Abstract
For nearly a decade, interest groups, from politicians to economists to physicians, have touted digitization of the nation’s health information. One frequently mentioned benefit is the transmission of information electronically from laboratories to public health personnel, allowing them to rapidly analyze and act on these data.
Switching from paper to electronic laboratory reports (ELRs) was thought to solve many public health surveillance issues, including workload, accuracy, and timeliness. However, barriers remain for both laboratories and public health agencies to realize the full benefits of ELRs.
The New York City experience highlights several successes and challenges of electronic reporting and is supported by peer-reviewed literature. Lessons learned from ELR systems will benefit efforts to standardize electronic medical records reporting to health departments.
LABORATORY REPORTS SUPPORT passive public health surveillance, providing highly specific data about health conditions in a community. Efficient electronic exchange of laboratory information can facilitate time-sensitive decision-making.1 This is particularly true for infectious diseases, which require timely, accurate data to confirm diagnoses, detect outbreaks, and prevent transmission of disease to additional people. As public health agencies expand their mission to address chronic diseases, such as diabetes, laboratory reporting will also have an important role. At present, electronic laboratory reports (ELRs) offer a more accurate, complete, and efficient data source for public health surveillance than do paper reports.2 Significant progress has been made in using ELRs, but challenges still exist for this public health reporting system.
Since early in the 21st century, clinical laboratories have been transitioning from a system of mailing or faxing test results to exclusively transmitting data electronically to health departments. After September 11, 2001, Congress set up the Terrorism Preparedness and Emergency Response funds to support the public health emergency preparedness activities of the Centers for Disease Control and Prevention (CDC). This revenue initiated many state and local ELR systems, but funds have declined from $970 million in FY 2003 to $657 million in FY 2012.3 The National Electronic Disease Surveillance System coordinated by CDC provides standards and software and hardware resources to state and local health departments to implement standards-based ELR systems between clinics, health departments, and CDC. This national surveillance program has both accelerated ELR adoption, by providing standards, and delayed development, because of funding shortages and a lack of infrastructure support.4 In New York City, local public health legislation also facilitated ELR adoption.5 Improvements in technology, such as the incorporation of some messaging syntax standards into laboratory information management systems, have accelerated the shift to ELRs.2 In 2010, 42 US states reported having general communicable disease surveillance systems that incorporate ELRs,4 but how complete these systems are is unclear.
Federal legislation such as the Health Information Technology for Economic and Clinical Health Act, part of the American Reinvestment and Recovery Act of 2009, further advanced the use of ELRs in health care facilities that employ electronic medical records (EMRs). This act created Meaningful Use (MU), a federal program with financial incentives to implement, upgrade, and demonstrate meaningful use of certified electronic health record technology. ELRs were included as part of the stage 1 MU incentives.4,6 MU, however, does not provide financial incentives for commercial clinical laboratories to make technology upgrades. A broader goal of the health information technology legislation and MU is for health care providers to eventually communicate with public health agencies electronically, rather than by paper or phone. For several reasons, EMR adoption has been a challenge.7 An efficient ELR surveillance system will be a valuable resource for public health, and the lessons learned from ELR implementation, such as the establishment of standards, will help inform the subsequent use of EMRs for public health surveillance.
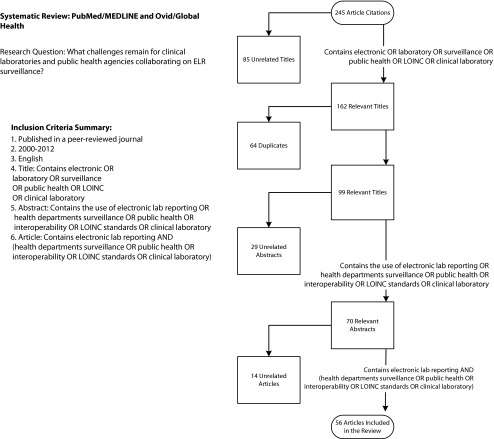
After reviewing the peer-reviewed literature addressing the topic of ELRs published between January 2000 and July 2012, we identified both substantial accomplishments and remaining challenges. The decision logic for the literature review and article inclusion is presented in Figure 1. To outline the issues, we studied the New York City Department of Health and Mental Hygiene (DOHMH) experience and followed the flow of a report from the clinical laboratory to the public health department. Along this cascade of information we identified major strides, delays, and possible solutions.
FIGURE 1—
Literature review inclusion decision tree for electronic laboratory reports in public health.
Note. ELR = electronic laboratory report; LOINC = Logical Observation Identifiers Names and Codes. The term SNOMED was not included in the search criteria because it did not noticeably improve the results of the search over using the term LOINC alone.
STRIDES FORWARD
Across the nation, states have been steadily transitioning clinical laboratory reporting to ELRs. Laboratories are required to report certain test results to health departments, as indicated in local jurisdiction health codes and national initiatives such as MU.4 According to a 2009 Council of State and Territorial Epidemiologists survey of the 50 states and the District of Columbia, epidemiologists reported that 27 states, or 53%, had technology capacity for automated ELR transmission.8 Unfortunately, the survey definition of the term “automated” was unclear. In a more recent 2011 national survey of 49 states and 4 cities, more than 80% of states surveyed had established some level of ELR information exchange with laboratories, and the remainder were in either the testing or the planning phase.9 Although the surveys were conducted by different researchers reaching out to different subjects, this increase in capacity suggests ELR infrastructure progress.
ELR implementation has led to more complete and faster public health surveillance of certain notifiable diseases.4,10–13 We analyzed 6 separate studies and found that use of ELRs resulted in an 8.5-day average decrease in overall reporting time (range = 4–17 days).11,13–17 Three studies also cited significant increases in the volume of reports.11,13,15 For example, when the DOHMH used ELRs for Salmonella cases, up to 76% more cases were reported than had previously been received on paper. At the DOHMH, the median improvement in reporting time with ELRs instead of paper reports was 11 days (range = 3–42 days) for a sample of common reportable diseases.13 In addition, the DOHMH keeps an electronic audit trail of ELRs. This leads to faster detection of laboratories that fail to send reports and decreases the time it takes to notice communication delays or underreporting.
Faster laboratory reporting may prevent further disease spread. For example, when a syphilis laboratory test result is received and analyzed, a public health case worker may contact the syphilis patient, identify the patient’s sexual partners, and contact the partners to treat them for syphilis, potentially preventing further disease transmission. Expedited laboratory reporting helps the health department shorten the window during which syphilis patients and their potentially infected partners can unknowingly spread this infection to others.13 ELRs also improve the tracking of high-volume chronic diseases. For example, the DOHMH now receives hemoglobin A1C results via ELRs to monitor blood sugar control and diabetes diagnoses in the city.18
COMMUNICATION DELAYS
Despite improved communication, laboratories still face the challenge of transmitting a single test result message to several different groups: doctors, patients, other laboratories, insurance companies, and public health agencies in multiple jurisdictions. Each ELR-receiving group may have its own semantic standards and reporting systems. Laboratory diagnostics, particularly for infectious diseases, are evolving rapidly, with clinical laboratories adopting many new multiplex test platforms. These new molecular assays are complicating the mapping of test results for ELRs that were designed to work with previous, not current or future, technologies.19 If a laboratory implements a new assay that generates results with a different laboratory test or outcome code, ELRs need to be reconfigured. Confounding the modification to ELRs is the ambiguity about new assays and how to compare results from these tests to the existing standards. Delay in adjustment of ELR code could lead to missed cases or misclassification.20 Although many of these problems existed with paper reporting, ELRs have changed the volume and work flow.
Technical capabilities are not the only major roadblock for sending ELRs. Health departments report that the number one barrier to ELR use is that laboratories have other competing information technology priorities.9 Because sending ELRs to public health agencies accounts for only a small proportion of all outgoing reports and does not generate revenue for the laboratory, it may be a lower priority for the laboratory than improving its reporting to health care providers and patients. This is especially true in smaller clinical laboratories with limited resources.21 Variations in laboratory resources can lead to variations in the quality of reports sent to public health agencies.
Although ELRs are faster than paper reporting, health departments may not always be able to interpret the data sent from laboratories.22 An analogy is to consider an ELR as a paper letter in an envelope. The envelopes carrying the message (the laboratory result) are uniform, but the messages contained inside are written in different languages. When this happens, the health department’s information system either misinterprets the message or simply cannot read it. This leads to a lengthy human review of ELRs and time lost in processing nonstandardized data.
Many of the following issues existed in the era of paper laboratory reporting; ELR use has automated some data processes but complicated others by increasing the reporting volume. Massive amounts of data in varying formats can quickly become difficult for health departments to manage, altering both work flow and load. First, some ELRs may lack basic information and need follow-up, such as retrieving the patient’s address or the specimen source.2 When the laboratory sends a positive test result for syphilis but no patient contact information, a public health case worker can do little with this information. It is up to health department staff to track down missing information to complete a report. In New York City, even though laboratories have been certified to send ELRs, informatics staff have to continually monitor the data to ensure quality.13 This puts an additional burden on public health staff to keep up on ELR changes and errors at the laboratory.
Second, many health departments accept a variety of nonstandard syntax formats. When laboratories use their own local codes in the ELR, these nonstandardized reports cannot be acted on quickly. To process nonstandardized ELRs, health departments have to maintain thousands of ever-changing rules in a computer program that sorts data. It takes substantial time and resources for programmers to decide on the logic and program rules that filter ELRs, without losing important cases. For example, in May 2009, a laboratory sent the DOHMH an ELR with the result of a test for Streptococcus with the local test result code ECLRS460. In July 2009, the same laboratory changed the Streptococcus code to 10003 without notifying the health department. This new test result name was not recognized by the health department information system sorting rules. Therefore, all messages containing this code required the health department to identify this problem, contact the laboratory for clarification, then update the computer sorting rules. In January 2013, the DOHMH received roughly 800 ELRs that could not be automatically sorted by the computer; therefore, public health staff had to manually review each message to decide whether it was important, a process that takes humans days but, if correctly processed by a computer, should take seconds. If New York City's experience is multiplied across the country in hundreds of laboratories and with thousands of disease codes, continuous ELR follow-up could massively delay action by public health workers.
Third, ELR use increases the volume of reports and can impose an additional burden on public health staff; increasing the data volume makes it harder to ensure data quality. Some health departments have found that receiving large amounts of laboratory data can lead to more false positives, which may be hard to distinguish from true positive cases that need to be acted on immediately.12,23,24 For example, a false positive tuberculosis ELR sent to the health department may alert a public health worker to initiate a case investigation, leading to wasted efforts.10,23–25 It is now easier for laboratories to send duplicate reports, and it is difficult and time consuming for health departments to sort a larger volume of duplicate reports. The resulting unnecessary follow-up could drain public health investigation resources.
Fourth, information technology infrastructure upgrades and workforce development are needed. Health departments must secure additional data storage for sensitive health messages as well as maintain information systems capable of sorting large amounts of data.26 In Massachusetts, the state government purchased an information system to assist laboratories with the task of mapping local codes to the state’s preferred format. However, it has required an investment in specialized applications and support staff,26 an outlay that many cash-strapped public health agencies cannot afford. According to a Council of State and Territorial Epidemiologists survey, the cost for the start-up, engagement, and maintenance phases of ELR systems in 9 states ranged from $221 000 to $633 500 per year.27 Although ELRs are more timely and abundant, they can also redirect scarce health department resources. From the workforce perspective, finding highly skilled staff and funds for development is a major challenge for public health informatics. The market for highly trained informatics workers extends across the business and government spectrum. It is no surprise, therefore, that health departments, which can rarely offer salaries or opportunities for upward mobility that match those of private industry, list lack of health department staff as the second most important barrier to ELR systems.27 Therefore, investments in technology upgrades and workforce training are continually needed.
INTEROPERABILITY SOLUTIONS
System interoperability is the capacity of computers in different institutions to exchange data and recognize the structure, format, and terms used in messages. Reaching this level of communication requires adherence to the structured message syntax—the envelope—and the standardized data language semantics—the letter. Getting agreement from all relevant parties involves a high level of coordination,28,29 but will ultimately lead to long-term benefits for patients, providers, and public health.
The CDC Public Health Information Network identifies 2 key components to consider when setting ELR standards. The first is the messaging syntax standard, such as Health Level 7 International (HL7), which provides the envelope for the health message.29 It is the most prevalent standard for electronic health care data exchange between multiple clinical information systems and provides syntax but not semantic standards.30 Close to a third of health departments in a national survey reported receiving all ELRs in this format. This means that roughly two thirds are receiving other ELR formats from laboratories, requiring systems that can process multiple formats.9
The second component of electronic reporting is the semantics contained within the message. The Logical Observation Identifiers Names and Codes (LOINC) is an international coding vocabulary for identifying laboratory tests and clinical observations. A LOINC committee managed by the Regenstrief Institute (Indianapolis, IN) frequently updates the code database in response to comments from users.31 LOINC is used by several large reference laboratories and federal agencies, including CDC and the Department of Veterans Affairs.31 The Regenstrief Institute provides a free application to assist with the translation of local codes to LOINC.
In many cases, LOINC laboratory test codes work in tandem with another code set, the Systematized Nomenclature of Medicine–Clinical Terms (SNOMED-CT; International Health Terminology Standards Development Organisation, Copenhagen, Denmark). SNOMED-CT is an inclusive health care coding system with broad coverage of clinical medicine, including anatomy, diseases, and procedures (laboratory procedures and others).32 SNOMED-CT is updated semiannually in response to requests for changes from users.33 Together, LOINC and SNOMED-CT attempt to create a comprehensive system for the world of medical tests and outcomes. Both LOINC and SNOMED-CT vocabularies are aggregated in a searchable resource called the Public Health Information Network Vocabulary Access and Distribution System.34
Even though standardization improves communication, these standards are by no means perfect, as studies have shown.35–38 Nevertheless, more widespread adoption will help these vocabularies evolve and adapt to improve public health surveillance. In other words, getting everyone to speak the same language, no matter how imperfect that language is, will improve communication more rapidly than allowing multiple different languages to compete for widespread use.
Standards need to be applied from the beginning, when the health care provider requests a laboratory test for a patient. Increasingly, that request will be done via the EMR. For that reason, EMR and laboratory information system developers, laboratory equipment vendors, and test kit manufacturers should include LOINC and SNOMED identification with their products.31,39 Ideally, patient information needed for public health investigations, such as pregnancy status, could come from the EMR requisition and travel with the ELR. To move past stalled semantic standards adoption, future versions of MU will ask that EMRs and laboratories use LOINC and SNOMED-CT standards.40 We understand that EMR developers will add these standards to a long list of other capability requests, but interoperability should be a priority, because it will ultimately benefit patients and public health.
As a short-term solution, laboratories can retain their local test code vocabulary and map these to accepted standard codes for ELRs.31 This solution is time consuming, but it allows laboratories to preserve their local test language while expediting electronic communication with other institutions. The approval process for codes for new diseases and tests is lengthy, and because laboratory procedures can change, mapping laboratory codes is an ongoing task.2,41 Still, the number of new terms added each year is minimal.42 The greatest time investment comes from the initial adoption and mapping of local laboratory codes to national standards. Ultimately, the long-term goal is for laboratories to incorporate standardized language directly into their information management system.
The barriers posed by ELRs need creative minds to propose solutions. To attract new talent and accelerate health information technology innovation, reportable ELR standards could be made available to developers through challenges such as Challenge.gov, a White House initiative that hosts competitions that use government data to solve complex problems. We propose public challenges to source ideas for software applications that integrate semantic standards into EMRs and laboratory information systems. These competitions have generated hundreds of designs and tools for using medical data.43 Data challenges also attract talented computer scientists to the field of public health informatics, which could ultimately advance workforce recruitment.
CONCLUSIONS
We found evidence from multiple sources that ELR implementation has reduced reporting time and increased reporting volume, but that many obstacles remain. ELR use can affect the workload and work flow of public health practice. Information system investments alone cannot solve ELR issues. Government agencies should endeavor to retain skilled staff and redirect information technology resources to handle the flood of data sent from clinical laboratories.
Although MU calls for the use of semantic standards, it is unclear whether the financial incentives from MU will reach the clinical laboratories. Developing tools for laboratories to efficiently adopt standards-based ELR may accelerate this transition. Standards organizations will continue to adapt to an ever-changing roster of health care codes and maintain an open forum for input from the standards consumers.
With continued collaboration from all involved parties, these challenges can be met and ultimately improve public health surveillance. Furthermore, EMR reporting to public health agencies may prove an even greater challenge than ELR implementation. Refining the ELR system now will serve as a model for the eventual exchange of data from provider EMRs to public health agencies. Government health agencies should promote reporting standards at an early stage and remain cautiously optimistic about the future of electronic disease reporting.
Acknowledgments
This study was supported in part by an appointment to the Applied Public Health Informatics Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by the Centers for Disease Control and Prevention (cooperative agreement 3U38HM000414-04W1).
We thank the Association of State and Territorial Health Officials, Public Health Informatics Institute, Laura Goodman, Robin Hennessy, Molly Kratz, Afua Saunders Kim, and the reviewers for their suggestions and guidance.
Human Participant Protection
No protocol approval was required because no human participants were involved.
References
- 1.Dullabh P, Moiduddin A. White Paper: Electronic Exchange of Clinical Laboratory: Information Issues and Opportunities. Washington, DC: Office of the Assistant Secretary for Planning and Evaluation, US Dept of Health and Human Services; 2008. [Google Scholar]
- 2.Wurtz R, Cameron BJ. Electronic laboratory reporting for the infectious diseases physician and clinical microbiologist. Clin Infect Dis. 2005;40(11):1638–1643. doi: 10.1086/429904. [DOI] [PubMed] [Google Scholar]
- 3.Kahn A. Public Health Preparedness: 2012 State-By-State Report on Laboratory, Emergency Operations Coordination, Emergency Public Information Coordination and Warning Capabilities. Atlanta, GA: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 4.Centers for Disease Control and Prevention. State electronic disease surveillance systems—United States, 2007 and 2010. MMWR Morb Mortal Wkly Rep. 2011;60(41):1421–1423. [PubMed] [Google Scholar]
- 5. Department of Health and Mental Hygiene. Notice of intention to amend Article 13 of the New York City health code. Notice of public hearing. 2005. Available at: http://www.nyc.gov/html/doh/downloads/pdf/public/notice-intention-art-13.pdf. Accessed March 7, 2013.
- 6.Office of the National Coordinator for Health Information Technology; Department of Health and Human Services. Health information technology: standards, implementation specifications, and certification criteria for electronic health record technology, 2014 edition; revisions to the permanent certification program for health information technology. Final rule. Fed Regist. 2012;77(171):54163–54292. [PubMed] [Google Scholar]
- 7.Kellermann AL, Jones SS. What it will take to achieve the as-yet-unfulfilled promises of health information technology. Health Aff (Millwood) 2013;32(1):63–68. doi: 10.1377/hlthaff.2012.0693. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Assessment of epidemiology capacity in state health departments—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(49):1373–1377. [PubMed] [Google Scholar]
- 9.Magnuson JA. National electronic laboratory reporting (ELR) snapshot survey: summary of results. 2011 Available at: http://www.coast2coastinformatics.com/2011NationalELRSurvey-DataSummary.pdf. Accessed May 16, 2013. [Google Scholar]
- 10.Centers for Disease Control and Prevention. Potential effects of electronic laboratory reporting on improving timeliness of infectious disease notification—Florida, 2002–2006. MMWR Morb Mortal Wkly Rep. 2008;57(49):1325–1328. [PubMed] [Google Scholar]
- 11.Overhage JM, Grannis S, McDonald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98(2):344–350. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overhage JM, Suico J, McDonald CJ. Electronic laboratory reporting: barriers, solutions and findings. J Public Health Manag Pract. 2001;7(6):60–66. doi: 10.1097/00124784-200107060-00007. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TQ, Thorpe L, Makki HA, Mostashari F. Benefits and barriers to electronic laboratory results reporting for notifiable diseases: the New York City Department of Health and Mental Hygiene experience. Am J Public Health. 2007;97(suppl 1):S142–S145. doi: 10.2105/AJPH.2006.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen I, Rasmussen M. Electronic interchange of lab test orders and results between laboratories reduces errors and gives full traceability. Stud Health Technol Inform. 2010;155:65–68. [PubMed] [Google Scholar]
- 15.Moore KM, Reddy V, Kapell D, Balter S. Impact of electronic laboratory reporting on hepatitis A surveillance in New York City. J Public Health Manag Pract. 2008;14(5):437–441. doi: 10.1097/01.PHH.0000333877.78443.f0. [DOI] [PubMed] [Google Scholar]
- 16.Panackal AA, M’Ikanatha NM, Tsui FC et al. Automatic electronic laboratory-based reporting of notifiable infectious diseases at a large health system. Emerg Infect Dis. 2002;8(7):685–691. doi: 10.3201/eid0807.010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward M, Brandsema P, van Straten E, Bosman A. Electronic reporting improves timeliness and completeness of infectious disease notification, the Netherlands, 2003. Euro Surveill. 2005;10(1):27–30. [PubMed] [Google Scholar]
- 18.Chamany S, Silver LD, Bassett MT et al. Tracking diabetes: New York City’s A1C Registry. Milbank Q. 2009;87(3):547–570. doi: 10.1111/j.1468-0009.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronquist AB, Mody RK, Atkinson R et al. Impacts of culture-independent diagnostic practices on public health surveillance for bacterial enteric pathogens. Clin Infect Dis. 2012;54(suppl 5):S432–S439. doi: 10.1093/cid/cis267. [DOI] [PubMed] [Google Scholar]
- 20.DeMaria A. Guiding principles for considering new diagnostic laboratory methodology and results in infectious disease case definitions of conditions under standardized surveillance. 2013 Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/13-ID-10.pdf. Accessed October 4, 2013. [Google Scholar]
- 21.Pinner RW, Jernigan DB, Sutliff SM. Electronic laboratory-based reporting for public health. Mil Med. 2000;165(7, suppl 2):20–24. [PubMed] [Google Scholar]
- 22.Bouhaddou O, Warnekar P, Parrish F et al. Exchange of computable patient data between the Department of Veterans Affairs (VA) and the Department of Defense (DoD): terminology mediation strategy. J Am Med Inform Assoc. 2008;15(2):174–183. doi: 10.1197/jamia.M2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M’ikanatha NM, Southwell B, Lautenbach E. Automated laboratory reporting of infectious diseases in a climate of bioterrorism. Emerg Infect Dis. 2003;9(9):1053–1057. doi: 10.3201/eid0909.020486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Effect of electronic laboratory reporting on the burden of lyme disease surveillance—New Jersey, 2001–2006. MMWR Morb Mortal Wkly Rep. 2008;57(2):42–45. [PubMed] [Google Scholar]
- 25.Backer HD, Bissell SR, Vugia DJ. Disease reporting from an automated laboratory-based reporting system to a state health department via local county health departments. Public Health Rep. 2001;116(3):257–265. doi: 10.1093/phr/116.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Massachusetts Department of Health. Electronic laboratory reporting. 2012. Available at: http://www.mass.gov/eohhs/gov/departments/dph/programs/id/isis/meaningful-use-and-public-health-objectives.html. Accessed July 30, 2012.
- 27. Council for State and Territorial Epidemiologists and Centers for Disease Control and Prevention. CSTE/CDC ELR Task Force; Resource Capacity Assessment Workgroup. ELR roadmap state cost summary. 2012. Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/SurveillanceInformatics/ELRRoadmapStateCostSummaryan.pdf?hhSearchTerms=%22Resource+and+Capacity+Assessment+Workgroup%22. Accessed July 13, 2012.
- 28. Centers for Disease Control and Prevention. Public Health Information Network. Available at: http://www.cdc.gov/phin. Accessed August 22, 2012.
- 29. Health Level Seven International. About HL7. 2013. Available at: http://www.hl7.org/about/index.cfm?ref=nav. Accessed August 21, 2012.
- 30.Zarcone P, Nordenberg D, Meigs M, Merrick U, Jernigan D, Hinrichs SH. Community-driven standards-based electronic laboratory data-sharing networks. Public Health Rep. 2010;125(suppl 2):47–56. doi: 10.1177/00333549101250S206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald CJ, Huff SM, Suico JG et al. LOINC, a universal standard for identifying laboratory observations: a 5-year update. Clin Chem. 2003;49(4):624–633. doi: 10.1373/49.4.624. [DOI] [PubMed] [Google Scholar]
- 32.Brown SH, Rosenbloom ST, Bauer BA et al. Direct comparison of MEDCIN and SNOMED CT for representation of a general medical evaluation template. AMIA Annu Symp Proc. 2007;11:75–79. [PMC free article] [PubMed] [Google Scholar]
- 33. International Health Terminology Standards Development Organisation. Available at: http://www.ihtsdo.org. Accessed September 4, 2012.
- 34.PHIN Vocabulary Access and Distribution System (VADS) 3.3.9 Release Notes Version 1.0. Atlanta, GA: Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 35.Fidahussein M, Friedlin J, Grannis S. Practical challenges in the secondary use of real-world data: the notifiable condition detector. AMIA Annu Symp Proc. 2011;2011:402–408. [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Progress in improving state and local disease surveillance—United States, 2000–2005. MMWR Morb Mortal Wkly Rep. 2005;54(33):822–825. [PubMed] [Google Scholar]
- 37.Grannis S, Vreeman D. A vision of the journey ahead: using public health notifiable condition mapping to illustrate the need to maintain value sets. AMIA Annu Symp Proc. 2010;2010:261–265. [PMC free article] [PubMed] [Google Scholar]
- 38.Lin MC, Vreeman DJ, McDonald CJ, Huff SM. Auditing consistency and usefulness of LOINC use among three large institutions—using version spaces for grouping LOINC codes. J Biomed Inform. 2012;45(4):658–666. doi: 10.1016/j.jbi.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichert JC, Glasgow M, Narus SP, Clayton PD. Using LOINC to link an EMR to the pertinent paragraph in a structured reference knowledge base. Proc AMIA Symp. 2002:652–656. [PMC free article] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention. Summary of public health objectives in stage 2 meaningful use ONC and CMS final rules. Available at: http://www.cdc.gov/ehrmeaningfuluse/Docs/Summary%20of%20PH%20Objectives%20in%20Stage%202%20MU%20ONC%20and%20CMS%20Final%20Rules.pdf. Accessed August 22, 2012.
- 41.Steindel S, Loonsk JW, Sim A, Doyle TJ, Chapman RS, Groseclose SL. Introduction of a hierarchy to LOINC to facilitate public health reporting. Proc AMIA Symp. 2002:737–741. [PMC free article] [PubMed] [Google Scholar]
- 42.Zunner C, Bürkle T, Prokosch HU, Ganslandt T. Mapping local laboratory interface terms to LOINC at a German university hospital using RELMA V.5: a semi-automated approach. J Am Med Inform Assoc. 2013;20(2):293–297. doi: 10.1136/amiajnl-2012-001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. ChallengePost, US General Services Administration. A partnership between the public and the government to solve important challenges. Available at: http://www.Challenge.gov. Accessed January 8, 2013.