Abstract
Objectives. We examined the impact of geographic residency status and census tract (CT)-level socioeconomic status (SES) on colorectal cancer (CRC) outcomes.
Methods. This was a retrospective cohort study of patients diagnosed with CRC in Georgia for the years 2000 through 2007. Study outcomes were late-stage disease at diagnosis, receipt of treatment, and survival.
Results. For colon cancer, residents of lower-middle-SES and low-SES census tracts had decreased odds of receiving surgery. Rural, lower-middle-SES, and low-SES residents had decreased odds of receiving chemotherapy. For patients with rectal cancer, suburban residents had increased odds of receiving radiotherapy, but low SES resulted in decreased odds of surgery. For survival, rural residents experienced a partially adjusted 14% (hazard ratio [HR] = 1.14; 95% confidence interval [CI] = 1.07, 1.22) increased risk of death following diagnosis of CRC that was somewhat explained by treatment differences and completely explained by CT-level SES. Lower-middle- and low-SES participants had an adjusted increased risk of death following diagnosis for CRC (lower-middle: HR = 1.16; 95% CI = 1.10, 1.22; low: HR = 1.24; 95% CI = 1.16, 1.32).
Conclusions. Future efforts should focus on developing interventions and policies that target rural residents and lower SES areas to eliminate disparities in CRC-related outcomes.
For men and women in the United States, colorectal cancer (CRC) ranks third in incidence and mortality among cancers, with an estimated 142 820 new cases and 50 830 deaths in 2013.1 Reflecting the US population distribution according to geography2 and evidence of similar incidence rates3,4 for rural residents, approximately 20% of incident CRC cases are expected to occur in rural populations. Although CRC incidence is equivalent for rural and urban residents, CRC mortality is higher in rural populations,5 and the causes of rural versus urban disparities in CRC mortality are not well understood. Compared with their suburban and urban counterparts, rural citizens are more likely to be older, live in poverty, have less education, lack health insurance, and have no regular health care provider.6–9 These facets of rural living pose challenges to accessing health promotional messages and high-quality primary care, not to mention treatment of cancer.10,11
Those of lower socioeconomic status (SES) have worse health-related outcomes than their more affluent counterparts, and SES often has a gradient effect on health.12 A challenge in studying the association between rurality and health is being able to disentangle the confounding effect of SES associated with geographic residency.13 As we previously demonstrated for a sample of urban and rural residents of Georgia with CRC, rural residence was associated with an increased risk of death following diagnosis.14 A limitation of that study was an inability to account for SES differences between urban and rural populations. If adjustment for SES explains the poorer survival that is associated with rural residence, this explanation provides an opportunity to investigate mediators of the SES effect as potential avenues for intervention.15 Identification of these mediating factors will facilitate the development of focused interventions with the goal of eliminating rural CRC-related disparities.16,17
Building on our previous work,14 we evaluated the independent and combined effects of rurality and area-level SES on CRC outcomes. In our previous study, (1) we were unable to evaluate the independent and potential confounding effect of SES on rurality,2 (2) our study population was a sample of the Georgia CRC population, and (3) residents were classified as urban or rural at the county level, which may have resulted in misclassification. In the present study, the exposures of interest were geographic residency status (rural, suburban, or urban) and area-level SES, both at the census tract (CT) level. In addition, the study population represents the entire state of Georgia rather than a sample. The primary study outcome was overall survival. Secondarily, we wanted to evaluate the effect of SES and geography adjusted for SES on the odds of late-stage disease at diagnosis and receipt of first-course treatment.
The findings of this study are meant to bring importance to a highly relevant area of public health research: disparities related to rural versus urban cancer outcomes, and specifically to rural CRC outcomes. As a result, interventions may be designed and policies developed to address the difficulties of accessing and providing high-quality cancer care in rural areas of the United States.11 It is through the combination of applying what is learned from epidemiological findings to community-level interventions and policymaking that the elimination of health disparities will occur.18
METHODS
This was a retrospective cohort study of patients in Georgia diagnosed with colon or rectal cancer for the years 2000 through 2007 (n = 30 100). The termination date for follow-up was January 1, 2012. The Georgia Comprehensive Cancer Registry (GCCR), the source of our study population, attempts to obtain data on all incident cases of cancer diagnosed in the state and has 98% case ascertainment.19 We sequentially employed the following inclusion–exclusion criteria: the diagnosis of CRC had to be the first lifetime diagnosis of cancer (excluding nonmelanoma skin cancer), and we excluded patients with multiple recordable primary tumors (sequence code ≥ 02; n = 4576 excluded), patients with unknown tumor stage (n = 1015 excluded), patients not aged between 45 and 85 years (n = 3491 excluded), and patients with race defined as other than African American or White (n = 305 excluded). We excluded additional study participants who had appendiceal tumors (n = 225), missing CT information (n = 43, as described in the nest subsection), and missing diagnosis date (n = 1). We excluded participants who were younger than 45 years of age for 2 reasons: (1) CRCs diagnosed in younger age groups are more likely to have a genetic basis20 and (2) some recommend that African Americans begin screening at 45 years of age.21
Study Variables
The GCCR collects demographic, tumor-related, treatment-related, and follow-up information on all cancer patients diagnosed in the state. The individual-level variables of interest included race, Hispanic ethnicity, gender, age at diagnosis, date of diagnosis, tumor-related information (location, stage, grade), first course of treatment received, last date of follow-up, and vital status at last follow-up. For tumor stage, we used the Surveillance Epidemiology and End Results (SEER) summary (2000–2003) and collaborative (2004–2007) staging classification system.22 The staging classification followed SEER guidelines for colorectal tumors, and we categorized tumors into 1 of 4 categories: localized, regional, regional with lymph node involvement, or distant.22 Late-stage designation corresponded to regional tumors with lymph node involvement and distant disease. We used the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) to identify the anatomical site of the cancer.23 We identified colon cancers by ICD-O-3 codes C18-C18.9 and C26.0 (bowel, not otherwise specified). We identified rectosigmoid cancers by the code C19.9 and rectal cancers by the code C20.9. In the analysis, we categorized rectosigmoid tumors as rectal. We dichotomized treatment as yes or no, with a separate category for missing or unknown.
The GCCR also records the CT corresponding to the residential address for all cancer patients. We merged, by CT, the data obtained from the GCCR with Census 2000 data to obtain a measure of CT-level SES according to the percentage of the population living below the federal poverty level on the basis of household income and family size. Use of CT poverty level as a measure of area-level SES is based on an extensive amount of research by Krieger et al.24–26 as part of the Public Health Disparities Geocoding Project. Census-tract poverty level has been shown to be consistently related to health outcomes and is highly correlated with other CT-level measures of SES.16,27 As was done in previous studies,25,28 we categorized participants according to the percentage of the population living below the federal poverty level in the following manner: high SES (0%–4.9%), upper-middle SES (5.0%–9.9%), lower-middle SES (10.0%–19.9%), and low SES (≥ 20.0%). However, to have enough rural participants classified as living in higher SES census tracts, we combined the categories of high and upper-middle SES.29 Next, by again merging by CT, we obtained geographic residency status by Rural–Urban Commuting Area (RUCA) primary codes from the US Department of Agriculture.30 As was done in previous studies,31 RUCA codes for each CT were used to classify each study case as rural, suburban, or urban in the following manner: rural (RUCA codes 7–10), suburban (RUCA codes 2–6), and urban (RUCA code 1).
Statistical Analysis
We present descriptive statistics as frequencies and percentages for the categorical variables. We used the Kaplan–Meier method to estimate survivor functions and obtain the median death time with 95% confidence interval. We compared characteristics of study participants across rural, suburban, and urban CT-level designation, and we tested differences in proportions using the χ2 statistic. We tested differences in the survivor functions by the log-rank test. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant.
For late-stage disease and receipt of first-course treatment, we obtained odds ratios with 95% confidence intervals. For each outcome, we constructed multilevel hierarchical models, containing both individual- and CT-level variables. Models of first course of treatment received were run separately for colon and rectal cancer. We excluded from the analysis CRC cases with unknown treatment status and those diagnosed at autopsy. For participants with colon cancer, in the model for receipt of chemotherapy, we excluded patients with local disease as chemotherapy is not routinely recommended for these patients. For all models, on the basis of our previous research,14 we included the relevant demographic, tumor-related, and treatment-related variables in the initial model with geographic residency and CT-level SES. If a variable was not associated with the outcome in the multivariable model, we removed it. By this procedure, Hispanic ethnicity was not retained in all models.
For the survival analysis, we calculated survival time from the date of first colon or rectal cancer diagnosis until either the date of death, the last date of follow-up, or the termination date of the study. The GCCR lacks information on cause of death; thus, the outcome was overall survival. To remove patients who died because of complications from surgery, we excluded those with a survival time of less than 2 weeks. We used the Cox proportional hazards model to obtain hazard ratios with 95% confidence intervals for the relative risk of death. The model-building procedure was the same as described for late-stage disease and receipt of treatment. The proportional hazards assumption was evaluated and met for CT-level geography and SES. As an additional step, we evaluated confounding by treatment and SES for the rural effect by comparing the hazard ratio from the partially adjusted model without these variables with the hazard ratio obtained after including treatment followed by SES in the full model.32 When confounding was present, we calculated the proportion of the excess risk explained for the rural effect following adjustment for the confounder. We also evaluated effect measure modification of the hazard ratios for the effect of geography stratified by SES and vice versa.
RESULTS
Our final study population consisted of 20 444 CRC cases. In Table 1, characteristics of the study population are displayed according to CT-level rural, suburban, or urban designation. A higher proportion of rural residents (54.2%) than of suburban and urban residents (49.9%) died during the follow-up period. The median death time was also significantly lower for rural residents (68.8 months) than for suburban and urban residents (81.1 and 82.0 months, respectively). In terms of SES, a much higher percentage of rural residents (44.9%) lived in low-SES census tracts than did suburban and urban residents (17.6% and 16.3%, respectively).
TABLE 1—
Characteristics of the Study Population: Georgia Comprehensive Cancer Registry, 2000–2007
| Characteristics | Rural, No. (%) or Median (95% CI) | Suburban, No. (%) or Median (95% CI) | Urban, No. (%) or Median (95% CI) | P |
| Study population | 3213 (15.7) | 5567 (27.2) | 11 664 (57.1) | |
| Vital status | < .001 | |||
| Alive | 1471 (45.8) | 2787 (50.1) | 5846 (50.1) | |
| Deceased | 1742 (54.2) | 2780 (49.9) | 5818 (49.9) | |
| Death time, mo | 68.8 (63.6, 73.1) | 81.1 (76.4, 86.3) | 82.0 (78.4, 85.9) | |
| Location of cancer | .635 | |||
| Colon | 2293 (71.4) | 4003 (71.9) | 8422 (72.2) | |
| Rectum | 920 (28.6) | 1564 (28.1) | 3242 (27.8) | |
| Gender | < .001 | |||
| Female | 1470 (45.8) | 2550 (45.8) | 5722 (49.1) | |
| Male | 1743 (54.2) | 3017 (54.2) | 5942 (50.9) | |
| Race | < .001 | |||
| African American | 809 (25.2) | 989 (17.8) | 3765 (32.3) | |
| White | 2404 (74.8) | 4578 (82.2) | 7899 (67.7) | |
| Ethnicity | < .001 | |||
| Hispanic | 12 (0.4) | 33 (0.6) | 196 (1.7) | |
| Non-Hispanic | 3201 (99.6) | 5534 (99.4) | 11 468 (98.3) | |
| Census tract SES | < .001 | |||
| High or upper middle | 108 (3.4) | 1716 (30.8) | 7092 (60.8) | |
| Lower middle | 1661 (51.7) | 2874 (51.6) | 2672 (22.9) | |
| Low | 1444 (44.9) | 977 (17.6) | 1900 (16.3) | |
| Age at diagnosis, y | < .001 | |||
| 45–64 | 1398 (43.5) | 2609 (46.9) | 5668 (48.6) | |
| 65–74 | 1014 (31.6) | 1685 (30.2) | 3191 (27.4) | |
| 75–85 | 801 (24.9) | 1273 (22.9) | 2805 (24.0) | |
| Tumor stage | .376 | |||
| Localized | 1271 (39.6) | 2233 (40.1) | 4798 (41.1) | |
| Regional | 471 (14.6) | 821 (14.7) | 1591 (13.6) | |
| Regional with LN involvement | 823 (25.6) | 1406 (25.3) | 2934 (25.2) | |
| Distant | 648 (20.2) | 1107 (19.9) | 2341 (20.1) | |
| Tumor grade | < .001a | |||
| Well differentiated | 333 (10.4) | 727 (13.0) | 1149 (9.9) | |
| Moderately differentiated | 2038 (63.4) | 3378 (60.7) | 7240 (62.1) | |
| Poorly differentiated | 444 (13.8) | 750 (13.5) | 1547 (13.2) | |
| Undifferentiated | 15 (0.5) | 43 (0.8) | 98 (0.8) | |
| Unknown | 383 (11.9) | 669 (12.0) | 1630 (14.0) | |
| Surgery | .061a | |||
| No | 340 (10.6) | 505 (9.1) | 1144 (9.8) | |
| Yes | 2844 (88.5) | 5023 (90.2) | 10 448 (89.6) | |
| Unknown | 29 (0.9) | 39 (0.7) | 72 (0.6) | |
| Chemotherapy | .018a | |||
| No | 1969 (61.3) | 3294 (59.2) | 6952 (59.6) | |
| Yes | 1093 (34.0) | 2085 (37.5) | 4262 (36.5) | |
| Unknown | 151 (4.7) | 188 (3.3) | 450 (3.9) | |
| Radiation | .012a | |||
| No | 2766 (86.1) | 4742 (85.2) | 10 115 (86.7) | |
| Yes | 409 (12.7) | 774 (13.9) | 1432 (12.3) | |
| Unknown | 38 (1.2) | 51 (0.9) | 117 (1.0) | |
| Late-stage diagnosis | .825 | |||
| No | 1742 (54.2) | 3054 (54.9) | 6389 (54.8) | |
| Yes | 1471 (45.8) | 2513 (45.1) | 5275 (45.2) |
Note. CI = confidence interval; LN = lymph node; SES = socioeconomic status. The sample size was n = 20 444.
This comparison of rural, suburban, and urban excludes the unknown category.
Late-Stage Disease and Cancer Treatment
The results for having late-stage disease at diagnosis are shown in Table 2. There was no association between late-stage disease at diagnosis and geography or SES.
TABLE 2—
Adjusted Odds Ratios for Late-Stage Colon or Rectal Cancer Diagnosis: Georgia Comprehensive Cancer Registry, 2000–2007
| CT-Level Variable | AORa (95% CI) |
| Geographic residency status | |
| Urban (Ref) | 1.00 |
| Suburban | 1.02 (0.95, 1.09) |
| Rural | 1.03 (0.94, 1.13) |
| Socioeconomic status | |
| High or upper middle (Ref) | 1.00 |
| Lower middle | 1.05 (0.98, 1.13) |
| Low | 1.02 (0.93, 1.11) |
Note. AOR = odds ratio; CI = confidence interval; CT = census tract. There were 9259 late-stage diagnoses, representing 45.3% of the total sample of 20 444.
Adjusted for the variables listed and age, gender, race, ethnicity, and tumor grade.
The adjusted odds ratios (AORs) for receipt of surgery and chemotherapy for patients with colon cancer are shown in Table 3. Compared with residents living in urban areas, rural colon cancer patients had 16% decreased odds (AOR = 0.84; 95% confidence interval [CI] = 0.72, 0.98) of receiving chemotherapy. For SES, lower-middle and low SES were associated with 24% to 25% decreased odds (lower-middle SES: AOR = 0.76; 95% CI = 0.63, 0.91; low SES: AOR = 0.75; 95% CI = 0.61, 0.93) of receiving surgery. Residents of low-SES CTs also had 17% decreased odds (AOR = 0.83; 95% CI = 0.72, 0.96) of receiving chemotherapy. Lower-middle SES was associated with 11% decreased odds (AOR = 0.89; 95% CI = 0.79, 1.00) of receiving chemotherapy, which was borderline statistically significant.
TABLE 3—
Adjusted Odds Ratios for Receiving Surgery and Chemotherapy for Colon Cancer Cases: Georgia Comprehensive Cancer Registry, 2000–2007
| CT-Level Variables | Surgery, AORa (95% CI) | Chemotherapy, AORa (95% CI) |
| Geographic residency status | ||
| Urban (Ref) | 1.00 | 1.00 |
| Suburban | 1.10 (0.91, 1.32) | 0.99 (0.88, 1.12) |
| Rural | 1.07 (0.85, 1.33) | 0.84 (0.72, 0.98) |
| Socioeconomic status | ||
| High or upper middle (Ref) | 1.00 | 1.00 |
| Lower middle | 0.76 (0.63, 0.91) | 0.89 (0.79, 1.00) |
| Low | 0.75 (0.61, 0.93) | 0.83 (0.72, 0.96) |
Note. AOR = adjusted odds ratio; CI = confidence interval; CT = census tract. For surgery, the total study sample was n = 14 642; 92.1% underwent surgery. For chemotherapy, the sample size was n = 9 160; 49.7% received chemotherapy.
Adjusted for the variables listed and for age, gender, race, disease stage, and tumor grade.
Treatment outcomes for patients with rectal cancer are shown in Table 4. Compared with urban residents, suburban residents had 15% increased odds (AOR = 1.15; 95% CI = 1.00, 1.33) of receiving radiotherapy. For SES, compared with residents of high-SES and upper-middle-SES census tracts, low-SES residents had 31% decreased odds (AOR = 0.69; 95% CI = 0.54, 0.88) of undergoing surgery.
TABLE 4—
Adjusted Odds Ratios for Receiving Surgery, Chemotherapy, and Radiation for Rectal Cancer Cases: Georgia Comprehensive Cancer Registry, 2000–2007
| CT-Level Variables | Surgery, AORa (95% CI) | Chemotherapy, AORa (95% CI) | Radiotherapy, AORa (95% CI) |
| Geographic residency status | |||
| Urban (Ref) | 1.00 | 1.00 | 1.00 |
| Suburban | 0.92 (0.75, 1.14) | 1.13 (0.98, 1.32) | 1.15 (1.00, 1.33) |
| Rural | 0.81 (0.63, 1.04) | 0.88 (0.72, 1.06) | 0.90 (0.75, 1.08) |
| Socioeconomic status | |||
| High or upper middle (Ref) | 1.00 | 1.00 | 1.00 |
| Lower middle | 0.95 (0.77, 1.17) | 0.98 (0.84, 1.14) | 1.08 (0.94, 1.25) |
| Low | 0.69 (0.54, 0.88) | 0.99 (0.83, 1.20) | 1.15 (0.96, 1.37) |
Note. AOR = adjusted odds ratio; CI = confidence interval CT = census tract. For surgery, the total study sample was n = 5 638; 85.4% underwent surgery. For chemotherapy and radiation, the sample size was n = 5 720; 49.2% received chemotherapy and 40.4% received radiation.
Adjusted for the variables listed and for age, gender, race, disease stage, and tumor grade.
Colorectal Cancer Survival
The results of the survival analysis for patients with CRC are depicted in Table 5. We combined colon and rectal cancer patients because of the similarity of the results obtained when we stratified them by tumor type. Because we found differences in treatment for rural residents, we evaluated the proportion of the excess risk of death for rural residents that could be accounted for by treatment differences and SES. In Table 5, we report the following: model 1—the unadjusted hazard ratios for geography and SES; model 2—partially adjusted results without treatment or SES (adjusted for age, gender, race, disease stage, tumor grade, and geography); model 3—adjusted results minus SES (adding treatment to model 2); and model 4—the fully adjusted results for all stages. We also stratified the fully adjusted results according to lymph node negative and lymph node positive or distant tumors. In model 2, compared with urban residents, rural residents had 14% higher risk of death (hazard ratio [HR] = 1.14; 95% CI = 1.07, 1.22) following diagnosis. When we compared this result with model 3, rural residence continued to be associated with a 10% increased risk of death (HR = 1.10; 95% CI = 1.04, 1.18). We obtained the proportion of excess risk explained by treatment by using the following formula33:
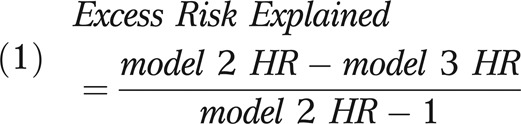 |
TABLE 5—
Hazard Ratios for Death Among Colorectal Cancer Cases, Overall and by Disease Stage: Georgia Comprehensive Cancer Registry, 2000–2007
| CT-Level Variables | Model 1: Unadjusted All Stages, HR (95% CI) | Model 2: Partially Adjusteda All Stages, HR (95% CI) | Model 3: Partially Adjustedb All Stages Plus Treatment, HR (95% CI) | Model 4: Fully Adjustedc All Stages, HR (95% CI) | Adjustedc Localized and Regional Stages, HR (95% CI) | Adjustedc Regional + LN Positive and Distant Stages, HR (95% CI) |
| Geographic residency status | ||||||
| Urban (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Suburban | 1.01 (0.97, 1.06) | 1.04 (0.99, 1.09) | 1.03 (0.98, 1.08) | 0.98 (0.93, 1.03) | 1.00 (0.92, 1.08) | 0.97 (0.91, 1.04) |
| Rural | 1.13 (1.07, 1.20) | 1.14 (1.07, 1.22) | 1.10 (1.04, 1.18) | 1.00 (0.93, 1.07) | 0.95 (0.85, 1.05) | 1.02 (0.94, 1.12) |
| Socioeconomic status | ||||||
| High or upper middle (Ref) | 1.00 | … | … | 1.00 | 1.00 | 1.00 |
| Lower middle | 1.20 (1.15, 1.25) | … | … | 1.16 (1.10, 1.22) | 1.16 (1.07, 1.26) | 1.15 (1.08, 1.23) |
| Low | 1.33 (1.25, 1.40) | … | … | 1.24 (1.16, 1.32) | 1.27 (1.15, 1.40) | 1.21 (1.11, 1.32) |
Note. CI = confidence interval; CT = census tract; HR = hazard ratio; LN = lymph node. For models 1-4, the total study sample was n = 19 992; 49.6% died. For local and regional stages, the sample size was n = 11 037; 34.3% died. For regional + LN positive and distant stages, the sample size was n = 8 955; 68.3% died.
Adjusted for age, gender, race, disease stage, tumor grade, and geography.
Adjusted for age, gender, race, disease stage, tumor grade, geography, and treatment (surgery, chemotherapy, or radiation).
Adjusted for age, gender, race, disease stage, tumor grade, geography, treatment (surgery, chemotherapy, or radiation), and socioeconomic status.
By this formula, treatment accounted for 26.2% of the excess risk associated with rural residence (a slight difference from the reported hazard ratios because of rounding). In the fully adjusted model that included SES (model 4), there was no association between rurality and survival (HR = 1.00; 95% CI = 0.93, 1.07). Thus, SES completely explained the remaining excess risk associated with rural residence. Although SES confounded the increased risk associated with rural residence, geography did not confound the association of SES with survival. In the fully adjusted model, lower-middle SES was associated with a 16% increased risk of death (HR = 1.16; 95% CI = 1.10, 1.22) and low SES was associated with a 24% increased risk of death (HR = 1.24; 95% CI = 1.16, 1.32; test of trend: P < .001). This effect was consistent when we compared the stage-specific results.
For the evaluation of effect measure modification in the fully adjusted model, there was no association between geography and survival at any level of SES (data not shown). For the effect of SES according to geography, the detrimental effect of living in a low-SES CT was somewhat greater for urban residents (urban: HR = 1.28; 95% CI = 1.17, 1.39; rural: HR = 1.16; 95% CI = 0.90,1.49; suburban: HR = 1.15; 95% CI = 1.01,1.30).
DISCUSSION
Using data on cases of CRC diagnosed in Georgia for the years 2000 through 2007, we found that geographic residency status was associated with receipt of treatment and that rurality, specifically, was associated with a partially adjusted increased risk of death that was somewhat explained by treatment differences, with the remaining risk completely explained by CT-level SES. Census tract–level SES was associated with receipt of treatment of both colon and rectal cancer. The effect of CT-level SES on survival demonstrated a gradient effect in that declining SES was associated with an increasing risk of death for CRC patients in Georgia.
Our results concerning late-stage disease are consistent with some,14,34 though not all,35,36 previous investigations. There was no association between geographic residency status or SES and late-stage CRC at diagnosis. This is encouraging, indicating that rural and lower-SES patients are not being diagnosed with more advanced tumors. However, it could also be related to the fact that screening rates for CRC are suboptimal for all population groups.37 In an investigation of CRC screening rates using the Behavior Risk Factor Surveillance System, Cole et al.38 reported that screening rates for rural residents were lower than those for urban residents. Possible explanations for our findings are that (1) the magnitude of the screening disparity by geography in Georgia was not large enough to translate into differences in stage of diagnosis, or that (2) screening rates for high-risk urban and rural residents were low for both groups, thereby demonstrating a null finding. Our findings are consistent with our earlier study involving a sample of Georgia residents with CRC14 and with the results of others34 who did not find an association with late-stage tumor diagnosis according to geography. However, some investigators have reported that rural residency is associated with advanced disease stage at diagnosis.35 In contrast to others,36 we did not find that declining SES was associated with more advanced stage at diagnosis. It is likely that cultural, socioeconomic, and demographic differences, as well as differing study designs and definitions of rural, suburban, and urban, explain the discrepant findings regarding the effect of rurality and SES on late-stage disease at diagnosis.39,40
We found disparities regarding the receipt of treatment. As Hao et al.31 demonstrated for stage III colon and stage II and III rectal cancer patients in Georgia, we found that rural residents in our study experienced decreased odds of receiving chemotherapy, but this was confined to colon cancer patients. This result has also been reported by others,41 although some investigators have not found a difference according to geographic residency status.42 Compared with urban residency, suburban residency was positively associated with receiving radiotherapy for rectal cancer. Several studies have reported that suburban residency is associated with better health outcomes.7,15,43 Colon cancer patients living in lower-middle–SES and low-SES CTs had decreased odds of receiving surgery and chemotherapy, the latter of which has been reported by others.44 Rectal cancer patients living in low-SES CTs also experienced decreased odds of having surgery. These results are consistent with studies that have shown lower SES to be associated with a decreased likelihood of receiving cancer treatment.45,46
Our findings regarding survival after diagnosis of CRC and the association with geographic residency status are revealing. In the bivariate analysis as well as the fully adjusted model without SES, rural residence was associated with a statistically significant increased risk of death. Treatment differences accounted for a portion of this increased risk. After we accounted for CT-level SES, the increased risk of death associated with rural residence disappeared, indicating that the confounding effect of CT-level SES accounted for most of this increase. The fact that SES, and to a smaller degree treatment, explains the increased risk of death associated with rurality does not diminish the public health implications of this finding; rural residence is associated with an increased risk of death for CRC patients, but it is differential treatment and CT-level poverty that is accounting for this rural difference. Further investigation is warranted to fully characterize the determinants of receiving treatment and the components of SES, at both the area and individual level, that are contributing to the rural survival disparity. In addition, identifying the mediators of the SES effect will facilitate the development of customized interventions to reduce CRC-related disparities associated with rural living.15
In terms of survival following diagnosis of CRC and the association with SES, our findings are troubling but not surprising. We found a linear relationship between decreasing CT-level SES and decreasing survival. This graded relationship according to SES was reported by Le et al.,47 who demonstrated that declining SES was associated with decreasing survival for patients with colon and rectal cancer at 1, 5, and 10 years postdiagnosis. Reasons given for the poorer survival among lower-SES individuals and those living in lower-SES areas include diminished access to health care, lower health literacy, higher prevalence of unhealthy behaviors, lack of health insurance, being diagnosed with advanced stage disease, and failure to receive appropriate treatment.3,48
The characteristics of rural citizens, rural living, rural primary care providers (PCPs), and the health care system provide many opportunities for intervention. Rural citizens and those of lower SES are less likely to have an ongoing relationship with a PCP and therefore less likely to obtain wellness and preventive care.9,49,50 In addition, seeking preventive care and even treatment of cancer may not be viewed as important in the context of competing demands such as employment, family obligations, and the burden and costs associated with traveling longer distances to receive care.9,51,52 Implementation of the Patient Protection and Affordable Care Act, which aims to provide insurance to citizens who are currently uninsured or underinsured, should remove a significant financial impediment to rural and lower-SES citizens receiving the care they need.53,54 However, there are often substantial travel burdens, lodging costs, missed work, and family responsibilities that are especially onerous for rural patients of lower means, which could still represent a substantial barrier.55,56 Insurance status may simply be one component in determining whether an individual undergoes screening, receives appropriate and prompt treatment, and adheres to surveillance guidelines following treatment.57
Access to health care is more difficult for rural residents because of fewer primary care physicians per capita and lack of specialists.6,11,58 National-level policies have been established to address access disparities due to geography and SES. One such policy has been to define Health Professional Shortage Areas and increase the number of primary care physicians practicing in these areas. By definition, Health Professional Shortage Areas lack access to health care and disproportionately occur in rural and lower-SES areas of the country.6 After they complete medical training, the National Health Service Corps offers loan repayment and scholarships to primary care physicians providing services to underserved populations.59 An additional example of a proactive government policy to combat the shortage of physicians and incentivize retention in rural areas is the increased Medicare reimbursement to physicians practicing in Health Professional Shortage Areas.6 Beyond government, medical schools and residency programs can play a large role by promoting primary care and emphasizing the necessity of providing care to those with the greatest need.60,61
Targeting practicing rural physicians is another avenue to reduce rural disparities in cancer outcomes. Rural physicians may have a patient population with a higher morbidity burden such that screening for cancer is not seen as a priority in the context of more pressing concerns and time constraints.8,18 In addition, rural physicians may have little knowledge of cancer treatment.62–64 In the realm of secondary (i.e., screening) and tertiary (i.e., prompt, appropriate treatment with proper surveillance) prevention, telehealth educational opportunities are a way to connect rural PCPs to academic medical centers in an effort to make sure they stay up-to-date with regard to screening and treatment guidelines.65 The implementation of patient reminders into the practice of rural PCPs—thereby ensuring that their patients receive screening examinations according to recommended guidelines—could promote the active involvement of PCPs in secondary CRC prevention (and also in primary prevention, by removal of precancerous adenomatous polyps). This method could also be used by cancer specialists to ensure appropriate follow-up care in the years following diagnosis and treatment. Many providers are currently using such reminders66 to prevent patients from “falling through the cracks” of the US health care system. In short, multilevel interventions targeting rural residents, rural PCPs, cancer specialists, and implementation of the Patient Protection and Affordable Care Act are all necessary components to institute a system-level change in the way cancer care is delivered to rural residents.67
Our study has several strengths. The GCCR has high case ascertainment,19 and the data for this study represented the statewide population of CRC patients in Georgia for the years 2000 through 2007. In addition, we obtained a CT-level measure of poverty by merging our data with Census 2000 data and a measure of rurality from US Department of Agriculture RUCA codes. This addressed a limitation of our previous study, in which the area-level variable was the county of residence; the lack of SES variation by geography precluded SES adjustment.14 Furthermore, although our study population consisted of CRC cases from Georgia, the issues faced by rural and lower-SES colorectal cancer patients are likely applicable to many parts of the United States. We encourage investigators to examine rural cancer disparities in other parts of the country to gain a greater understanding of the issues faced by rural and low-SES residents in receiving high-quality cancer care. We evaluated losses to follow-up by considering patients who were alive and whose last date of contact was more than 1 year earlier. There were few losses to follow-up (3.2%–4.4%) by geography, and rural participants were the least likely to be lost to follow-up. Last, we compared agreement in the SES categorization of CTs between Census 2000 and Census 2010. In general, there was good agreement between these 2 time points. Between Census 2000 and Census 2010, on average, there was a 2% increase in the proportion of households in a CT below the federal poverty level. Thus, if any bias occurred, its impact was likely minimal.
The results of this study should be considered within the context of acknowledged limitations. We lacked information on individual-level indicators of SES, as these data are not routinely collected by cancer registries.27 When the impact of area-level SES in the absence of individual-level SES indicators is measured, the area-level SES variable is, to some degree, accounting for both area and individual effects.68 The area effect is likely moderated by individual SES, which is further mediated by factors such as having health insurance, health literacy, and the patient–provider relationship.69,70 Another limitation concerns information on the first course of treatment. As has been documented for cancer registry data,71 it is likely that treatment information was missing or incomplete for some participants. Rural patients were slightly more likely to have missing information on receipt of chemotherapy (4.7%, vs 3.4% and 3.9% for suburban and urban patients, respectively). If rural patients were also more likely to have been misclassified as not having received treatment, the effect measures in the analysis for the odds of treatment would be biased away from the null. In addition, we simply dichotomized (yes or no) the receipt of surgery, chemotherapy, or radiotherapy.
The purpose of our study was to evaluate geography and SES as determinants of CRC outcomes from diagnosis to survival. We acknowledge that our dichotomous categorization of treatment was not as refined as it could have been and resulted in some loss of information. We invite other investigators to explore treatment differences according to SES and geography in greater detail. An additional limitation concerns the lack of information on cause of death for the patients that died during follow-up, which precluded an assessment of CRC-related death. However, in our previous study,14 we were able to compare the hazard ratios associated with rural residence for overall survival, cancer-specific survival, and other causes of death. These results were remarkably similar (HR range = 1.17–1.18) and indicate that the factors determining the increased risk of CRC-related death for rural residents, whether individual- or macro-level factors, are also operative for all causes of death. Finally, for some of our stratified analyses, reductions in sample size for the exposures of interest resulted in wider confidence intervals.
In the current study, we sought to address the issue of rurality and CT-level SES as determinants of CRC outcomes. We found that being rural and living in lower SES CTs independently contributed to decreased odds for receipt of treatment. Unlike the rural survival disparity, the disparity in treatment (chemotherapy in colon cancer) persisted after adjustment for SES. In terms of survival, there was a gradient effect of decreasing SES associated with poorer survival that persisted following adjustment for geography and individual-level characteristics. The socioeconomic disparities in CRC survival indicate that interventions and policies are needed to address the consistently worse outcomes borne by those living in lower-SES areas. The finding that rural citizens experienced an unadjusted increased risk of death following diagnosis of CRC, which was partially explained by treatment and completely explained by CT-level SES, provides an opportunity for further study. We are currently investigating what components of SES (individual, collective, or contextual effects) that are associated with rurality are responsible for treatment disparities and the increased risk of death following a diagnosis of CRC. Once these various actors are identified, focused interventions may be designed to targeted rural populations who bear a disproportionate burden of disease. Multilevel interventions targeting rural residents, rural PCPs, cancer specialists, and the Patient Protection and Affordable Care Act are required to achieve a system-level change if the goal of eliminating health disparities according to geography and SES is to become a reality.67
Acknowledgments
This work was funded by a faculty research award from Georgia Southern University.
Human Participant Protection
The research study was approved by the institutional review boards of Georgia Southern University and the Georgia Department of Public Health.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.US Census Bureau, Geography Division. 2010 Census urban and rural classification and urban area criteria. Available at: http://www.census.gov/geo/reference/ua/urban-rural-2010.html. Accessed November 5, 2012.
- 3.Clegg LX, Reichman ME, Miller BA et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. J Rural Health. 1992;8(3):212–220. doi: 10.1111/j.1748-0361.1992.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural–urban, and racial inequalities in US cancer mortality: part i–all cancers and lung cancer and part ii–colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2012 doi: 10.1155/2011/107497. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Research Council. Quality Through Collaboration: The Future of Rural Health Care. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 7.Eberhardt MS, Ingram DD, Makuc DM. Urban and Rural Health Chartbook. Health, United States, 2001. Hyattsville, MD: National Center for Health Statistics; 2001. [Google Scholar]
- 8.Harris R, Leininger L. Preventive care in rural primary care practice. Cancer. 1993;72(3 suppl):1113–1118. doi: 10.1002/1097-0142(19930801)72:3+<1113::aid-cncr2820721328>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Hunsaker M, Kantayya VS. Building a sustainable rural health system in the era of health reform. Dis Mon. 2010;56(12):698–705. doi: 10.1016/j.disamonth.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Given BA, Given CW, Harlan AN. Strategies to meet the needs of the rural poor. Semin Oncol Nurs. 1994;10(2):114–122. doi: 10.1016/s0749-2081(05)80065-8. [DOI] [PubMed] [Google Scholar]
- 11.Lyckholm LJ, Hackney MH, Smith TJ. Ethics of rural health care. Crit Rev Oncol Hematol. 2001;40(2):131–138. doi: 10.1016/s1040-8428(01)00139-1. [DOI] [PubMed] [Google Scholar]
- 12.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 13.Baade PD, Turrell G, Aitken JF. Geographic remoteness, area-level socio-economic disadvantage and advanced breast cancer: a cross-sectional, multilevel study. J Epidemiol Community Health. 2011;65(11):1037–1043. doi: 10.1136/jech.2010.114777. [DOI] [PubMed] [Google Scholar]
- 14.Hines RB, Markossian TW. Differences in late-stage diagnosis, treatment, and colorectal cancer-related death between rural and urban African Americans and whites in Georgia. J Rural Health. 2012;28(3):296–305. doi: 10.1111/j.1748-0361.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 15.Henry KA, Niu X, Boscoe FP. Geographic disparities in colorectal cancer survival. Int J Health Geogr. 2009;8:48. doi: 10.1186/1476-072X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N. Defining and investigating social disparities in cancer: critical issues. Cancer Causes Control. 2005;16(1):5–14. doi: 10.1007/s10552-004-1251-5. [DOI] [PubMed] [Google Scholar]
- 17.Cummins S, Curtis S, Diez-Roux AV, Macintyre S. Understanding and representing “place” in health research: a relational approach. Soc Sci Med. 2007;65(9):1825–1838. doi: 10.1016/j.socscimed.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan LW, Richmond J. Foreword: building a healthy South—the challenge is now. Ethn Dis. 2002;12(1):S2-2–4. [PubMed] [Google Scholar]
- 19.Clarkson LS. An Evaluation of the Georgia Comprehensive Cancer Registry: Improving an Established System. Atlanta: Georgia Dept of Human Resources, Division of Public Health; 2007. [Google Scholar]
- 20.Winawer S, Fletcher R, Rex D et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Johnson DA, Anderson JC et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 22.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual—2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH publication 01–4969. [Google Scholar]
- 23.International Classification of Diseases for Oncology, Third Edition. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 24.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 27.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in US incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 28.Niccolai LM, Julian PJ, Bilinski A et al. Geographic poverty and racial/ethnic disparities in cervical cancer precursor rates in Connecticut, 2008–2009. Am J Public Health. 2013;103(1):156–163. doi: 10.2105/AJPH.2011.300447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausauer AK, Keegan TH, Chang ET, Glaser SL, Howe H, Clarke CA. Recent trends in breast cancer incidence in US white women by county-level urban/rural and poverty status. BMC Med. 2009;7:31. doi: 10.1186/1741-7015-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Agriculture, Economic Research Service. Rural–urban commuting area codes. Available at: http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#.UnqX3fkqh8E. Accessed October 8, 2012.
- 31.Hao Y, Landrine H, Jemal A et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health. 2011;65(3):211–217. doi: 10.1136/jech.2009.096008. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 33.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 3rd ed. Burlington, MA: Jones & Bartlett Learning; 2014. [Google Scholar]
- 34.Campbell RJ, Ferrante JM, Gonzalez EC, Roetzheim RG, Pal N, Herold A. Predictors of advanced stage colorectal cancer diagnosis: results of a population-based study. Cancer Detect Prev. 2001;25(5):430–438. [PubMed] [Google Scholar]
- 35.Elliott TE, Elliott BA, Renier CM, Haller IV. Rural–urban differences in cancer care: results from the Lake Superior Rural Cancer Care Project. Minn Med. 2004;87(9):44–50. [PubMed] [Google Scholar]
- 36.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988–2000. Cancer. 2006;107(5 suppl):1189–1195. doi: 10.1002/cncr.22016. [DOI] [PubMed] [Google Scholar]
- 37.Semrad TJ, Tancredi DJ, Baldwin LM, Green P, Fenton JJ. Geographic variation of racial/ethnic disparities in colorectal cancer testing among Medicare enrollees. Cancer. 2011;117(8):1755–1763. doi: 10.1002/cncr.25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole AM, Jackson JE, Doescher M. Urban–rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the Centers for Disease Control’s Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1(3):350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamm L, Hutchison L. Rural health priorities in America: where you stand depends on where you sit. J Rural Health. 2003;19(3):209–213. doi: 10.1111/j.1748-0361.2003.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 40.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sankaranarayanan J, Watanabe-Galloway S, Sun J, Qiu F, Boilesen E, Thorson AG. Rurality and other determinants of early colorectal cancer diagnosis in Nebraska: a 6-year cancer registry study, 1998–2003. J Rural Health. 2009;25(4):358–365. doi: 10.1111/j.1748-0361.2009.00244.x. [DOI] [PubMed] [Google Scholar]
- 42.Ayanian JZ, Zaslavsky AM, Fuchs CS et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21(7):1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 43.Clifford WB, Brannon YS. Rural–urban differentials in mortality. Rural Sociol. 1985;50(2):210–224. [Google Scholar]
- 44.Morris M, Platell C, Fritschi L, Iacopetta B. Failure to complete adjuvant chemotherapy is associated with adverse survival in stage III colon cancer patients. Br J Cancer. 2007;96(5):701–707. doi: 10.1038/sj.bjc.6603627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchholz TA, Theriault RL, Niland JC et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network Centers. J Clin Oncol. 2006;24(3):361–369. doi: 10.1200/JCO.2005.02.3127. [DOI] [PubMed] [Google Scholar]
- 46.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 47.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 48.Coughlin SS, Richards TB, Thompson T et al. Rural/nonrural differences in colorectal cancer incidence in the United States, 1998–2001. Cancer. 2006;107(5 suppl):1181–1188. doi: 10.1002/cncr.22015. [DOI] [PubMed] [Google Scholar]
- 49.Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37(5):475–484. doi: 10.1016/s0091-7435(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 50.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97(6):1528–1540. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 51.Mathews M, West R, Buehler S. How important are out-of-pocket costs to rural patients’ cancer care decisions? Can J Rural Med. 2009;14(2):54–60. [PubMed] [Google Scholar]
- 52.Beyer KM, Comstock S, Seagren R, Rushton G. Explaining place-based colorectal cancer health disparities: evidence from a rural context. Soc Sci Med. 2011;72(3):373–382. doi: 10.1016/j.socscimed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Fitzpatrick AL, Powe NR, Cooper LS, Ives DG, Robbins JA. Barriers to health care access among the elderly and who perceives them. Am J Public Health. 2004;94(10):1788–1794. doi: 10.2105/ajph.94.10.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bellamy GR, Bolin JN, Gamm LD. Rural Healthy People 2010, 2020, and beyond: the need goes on. Fam Community Health. 2011;34(2):182–188. doi: 10.1097/FCH.0b013e31820dea1c. [DOI] [PubMed] [Google Scholar]
- 55.Geller BM, Mace J, Vacek P, Johnson A, Lamer C, Cranmer D. Are cancer survivors willing to participate in research? J Community Health. 2011;36(5):772–778. doi: 10.1007/s10900-011-9374-6. [DOI] [PubMed] [Google Scholar]
- 56.Bettencourt BA, Schlegel RJ, Talley AE, Molix LA. The breast cancer experience of rural women: a literature review. Psychooncology. 2007;16(10):875–887. doi: 10.1002/pon.1235. [DOI] [PubMed] [Google Scholar]
- 57.Ko CW, Kreuter W, Baldwin LM. Persistent demographic differences in colorectal cancer screening utilization despite Medicare reimbursement. BMC Gastroenterol. 2005;5:10. doi: 10.1186/1471-230X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coughlin SS, Thompson TD, Seeff L, Richards T, Stallings F. Breast, cervical, and colorectal carcinoma screening in a demographically defined region of the southern US. Cancer. 2002;95(10):2211–2222. doi: 10.1002/cncr.10933. [DOI] [PubMed] [Google Scholar]
- 59.Hart LG, Salsberg E, Phillips DM, Lishner DM. Rural health care providers in the United States. J Rural Health. 2002;18(suppl):211–232. doi: 10.1111/j.1748-0361.2002.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 60.Bennett K, Phillips J, Teevan B. Closing the gap: finding and encouraging physicians who will care for the underserved. Virtual Mentor. 2009;11(5):390–398. doi: 10.1001/virtualmentor.2009.11.5.pfor1-0905. [DOI] [PubMed] [Google Scholar]
- 61.Ross R. Fifteen-year outcomes of a rural residency: aligning policy with national needs. Fam Med. 2013;45(2):122–127. [PubMed] [Google Scholar]
- 62.Hatzell TA, Ricketts TC, Tropman SE, Paskett ED, Cooper MR. Rural physicians’ understanding of the state-of-the-art in breast, colon and rectum cancer treatment. Cancer Causes Control. 1999;10(4):261–267. doi: 10.1023/a:1008996227202. [DOI] [PubMed] [Google Scholar]
- 63.Howe HL, Katterhagen JG, Yates J, Lehnherr M. Urban–rural differences in the management of breast cancer. Cancer Causes Control. 1992;3(6):533–539. doi: 10.1007/BF00052750. [DOI] [PubMed] [Google Scholar]
- 64.Dragun AE, Huang B, Tucker TC, Spanos WJ. Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer: impact on overall survival. Cancer. 2011;117(12):2590–2598. doi: 10.1002/cncr.25821. [DOI] [PubMed] [Google Scholar]
- 65.Loberiza FR, Jr, Cannon AJ, Weisenburger DD et al. Survival disparities in patients with lymphoma according to place of residence and treatment provider: a population-based study. J Clin Oncol. 2009;27(32):5376–5382. doi: 10.1200/JCO.2009.22.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer GS, Hess R, Landeen BM et al. Electronic reminders to patients within an interactive patient health record. Telemed J E Health. 2013;19(6):497–500. doi: 10.1089/tmj.2012.0116. [DOI] [PubMed] [Google Scholar]
- 67.Warnecke RB, Oh A, Breen N et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steenland K, Henley J, Calle E, Thun M. Individual- and area-level socioeconomic status variables as predictors of mortality in a cohort of 179,383 persons. Am J Epidemiol. 2004;159(11):1047–1056. doi: 10.1093/aje/kwh129. [DOI] [PubMed] [Google Scholar]
- 69.Yu XQ, O’Connell DL, Gibberd RW, Armstrong BK. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996–2001. Cancer Causes Control. 2008;19(10):1383–1390. doi: 10.1007/s10552-008-9210-1. [DOI] [PubMed] [Google Scholar]
- 70.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer. 1999;86(11):2378–2390. [PubMed] [Google Scholar]
- 71.Du XL, Key CR, Dickie L et al. Information on chemotherapy and hormone therapy from tumor registry had moderate agreement with chart reviews. J Clin Epidemiol. 2006;59(1):53–60. doi: 10.1016/j.jclinepi.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


