise to voltage activated K channels which have distinct properties in comparison with Drosophila eag channels and other voltage activated K channels. Thus, the rat eag channel further extends the known diversity of K channels. Most notably, the kinetics of rat eag channel activation depend strongly on holding membrane potential. Hyperpolarization slows down the kinetics of activation; conversely depolarization accelerates the kinetics of activation. This novel K channel property may have important implications in neural signal transduction allowing neurons to tune their repolarizing properties in response to membrane hyperpolarization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Shen K. Z., Kavanaugh M. P., Warren R. A., Wu Y. N., Lagrutta A., Bond C. T., North R. A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992 Aug;9(2):209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Altenhofen W., Ludwig J., Eismann E., Kraus W., Bönigk W., Kaupp U. B. Control of ligand specificity in cyclic nucleotide-gated channels from rod photoreceptors and olfactory epithelium. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9868–9872. doi: 10.1073/pnas.88.21.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson N. S., Robertson G. A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991 Aug 2;253(5019):551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A., Grupe A., Ackermann A., Pongs O. Structure of the voltage-dependent potassium channel is highly conserved from Drosophila to vertebrate central nervous systems. EMBO J. 1988 Aug;7(8):2457–2463. doi: 10.1002/j.1460-2075.1988.tb03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann A., Pardo L. A., Stühmer W., Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca2+ and modulated by cAMP. Nature. 1993 Sep 30;365(6445):445–448. doi: 10.1038/365445a0. [DOI] [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D. P., Wei A., Salkoff L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channels. Science. 1993 Jul 9;261(5118):221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R., Ray S. C. Eukaryotic start and stop translation sites. Nucleic Acids Res. 1991 Jun 25;19(12):3185–3192. doi: 10.1093/nar/19.12.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Drysdale R., Warmke J., Kreber R., Ganetzky B. Molecular characterization of eag: a gene affecting potassium channels in Drosophila melanogaster. Genetics. 1991 Mar;127(3):497–505. doi: 10.1093/genetics/127.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Neurogenetics of membrane excitability in Drosophila. Annu Rev Genet. 1986;20:13–44. doi: 10.1146/annurev.ge.20.120186.000305. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Durell S. R., Warmke J., Drysdale R., Ganetzky B. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 1991 Nov 1;254(5032):730–730. doi: 10.1126/science.1658932. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Lu Z., Abramson T., MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994 Apr;66(4):1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Kaplan W. D., Trout W. E., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969 Feb;61(2):399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
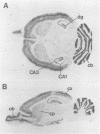
- Kues Wilfried A., Wunder Frank. Heterogeneous Expression Patterns of Mammalian Potassium Channel Genes in Developing and Adult Rat Brain. Eur J Neurosci. 1992;4(12):1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- McCormack K., Tanouye M. A., Iverson L. E., Lin J. W., Ramaswami M., McCormack T., Campanelli J. T., Mathew M. K., Rudy B. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2931–2935. doi: 10.1073/pnas.88.7.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
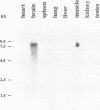
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
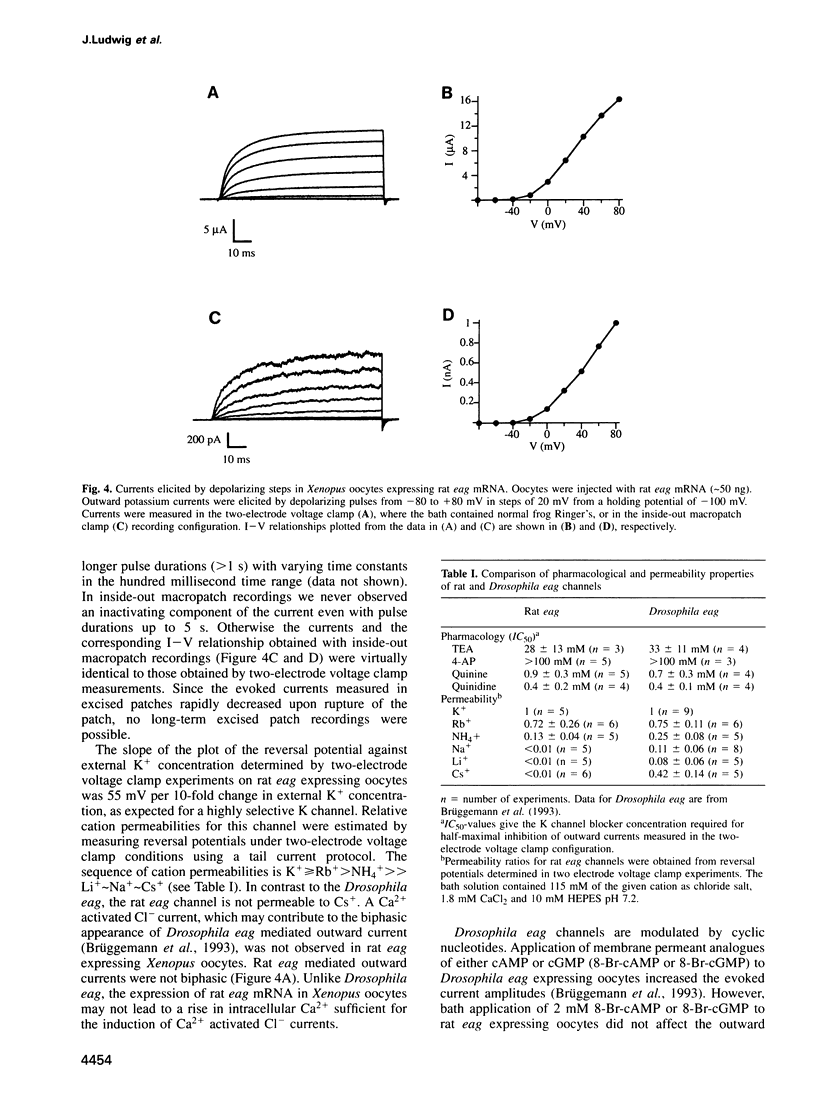
- Rettig J., Wunder F., Stocker M., Lichtinghagen R., Mastiaux F., Beckh S., Kues W., Pedarzani P., Schröter K. H., Ruppersberg J. P. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992 Jul;11(7):2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N. E., McCormack K., Tanouye M. A., Sigworth F. J. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992 Mar 27;255(5052):1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- Shabb J. B., Buzzeo B. D., Ng L., Corbin J. D. Mutating protein kinase cAMP-binding sites into cGMP-binding sites. Mechanism of cGMP selectivity. J Biol Chem. 1991 Dec 25;266(36):24320–24326. [PubMed] [Google Scholar]
- Shabb J. B., Ng L., Corbin J. D. One amino acid change produces a high affinity cGMP-binding site in cAMP-dependent protein kinase. J Biol Chem. 1990 Sep 25;265(27):16031–16034. [PubMed] [Google Scholar]
- Stocker M., Pongs O., Hoth M., Heinemann S. H., Stühmer W., Schröter K. H., Ruppersberg J. P. Swapping of functional domains in voltage-gated K+ channels. Proc Biol Sci. 1991 Aug 22;245(1313):101–107. doi: 10.1098/rspb.1991.0094. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Jan Y. N., Jan L. Y. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988 Apr 28;332(6167):837–839. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- Warmke J. W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke J., Drysdale R., Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991 Jun 14;252(5012):1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Shabb J. B., Corbin J. D. Predicted structures of the cGMP binding domains of the cGMP-dependent protein kinase: a key alanine/threonine difference in evolutionary divergence of cAMP and cGMP binding sites. Biochemistry. 1989 Jul 11;28(14):6122–6127. doi: 10.1021/bi00440a059. [DOI] [PubMed] [Google Scholar]
- Wei A., Covarrubias M., Butler A., Baker K., Pak M., Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990 May 4;248(4955):599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991 Jun 14;252(5012):1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Modulation of different K+ currents in Drosophila: a hypothetical role for the Eag subunit in multimeric K+ channels. J Neurosci. 1993 Nov;13(11):4669–4679. doi: 10.1523/JNEUROSCI.13-11-04669.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]