Abstract
Understanding the mutual relationship between the liver and the heart is important for both hepatologists and cardiologists. Hepato-cardiac diseases can be classified into heart diseases affecting the liver, liver diseases affecting the heart, and conditions affecting the heart and the liver at the same time. Differential diagnoses of liver injury are extremely important in a cardiologist’s clinical practice calling for collaboration between cardiologists and hepatologists due to the many other diseases that can affect the liver and mimic haemodynamic injury. Acute and chronic heart failure may lead to acute ischemic hepatitis or chronic congestive hepatopathy. Treatment in these cases should be directed to the primary heart disease. In patients with advanced liver disease, cirrhotic cardiomyopathy may develop including hemodynamic changes, diastolic and systolic dysfunctions, reduced cardiac performance and electrophysiological abnormalities. Cardiac evaluation is important for patients with liver diseases especially before and after liver transplantation. Liver transplantation may lead to the improvement of all cardiac changes and the reversal of cirrhotic cardiomyopathy. There are systemic diseases that may affect both the liver and the heart concomitantly including congenital, metabolic and inflammatory diseases as well as alcoholism. This review highlights these hepatocardiac diseases
Keywords: Cardiac cirrhosis, Ischemic hepatitis, Fatty liver, Liver cirrhosis, Heart failure
Core tip: Acute and chronic heart failure may lead to acute ischemic hepatitis or chronic congestive hepatopathy. Treatment in these cases should be directed to the primary heart disease. In patients with advanced liver disease, cirrhotic cardiomyopathy may develop including hemodynamic changes, diastolic and systolic dysfunctions, reduced cardiac performance and electrophysiological abnormalities. Cardiac evaluation is important for patients with liver diseases especially before and after liver transplantation. Liver transplantation may lead to improvement of all cardiac changes and reversal of cirrhotic cardiomyopathy. There are systemic diseases that may affect both liver and heart concomitantly including congenital, metabolic, inflammatory diseases and alcoholism.
INTRODUCTION
The heart and liver are organs that are closely related both in health and disease. According to traditional medicine, each body has its organ own specific temperament composed of four qualities (elements): “warmth”, “coldness”, “wetness” and “dryness”. “Wetness” and “dry-ness” are considered on a spectrum of “tissue moistures” and “warmth” and “coldness” may be regarded as the basic metabolism of the organ. In his famous book ,“Canon” (The Law), Avicenna pointed to some of the interactive effects occurring in the heart and the liver. Some of the most important include: (1) dominance of the “heart warmth” over “liver coldness” and (2) the dominance of “liver dryness” over “heart wetness”. The impact and position of “heart temperament” as well as its effect on “liver intemperaments” may be definitive in diagnosis and assessment of the general prognosis of liver disease and in the treatment process[1,2].
Chronic liver diseases may affect cardiac functions in the absence of other heart disease. These effects are called cirrhotic cardiomyopathy and may aggravate the course during orthotropic liver transplantation (OLT). Most of these effects are reversed after OLT[3,4]. In case of ischemic hepatitis, patients with severe heart failure usually remain asymptomatic, while for patients with congestive hepatopathy, signs of right-sided heart failure could mask hepatic injury. However, changes in hepatic function, that are proven by laboratory tests are significant in predicting the survival of patients with severe heart failure. Therefore, the evaluation of cardiac and hepatic function is very important in patients with severe heart failure and hepatic injury. Their treatment options should be revised in order to ensure stable hemodynamics, as well as optimal liver function, and so in this way their survival and prognosis could be improved[5]. This review highlight the liver diseases affecting the heart, heart diseases affecting the liver and some systemic diseases affecting both heart and liver.
LIVER DISEASES AFFECTING THE HEART
Chronic hepatitis C virus
In hepatitis C virus (HCV) heart disease, most patients develop chronic inflammation of the myocardium and, later, dilated cardiomyopathy attributable to necrosis and loss of myocytes. However, because myocytes do not replicate, the proliferative stimuli induced by HCV infection may promote myocyte hypertrophy and hypertrophic cardiomyopathy[6]. A role of direct effect of HCV core proteins was suggested in the pathogenesis of cardiomyopathy[7]. Cardiac damage is a rare manifestation of HCV-related mixed cryoglobulinemia vasculitis. Despite favourable early outcomes, patients with cardiac damage had poorer survival than those without[8]. Chronic hepatitis C viral infection is independently associated with presence of metabolic conditions (insulin resistance, type 2 diabetes mellitus and hypertension) and congestive heart failure[9].
The connection between hyperlipidemia and atherosclerosis is not linear in people with hepatitis C. In a population-based study, although chronic HCV infection was associated with severe insulin resistance, the patients only had mild atherosclerosis, suggesting a unique characteristic of HCV-related metabolic abnormality. Chronic HCV-associated steatosis was suggested as a leading cause of coronary artery diseases through the modulation of atherogenic factors, such as inflammation and dysmetabolic milieu. Interestingly, interferon-based therapies in patients with chronic HCV were found to reduce the long-term risk of stroke. Thus, atherosclerosis in patients with hepatitis C is likely due to an inflammatory process rather than to a lipid related source[10-12]. Thus, even patients having healthy cholesterol and triglyceride levels in the presence of chronic hepatitis C infections should not engage in activities that could further increase the disease risk of their cardiovascular vessels.
Liver cirrhosis
Patients with liver cirrhosis (LC) frequently experience autonomic cardiovascular dysfunction, such as increased activity of the sympathetic nervous system and reduced vagal cardiac function, which has important implications for liver dysfunction and poor survival[13-15]. Baroreflex has been shown to be an important determinant of electrical stability in the heart and can predict increased mortality and end-organ damage[16-19]. Patients with liver cirrhosis have an enhanced activity of the sympathetic nervous system and hyperdynamic circulation showing increased cardiac output and reduced systemic vascular resistance. These changes may induce myocardial remodelling and LV hypertrophy (LVH), resulting in systolic and diastolic functional abnormalities and cardiomyopathy[20-22]. Cirrhotic cardiomyopathy was defined by a working group as a cardiac dysfunction in patients with cirrhosis characterized by impaired contractile responsiveness to stress and or altered diastolic relaxation with electrophysiological abnormalities in the absence of known cardiac disease[23]. The criteria for the diagnosis of cirrhotic cardiomyopathy are shown in Table 1[24].
Table 1.
Proposal for diagnostic and supportive criteria for cirrhotic cardiomyopathy agreed upon at a working party held at the 2005 World Congress of Gastroenterology
| A working definition of cirrhotic cardiomyopathy |
| A cardiac dysfunction in patients with cirrhosis characterised by impaired contractile responsiveness to stress and/or altered diastolic relaxation with electrophysiological abnormalities in the absence of other known cardiac disease |
| Diagnostic criteria |
| Systolic dysfunction |
| Blunted increase in cardiac output with exercise, volume challenge or pharmacological stimuli |
| Resting EF < 55% |
| Diastolic dysfunction |
| E/A ratio < 1.0 (age-corrected) |
| Prolonged deceleration time (> 200 ms) |
| Prolonged isovolumetric relaxation time (> 80 ms) |
| Supportive criteria |
| Electrophysiological abnormalities |
| Abnormal chronotropic response |
| Electromechanical uncoupling/dyssynchrony |
| Prolonged QTc interval |
| Enlarged left atrium |
| Increased myocardial mass |
| Increased BNP and pro-BNP |
| Increased troponin I |
BNP: Brain natriuretic peptide; E/A: Early diastolic/atrial filling ratio; EF: Left-ventricular ejection fraction.
Systolic dysfunction is related to the inability of the heart to meet its demands with respect to the generation of an adequate arterial blood pressure and cardiac output. This dysfunction can be unveiled by physical exercise that increases left ventricular pressure, volume, and left ventricular ejection fraction and heart rate in some cirrhotic patients. Similarly, the administration of vasoconstrictors, such as angiotensin II and terlipressin, increases the SVR and thereby the left ventricular afterload unmasking a latent left ventricular dysfunction in cirrhosis. In contrast, vasodilators, such as angiotensin-converting enzyme inhibitors and other afterload-reducing agents, should be used with caution due to the risk of further aggravation of the vasodilatory state[24]. Systolic dysfunction may have an impact on the development of complications, such as sodium and water-retention and ascites formation, as well as development and prognosis of renal dysfunction[25,26].
Diastolic dysfunction in cirrhosis is due to an increased stiffness of the myocardial wall owing to myocardial hypertrophy, fibrosis, and subendothelial edema. The prevalence of diastolic dysfunction has been reported to range from 45% to 56%. Diastolic dysfunction is most prominent in patients with severe decompensation, in whom, the combination of myocardial hypertrophy, contractile dysfunction, changes in heart volumes, and diastolic dysfunction may represent an essential element in cirrhotic cardiomyopathy[26-28]. The diastolic dysfunction may adversely affect the prognosis of patients with cirrhosis, by favouring the occurrence of complications and impairing the outcomes of manoeuvres that lead to rapid increases in preload, such as transjugular intrahepatic porto-systemic shunt (TIPS) insertion[24].
Patients with advanced cirrhosis usually exhibit tachycardia. The inability to increase the heart rate further contributes to an impaired ability to keep the cardiac output at a level adequate to meeting the needs of systemic circulation. At this point, the effective volemia suddenly worsens, similar to the events of post-paracentesis circulatory dysfunction and hepatorenal syndrome[29-31]. The prolongation of the electrocardiographic QT interval is common in cirrhosis, with a prevalence that exceeds 60% in patients with an advanced disease. In this case, drugs affecting QT should be avoided or used with caution and under close ECG monitoring[32]. Systemic and cardiac changes in patients with liver cirrhosis are shown in Figure 1.
Figure 1.
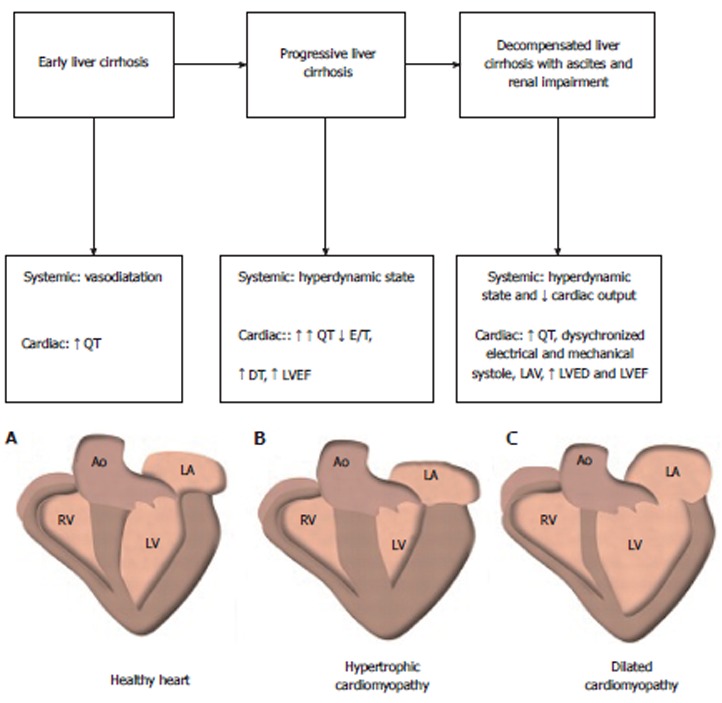
Proposal of changes in cardiac output during the course of the liver disease. DT: Deceleration time; LAV: Left atrial volume; LVEDV: Left end-diastolic volume; LVEF: Left ventricular ejection time.
Almost all cardiovascular abnormalities reverse a few months after liver transplantation[4,24,33].
Nonalcoholic fatty liver disease
It has been shown that the leading cause of death in patients with nonalcoholic fatty liver disease (NAFLD) is coronary events. In patients with diabetes mellitus, NAFLD is associated with cardiovascular disease (CVD) independent of the classical risk factors, glycaemic control, medications, and metabolic syndrome features. When diabetic patients with and without NAFLD were compared, those with NAFLD had a higher prevalence of coronary vascular disease, hypertension, central obesity, poor glycaemic control, and dyslipidaemia and greater carotid intimal thickness. Furthermore, with the development of steatohepatitis, the degree and severity of CVD became directly proportional to the severity of inflammation on liver biopsy. Cardiovascular mortality is also increased at least two-fold in non-alcoholic steatohepatitis (NASH). The presence of liver fat is associated with lower adiponectin levels and increased levels of fibrinogen, C-reactive protein (CRP), and plasminogen activator inhibitor 1 (PAI-1), which are markers of inflammation and risk factors of coronary vascular disease independent of BMI and intra-abdominal obesity. Patients with NAFLD also have significantly higher mean values of intima-media thickness and prevalence of plaques resulting in an increased risk of atherosclerosis in subjects with metabolic syndrome. It has also been shown that NASH predicts plasma inflammatory biomarkers independent of visceral adiposity and other potential confounders. These findings suggest that NASH is not simply a marker of CVD but may also be involved in its pathogenesis. Steatosis has been found to be the strongest independent risk predictor of vascular damage, followed by age and blood pressure. Patients with NAFLD and systolic BP ≥ 130 mmHg are 4.7 times more likely to have a positive treadmill test[34-36].
In a recent study, asymptomatic obese children with NAFLD exhibited features of early LV diastolic and systolic dysfunction. These abnormalities were more severe in those with NASH[37].
Primary biliary cirrhosis
Circulating cholesterol levels are elevated in most with primary biliary cirrhosis. Hypercholesterolemia in patients with primary biliary cirrhosis should be considered a cardiovascular risk factor only when other factors are present. Ursodeoxycholic acid, the standard treatment for primary biliary cirrhosis, improves cholestasis, thereby lowering the circulating levels of cholesterol. Thus, hypercholesterolemia in the absence of other cardiovascular risk factors does not require specific therapeutic interventions in patients with primary biliary cirrhosis.
Epidemiological studies have shown significantly increased all-cause mortality rates in comprehensive PBC patient groups, with a significant component of this increased mortality coming from non-liver-related causes[38,39]. These studies were not designed to address the cause of this increase in non-liver-related mortality. There is, however, convincing evidence from the same populations to suggest that malignant disease makes little or no contribution to this excess non liver mortality[38,40]. Given the importance of cardiovascular mortality in the general population, the possibility must be considered that cardiac mechanisms contribute to the excess non liver mortality rates seen in these populations. Autonomic dysfunction has been seen in PBC and was associated with an increased cardiac mortality risk in non-liver chronic disease states[41,42]. Furthermore, a significant peripheral muscle bioenergetics abnormality has also been reported in PBC[43]. Raising the possibility that similar bioenergetic abnormalities may also be present in the cardiac muscle. The effects of autonomic dysfunction may alter the perfusion patterns in tissues, potentially reducing muscle perfusion and contributing to peripheral mechanisms of fatigue. A generic tendency towards altered myocardial function was shown in PBC and did not typically appear to be symptomatic in terms of “classical” myocardial dysfunction symptoms[44].
Primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease of affecting the large bile ducts and is characterized by periductal fibrosis and stricture formation. Arteriosclerosis involves the accumulation of altered lipids and lipoproteins in large arteries; this drives inflammation and fibrosis and ultimately leads to the narrowing of the arteries and hypoperfusion of dependent organs and tissues. Knowledge of the causative factors is crucial to the understanding of disease mechanisms and the development of specific treatment. Based on the pathogenic similarities and common molecular, cellular, and morphological features that provide the conceptual framework for a deeper understanding of their pathogenesis between PSC and arteriosclerosis, it has been hypothesized that PSC represents “arteriosclerosis of the bile duct” initiated by toxic biliary lipids[45]. This hypothesis should stimulate translational research to facilitate the search for novel treatment strategies for both diseases.
Hepatocellular carcinoma
Cardiac complications of hepatocellular carcinoma hepatocellular carcinoma are rare. Cases of right atrial invasion of HCC had been reported[46], which led in some cases to right ventricular outflow obstruction and Budd Chiari syndrome[47,48]. Hepatocellular carcinoma patients with cardiac metastases are usually found in advanced stages. These patients have limited survival from the diagnosis of cardiac metastases. The most common causes of death are related to HCC itself or to the underlying liver disease. Only a few patients will die due to cardiac metastases[49]. The palliative treatments for tumor thrombi may include transcatheter chemotherapy, transarterial chemoembolization and radiation therapy with a partial improvement of patient symptoms[50].
Budd-chiari syndrome
Primary Budd-chiari syndrome (BCS) is a rare clinical entity characterized by hepatic venous outflow obstruction at various levels from the small hepatic veins to the inferior vena cava. There are three main types of BCS: Type I, occlusion of the IVC; type II, occlusion of the hepatic veins; and type III, occlusion of the IVC and the hepatic veins. The incidence of HCC combined with BCS varies among the types of BCS[50,51]. Type I BCS is more prone to inducing HCC and the incidence ranges between 10.7% and 43.5%. The mechanisms of HCC induction unknown. The therapeutic treatments of BCS combined with HCC includes TACE, surgery and more recently angioplasty followed by percutaneous microwave ablation[52-55].
Portal hypertension
Three important complications are associated with portal hypertension hepatopulmonary syndrome, portopulmonary hypertension, and hepatic hydrothorax.
The hepatopulmonary syndrome: This entity is defined by an oxygenation defect caused by the development of intrapulmonary vascular dilation in patients with either advanced liver disease and/or portal hypertension[56]. Angiogenesis was shown to be induced by an increased level of nitric oxide and vascular endothelial growth factor in patients with advanced liver disease or portal hypertension[57,58]. Patients with the hepatopulmonary syndrome (HPS) may present with the insidious onset of dyspnea or remain completely asymptomatic during the early stages. Dyspnea upon standing (platypnea) and hypoxemia exacerbated in the upright position (orthodeoxia) are present in almost 25% of HPS patients[59]. Patients with severe HPS may display digital clubbing and cyanosis.
Chest radiographs may be normal or show bibasilar nodular or reticulonodular opacities, reflecting diffuse vascular pulmonary dilation[60,61]. Pulmonary function tests typically demonstrate a reduced diffusion capacity for carbon monoxide[61]. There is no established medical therapy currently available for HPS. In patients with PaO2 < 60 mmHg at rest or with exertion, the administration of supplemental oxygen is appropriate, because chronic hypoxemia itself may contribute to the mortality in HPS[62,63]. The administration of garlic resulted in improvements in the PaO2, in two uncontrolled trials and a small randomized study[64].
Portopulmonary hypertension: Portopulmonary hypertension (POPH) is characterized by pulmonary arterial hypertension (PAH) that occurs in the setting of portal hypertension, with or without advanced liver disease[65]. The severity of POPH does not correlate with the degree of liver dysfunction or the severity of portal hypertension[66,67]. The pathophysiology of POPH is not fully understood. The histopathology of POPH is similar to that of idiopathic PAH, and is triggered by vascular injury as reflected by the development of plexiform arteriopathy, concentric intimal fibrosis, and proliferation and muscularization of the pulmonary arterioles[68]. Dyspnea on exertion is the most common initial symptom of POPH and fatigue, orthopnea, chest pain, peripheral edema, syncope, and dyspnea at rest may develop as the disease progresses[69,70]. Medical treatment includes the following: prostacyclin analogs (prostanoids), Endothelin receptor antagonist and Phosphodiesterase-5 inhibitors[71-73]. A single short-term study in patients with moderate to severe POPH, found that the use of β-blockers was associated with worsening exercise capacity[72].
Hepatic hydrothorax: This entity is characterized by a transudative pleural effusion in the absence of underlying cardiac or pulmonary disease. Its prevalence has been estimated to be 5%-10% in cirrhotics, based on retrospective observational data[73]. The most important mechanism leading to the passage of ascitic fluid from the peritoneal into the pleural cavity is the presence of diaphragmatic defects. These defects were corroborated by showing passage of 99mTc-human albumin from the abdominal into the pleural cavity, even in the absence of underlying ascites[74]. Symptoms include cough, dyspnea, chest discomfort, hypoxia, and in the most severe cases respiratory failure with or without ascites[74,75]. Spontaneous bacterial pleuritis (SBPL) results when hepatic hydrothorax (HH) becomes infected in the absence of pneumonia. Symptoms in SBPL vary from fever and pleuritic chest pain to subtle worsening of encephalopathy or renal function, necessitating a high index of suspicion. A PMN > 500 cells/mm3 is diagnostic for SBPL in a pleural effusion, although SBPL with PMN between 250-500 cells/mm3 is documented by positive pleural fluid culture[76]. Chest tube placement is contraindicated in SBPL, in the absence of empyema, due to the risk of protein loss, prolonged drainage, secondary infection and hepatorenal syndrome[77]. Treatment of HH includes the restriction of sodium intake with the administration of diuretics. This approach is effective in controlling HH, although fluid mobilization from the pleural cavity may be slower than from the peritoneal cavity and approximately 20% of patients develop refractory HH[77]. Percutaneous drainage, and chest tube placement can be used in some cases[77,78]. The standard of care treatment for refractory HH is TIPS placement with response rates of 70% to 80%[79,80]. Video assisted thoracoscopy (VATS) with pleurodesis is a potential treatment alternative for patients with refractory HH, who are not eligible for or who have failed TIPS[81-83].
Liver transplantation
Patients with cirrhosis requiring liver transplantation (LT) usually demonstrate increased cardiac output. Low systemic vascular resistance and bradycardia are also commonly seen in cirrhosis and can be aggravated by beta-blocker use. These physiologic changes increase the risk of cardiovascularn complications, in addition to altered hemodynamic stresses that LT patients face in the immediate post-operative period. Post-transplant reperfusion may result in cardiac death due to a multitude of causes, including arrhythmia, acute heart failure (HF), and myocardial infarction[84].
The unusually high perioperative mortality in transplant patients with CAD warrants a systematic evaluation in every patient that thought to have a greater risk of atherosclerotic coronary disease. No single test has a predictive value of 100%. Therefore, diagnostic protocols must account for the variation in prevalence that occurs in subsets of transplant candidates and the limitation of each type of test[85]. In contrast to ischemic heart disease, most patients with advanced liver disease have myocardial defects that cause systolic and diastolic impairments not always evident at rest. There are also underlying electrophysiological defects that cause an uncoupling of the mechanical and electrical activity. Diagnosis of “cirrhotic cardiomyopathy” is difficult because the findings can be subtle as some patients develop frank heart failure when exposed to pharmacological or physiological stressors such as during liver transplantation[85].
Almost all cardiovascular abnormalities can be reversed 6 to 12 mo after liver transplantation. Namely, indices of both systolic and diastolic function, cardiac workload, and exercise capacity can be substantially improved or normalized. QT interval prolongation can also revert after OLT, even though this occurs in about half of cases suggesting that liver disease may not be the only pathogenic factor[4,24,33].
CARDIAC CAUSES OF HEPATIC DISORDERS
Heart failure
The cardiac causes of hepatic dysfunction include constrictive pericarditis, severe pulmonary arterial hypertension (PAH), mitral stenosis, tricuspid regurgitation (TR), corpulmonale, ischemic cardiomyopathy, and postoperative consequences of the Fontan procedure for pulmonary atresia and hypoplastic left heart syndrome. All of these causes can lead to passive congestion due to the elevated right ventricular (RV) pressure and right sided heart failure. The outcomes of heart failure have dramatically improved, due to the increased efficiency of medical treatment, as a result, cardiac cirrhosis prevalence is declining[24,86].
Pathophysiology: In chronic heart failure (backward failure), the increase in venous pressure caused by RV dysfunction leads to the atrophy of hepatocytes and causes perisinusoidal edema which can impair the diffusion of oxygen and nutrients to the hepatocytes[87,88]. This backward failure is also responsible for the enhanced hepatic lymph formation, leading to ascites when its production rate exceeds the draining capacity of the lymphatic system. Moreover, increased pressure within the hepatic sinusoid favours bile duct damage by disrupting endothelial cells and the interhepatocyctic tight junctions that separate the extravascular space from the bile canaliculus. Finally, stagnant flow favors thrombosis within sinusoids, hepatic venules, and portal tracts; thereby contributing to liver fibrosis[24,89,90].
On gross examination, the congestive liver is enlarged, with a purple or reddish hue and prominent hepatic veins. The cut surface shows a classic nutmeg appearance, reflecting an alternating pattern of haemorrhage and necrosis of zone 3 with normal or slightly steatotic areas in zones 1 and 2. Microscopically, the hallmark features of hepatic venous hypertension are the prominence of the central veins, central vein haemorrhage, and sinusoidal engorgement[87,91,92]. Untreated, longstanding congestion can lead to cardiac fibrosis and,ultimately cardiac cirrhosis[93]. Acute HF (forward failure): most commonly arises in the context of profound systemic hypotension from acute cardiopulmonary collapse after myocardial infarction, exacerbation of HF, or pulmonary embolism. In the absence of established hypotension, ischemic hepatitis has been shown in instances of severe hypoxemia, such as obstructive sleep apnea, respiratory failure, and in conditions of increased metabolic demand, such as those seen in toxic/septic shock[94-96]. Ischemic liver injury is characterized by centrolobular necrosis of zone 3 hepatocytes in the absence of histological evidence of inflammation characteristic of viral hepatitis[97-101].
Oxygen consumption can be easily increased when the hepatic blood flow is decreased. The mechanism by which the liver protects itself from damage in hypoxia is increasing oxygen extraction by the hepatocytes up to 95% as the blood passes through the liver. When inadequate end-organ perfusion and tissue hypoxia is persistent or when acute shock develops this protecting mechanism against hypoxic liver damage is overwhelmed. Hepatocellular injury ensues, accompanied by a sharp elevation of the serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactic dehydrogenase (LDH), prolongation of the prothrombin time, and occasionally functional renal impairment. These abnormalities reach their peak 1 to 3 d after the onset of cardiogenic ischemic hepatitis and return to normal within 5 to 10 d from onset of the disorder[102].
These forward and backward factors often coexist and potentiate each other. Additionally, the presence of hepatic steatosis due to diabetes, obesity, or other causes may increase liver susceptibility to the ischaemic reperfusion injury[24,103].
Clinical presentations: As for the acute ischemic hepatitis, no specific symptoms but patients may present with symptoms of nausea, vomiting, anorexia, malaise, right-upper quadrant pain, jaundice, oliguria, and flapping tremors representing cerebral hypoperfusion rather than hepatic encephalopathy. Ischemic hepatitis is usually benign and self-limited. The clinical diagnosis of liver injury is almost always incidental when liver enzymes are found to be massively elevated 1 to 3 d after an episode of systemic hypotension. This condition may be associated with increased serum creatinine level from acute tubular necrosis[102].
Congestive hepatopathy: The term congestive hepatopathy replaced cardiac cirrhosis. Patients experience mild, dull right upper quadrant pain caused by the stretching of the liver capsule. Hepatomegaly with a firm, tender liver edge and peripheral edema are typically the most prominent findings in patients with chronic right-sided HF, but these may also occur rapidly in acute HF. Ascites may be present in up to 25% of these patients and splenomegaly is characteristically absent[86]. Jaundice is not commonly reported. In patients with considerable TR, a prominent systolic pulsation of the liver, attributable to an enlarged right atrial V wave, is often noted. A presystolic pulsation of the liver, attributable to an enlarged right atrial A wave, can occur in tricuspid stenosis, constrictive pericarditis, restrictive cardiomyopathy involving the RV, and pulmonary hypertension[5].
Laboratory data: As for the acute ischemic hepatitis, severe jaundice is common, with a bilirubin level as high as 15 to 20 mg/dL, elevation of AST to more than 10 times the upper reference range limit, a marked increase in serum LDH, an elevated ALP level, and prolongation of the prothrombin time. Increases in LDH tend to be massive and an ALT/LDH ratio of less than 1.5 helps distinguishing ischemic injury from other forms of acute hepatitis[104,105].
As for the congestive hepatopathy, the usual findings are moderate elevations of the biochemical parameters of liver function 2 to 3 times the upper normal reference level. These parameters include AST, ALT, LDH, gamma-glutamyl transpeptidase (GGT), and alkaline phosphatase (ALP). Hyperbilirubinemia, secondary to an increase in both the direct and indirect bilirubin, is also common. The total bilirubin level is rarely greater than 3 mg/dL. In patients with long-standing HR, albumin synthesis may be impaired, leading to hypoalbuminemia and intensifying the accumulation of fluid[102].
Treatment: Treatment of the cardiac problem is the key to improvement in hepatic dysfunction.
As for the AHF and ischemic hepatitis, correcting underlying circulatory or respiratory disturbances is the main treatment. It is recommended that doctors identify and remove any precipitating cause, such as medications with negative inotropic or hypotensive effects (certain antiarrhythmic drugs, calcium-channel blockers, and vasodilators), medications likely to cause impairment of renal function (high doses of angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers), or medications likely to accumulate with evolving renal failure (like digoxin)[106]. Oxygen should be administered as early as possible in hypoxemic patients to achieve an arterial oxygen saturation > 95%. Administration of intravenous diuretics is recommended with caution in acute HF patients in the presence of symptoms secondary to congestion and volume overload. Inotropic agents should be considered in patients with low output states and low systolic blood pressure. When needed, inotropic agents should be administered as early as possible and withdrawn as soon as adequate organ perfusion is restored and/or the congestion is reduced. Vasopressors are only indicated in cases of cardiogenic shock when the combination of an inotropic agent and fluid challenge fails to restore systolic blood pressure > 90 mmHg, with inadequate organ perfusion, despite an improvement in cardiac output[5,106].
As for the chronic HF and congestive hepatopathy, the main lines of treatment are angiotensin-converting enzyme (ACE) inhibitors and beta blockers. The addition of a low-dose aldosterone antagonist should be considered in all patients with an LV systolic dysfunction. ACE Inhibitors increase cardiac output and decrease LV filling pressure due to their vasodilatory effect. Some ACE inhibitors are prodrugs, which require transformation by the liver into active metabolites. These drugs include enalapril, ramipril, fosinopril, trandolapril, quinapril, benazepril, and moexipril. With liver dysfunction, decreases in the prodrug transformation and inactivation of the active drug may occur[107]. For those patients who cannot tolerate ACE inhibitors due to cough, angiotensin receptor blockers (ARBs) are recommended instead. ARBs reduce morbidity and mortality in patients with systolic HF. Losartan is metabolized to the active metabolite via hepatic carboxylation. In patients with hepatic impairment, the bioavailability is doubled and the total plasma clearance is halved. Therefore, lower initial doses are recommended. Valsartan undergoes little metabolic conversion. Caution is recommended in patients with mild to moderate liver dysfunction but dosage adjustments are generally not needed. Similar to valsartan, irbesartan does not require biotransformation, thus dosage modification is not necessary[108-111].
The use of b-blockers is associated with a 30% reduction in total mortality in HF. Propranolol should be administered cautiously in patients with hepatic impairment. No dose adjustments are necessary for atenolol, nadolol, esmolol, sotalol, or acebutolol[112-116].
Diuretics: Loop diuretics, such as furosemide, bumetanide, and torsemide, are used for volume management in HF because of their superior natriuretic effects compared with other classes of diuretics. For unknown reasons, the pharmacologic response in patients with liver dysfunction and HF is diminished, and there is a net decreased in sodium excretion when compared with healthy individuals taking the same dose. No adjustments are necessary if renal function is normal[115].
In patients with severe HF, amiodarone has proven to be effective for suppressing ventricular arrhythmias, reducing sudden death and cardiac mortality, and improving exercise tolerance and ejection fraction. This drug undergoes an extensive hepatic metabolism to active metabolite, but no dosage reduction is indicated in hepatic impairment[116].
Statins undergo extensive hepatic metabolism. In patients with active liver disease or persistent unexplained elevations in serum transaminases to above 3 times the upper limit of normal, the use of statins is contraindicated as they may worsen liver function[117].
For patients, who are refractory to medical therapy and who may be candidates for cardiac surgery, CH due to chronic HF can improve and reverse after temporary LV assistive device support or for selected patients or cardiac transplantation[118,119]. The differences between acute and chronic hepatic impairment are summarized in Table 2.
Table 2.
Comparison between acute and chronic hepatic complications of cardiac failure
| Chronic congestive hepatopathy | Acute ischemic hepatitis | |
| Aetiology | Chronic heart failure | Acute heart failure |
| Pathophysiology | Perisinusoidal edema | Tissue hypoxia |
| Increased lymph flow | Zone 3 necrosis | |
| Zone 3: alternating necrosis and hemorrhage | ||
| Sinusoidal thrombosis | ||
| Manifestations | Right hypochondrial pain | Asymptomatic or nonspecific |
| Edema, ascites, jaundice | (nausea, vomiting, jaundice, right hypochondrial pain) | |
| Laboratory data | ||
| Bilirubin | Mild increase | Marked elevation |
| ALT and AST | Normal mild elevation | Marked elevation |
| LDH | Normal or mild elevation | Marked elevation |
| Prothrombin time | Prolonged | Normal or prolonged |
| ALP | Normal or mild elevation | Increased |
| Albumin | Hypoalbuminemia | Normal |
| Traetment | ACE inhibitors | Oxygen therapy |
| b-blockers | Avoid precipitating factors | |
| Diuretic | Inotropic agents with caution | |
| Amiodarone | Vasopressor with caution | |
| Statins with caution | Diuretics in hypervolemia | |
| Prognosis | Slowly progressive course | Benign and usually self limited |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; LDH: Lactic dehydrogenase; ALP: Alkaline phosphatase; ACE: Angiotensin-converting enzyme.
Ventricular assist devices
Ventricular assist devices (LVADs) lead to volume shifts from the intrathoracic area to the systemic circulation, thus improving liver blood flow, as assessed by indocyanine green clearance. Studies have shown improvement in the liver function in patients with mild abnormalities in pre-implant liver tests, and no deterioration in those with normal baseline values, up to 6 mo[118,120]. However, pre-existing or post-LVAD severe liver dysfunction strikingly influences patients’ prognosis and endangers their survival[121]. Liver dysfunction can also occur or worsen after LVAD implantation. Pre-, peri-, and post-operative factors, such as large doses of vasopressors, prolonged cardiopulmonary bypass time, arterial hypotension, systemic inflammatory responses and, mainly, right ventricular failure predispose a patient to liver damage, often presenting with intrahepatic cholestasis[121]. The model for end-stage liver disease (MELD), a scoring system assessing the severity of chronic liver disease based on serum bilirubin, creatinine and INR for prothrombin time that is widely used to determine prognosis and prioritize the receipt of a liver transplant, is able to predict mortality and morbidity following LVAD. The severity and course of post-ventricular assist devices liver damage can be monitored by sequential assessment of MELD-XI, a modified MELD score excluding INR to overcome the problem posed by concomitant anticoagulation[24,122].
Heart transplantation
Chronic cardiac hepatopathy is common in patients evaluated for heart transplantation-x (HTx), and liver dysfunction predicts an adverse outcome following transplantation. At the same time, altered pre-HTx liver tests can significantly improve after surgery, suggesting that chronic cardiac hepatopathy is a potentially reversible disease. In a large cohort study over 10 years among patients who had received LTx, all cholestatic parameters, transaminases and LDH improved after the procedure. Interestingly, a complete reversal of cardiac cirrhosis 10 years after HTx has even been reported[123,124].
A careful assessment of liver function and detection of liver cirrhosis is required in all candidates for HTx. An ultrasound of the abdomen with an echo-Doppler study of portal and tributary veins should be performed. Endoscopy may be necessary to assess the presence of gastro-esophageal varices and congestive gastropathy in patients with portal hypertension. Liver biopsy may be needed in some cases. MELD or modified MELD scores should be calculated. Higher MELD scores predict higher postoperative complication rates, including reoperation for bleeding, bacterial infections, and in-hospital death[124].
Patients with chronic hepatitis C or chronic hepatitis B should be treated before HTx to avoid antiviral drug intake after HTx which may be associated with graft rejection[125]. Finally, patients with heart failure and irreversible cirrhosis could be offered combined heart and liver transplantation[126].
DISEASES AFFECTING BOTH THE HEART AND THE LIVER
There are many systemic diseases in addition to chronic alcoholism, that affect both the liver and the heart. This fact may have important implications, because the heart and the liver also interact particularly during surgical procedures, OLT, and TIPS insertion, thereby influencing the outcomes. The spectrum of these diseases include congenital , autoimmune, metabolic and infectious causes (shown in Table 3). We highlight some examples of these diseases.
Table 3.
Diseases affecting both the liver and the heart concomitantly
| Hepatic manifestations | Cardiac manifestations | |
| Congenital | ||
| Allagile syndrome | Cholestasis | Congenital heart defects |
| Situs Inversus totalis | Concerns with liver or heart transplantation | |
| Infections | ||
| Sepsis | Acute liver failure | Acute heart failure |
| Hepatitis C | Hepatitis | Myocarditis, cardiomyopathy |
| Cytomegalovirus | Hepatiitis | Myopericarditis |
| HIV | Hepatitis, granuloma | Myocarditis , cardiomyopathy |
| Malaria | Hepatic necrosis | Cariac failure |
| Dengue fever | Hepatic necrosis | Myocarditis |
| Amebiasis | Hepatitis, hepatic abscess | Pericarditis, effusion |
| Metabolic | ||
| Wilson disease | Cirrhosis, hepatitis | Left ventricular remodeling |
| Hemochromatosis | Cirrhosis, hepatitis | Cardiomyopathy |
| Systemic | ||
| SLE | Steatosis, hepatomegaly | Endocarditis, pericarditis |
| Amyloidosis | Hepatomegaly, cholestasis | Cardiomyopathy |
| Sarcoidosis | Granuloma, cholestasis | Conduction defects, HF |
| Chronic alcoholism | Cirrhosis | Cariomyopathy |
| Autoimmune | ||
| Grave’s disease | Hepatitis, cholestasis | HF |
| Autoimmune hepatitis | Hepatitis, cirrhosis | Carditis |
HF: Heart failure.
Congenital causes
The famous example is Alagile syndrome (AS), which is a multisystemic disease that is autosomal dominant, with variable expression. The major clinical manifestations are as follows: chronic cholestasis, congenital heart disease, posterior embryotoxon in the eye, characteristic facial phenotype, and butterfly vertebrae. AS is caused by mutations in JAGGED1 (more than 90%) and in NOTCH2. Cholestasis, pruritus and xanthomas have been successfully treated with choleretic agents (ursodeoxycholic acid) and other medications (cholestyramine, rifampin, naltrexone). In certain cases, partial external biliary diversion has also proven successful. Liver transplantation is indicated in children with cirrhosis and liver failure[127].
Infections
Cytomegalovirus (CMV) infection in inmunocompetent hosts generally is asymptomatic; whoever, it rarely can lead to severe organ complications. A rare, but serious complication of cytomegalovirus infection is the presence of myopericarditis concomitant with hepatitis with a possible role of oral valganciclovir in these patients[128].
Metabolic causes
Wilson disease: Wilson disease is an inherited autosomal recessive disorder of the copper metabolism resulting in the pathological accumulation of copper in the liver, brain and other tissues. One of the reported manifestations is cardiac involvement. Cardiac involvement in Wilson disease patients is characterized by LV parietal thickening with an increased prevalence of concentric LV remodelling. Children with Wilson diseases were asymptomatic upon cardiological examination, but had significantly lower mitral E velocities, mitral E/A ratios as estimated by pulsed wave Doppler echocardiography[129,130].
Hemochromatosis: Hemochromatosis is an autosomal recessive disorder affecting the white population. In this disorder, the inappropriate absorption and deposition of dietary iron may result in the development of hepatic and non-hepatic end-organ injury, leading to liver cirrhosis, hepatocellular carcinoma, diabetes, arthritis, skin pigmentation and cardiac diseases[131]. Cardiac involvement in hemochromatosis affects mainly the myocardium: iron overload of the myocytes reduces left ventricular distensibility. Heart failure is the most frequent manifestation of cardiac involvement. Diagnosis of cardiac involvement depends essentially on Doppler echocardiography showing abnormal left ventricular filling and, later, ventricular dilatation with left ventricular systolic dysfunction. Magnetic resonance imaging can quantify intrahepatic and intramyocardial iron levels. The two principal means of treatment by iron depletion are phlebotomy in primary hemochromatosis and excretion of iron by chemical chelation in secondary hemochromatosis. Early diagnosis and iron depletion improve survival by reducing the organ iron overload, especially in the liver and myocardium. The recent guidelines issued by Anaes (national agency for health evaluation) make it possible to identify risk factors for complications early, to determine the disease stage, and to provide appropriate management as a function of disease severity. Combined liver transplantation and cardiac surgery may be needed in cases of hemochromatosis with end stage liver disease and heart failure[132].
Autoimmune diseases: The atypical clinical presentations of Graves’ disease (GD) include anemia, vomiting, jaundice, and right heart failure. Hyperthyroidism may present with jaundice, and on the other hand, deep jaundice may develop with the onset of overt hyperthyroidism in previously compensated chronic liver disease patients. Jaundice may be caused by hepatitis or intrahepatic cholestasis. Pulmonary hypertension is reported to be associated with GD and to respond to its treatment. GD-related pulmonary hypertension may be so severe that it produce isolated right-sided heart failure, which is occasionally identified as the presenting manifestation of GD[133].
Chronic alcoholism: Patients with chronic alcoholism can be presented with both hepatic and cardiac complications. Actively drinking alcoholics with cirrhosis have significantly lower mean ejection fraction and shortening fraction, as well as a greater mean end-diastolic diameter and left ventricular mass than abstaining alcoholics with cirrhosis. Alcoholics admitted solely for cardiomyopathy have a higher prevalence of cirrhosis than unselected alcoholics without heart disease. Actively drinking alcoholics admitted only for cirrhosis show impaired cardiac performance, whereas abstaining alcoholics with liver disease tend to manifest normal cardiac function[134]. Patients with alcoholic cirrhosis should be screened for cardiomyopathy for at least three reasons: (1) asymptomatic systolic and diastolic dysfunctions can precede the overt manifestation of cardiomyopathy; (2) hyperdynamic circulatory syndrome may disguise the clinical expression of initial heart failure; and (3) prevention or treatment of some complications of cirrhosis, such as hepatorenal syndrome, is based on plasma expansion with albumin and the administration of vasoconstrictors. This would lead to deleterious effects if latent heart failure goes unrecognized[135].
CONCLUSION
Chronic liver diseases may induce systolic and diastolic dysfunctions in addition to electrophysiological changes, and the prolongation of QT interval in conditions of cirrhotic cardiomyopathy; all of these may improve completely after liver transplantations. Recent studies have found cardiac changes in patients with NAFLD, hepatitis C and primary biliary cirrhosis. On the contrary, acute and chronic heart failure have been shown to lead to acute hepatic injury and chronic congestive hepatopathy with manifestations of liver failure and laboratory data specific to ischemic hepatitis or congestive hepatopathy. There are systemic diseases that affect both the heart and the liver, thus necessitating good cardiac and hepatic evaluation. Collaboration between hepatologists and cardiologists is needed in these categories of patients for better diagnosis, treatment and prognosis. Liver and cardiac transplantation may solve this problem in some patients with heart and liver failure.
Footnotes
P- Reviewers: Fisher RA, Konstantinos T, Marta RR, Sandro V, Sobhonslidsuk A S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Khadem E, Nasiri Toosi M, Ilkhani R. Liver- Heart Inter- Relationship in Fatty Liver Disease Based on the Avicenna’s Point of View. Iran J Public Health. 2013;42:648–649. [PMC free article] [PubMed] [Google Scholar]
- 2.Khadem E Avicenna. Al Qanun Fi Al-Tibb (Arabic) Beirut: Alaalami library; 2005. pp. 128–129. [Google Scholar]
- 3.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–1134. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 4.Adigun AQ, Pinto AG, Flockhart DA, Gorski JC, Li L, Hall SD, Chalasani N. Effect of cirrhosis and liver transplantation on the gender difference in QT interval. Am J Cardiol. 2005;95:691–694. doi: 10.1016/j.amjcard.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Kavoliuniene A, Vaitiekiene A, Cesnaite G. Congestive hepatopathy and hypoxic hepatitis in heart failure: a cardiologist’s point of view. Int J Cardiol. 2013;166:554–558. doi: 10.1016/j.ijcard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96:144–147. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- 7.Omura T, Yoshiyama M, Hayashi T, Nishiguchi S, Kaito M, Horiike S, Fukuda K, Inamoto S, Kitaura Y, Nakamura Y, et al. Core protein of hepatitis C virus induces cardiomyopathy. Circ Res. 2005;96:148–150. doi: 10.1161/01.RES.0000154263.70223.13. [DOI] [PubMed] [Google Scholar]
- 8.Terrier B, Karras A, Cluzel P, Collet JP, Sène D, Saadoun D, Cacoub P. Presentation and prognosis of cardiac involvement in hepatitis C virus-related vasculitis. Am J Cardiol. 2013;111:265–272. doi: 10.1016/j.amjcard.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–652. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 10.Miyajima I, Kawaguchi T, Fukami A, Nagao Y, Adachi H, Sasaki S, Imaizumi T, Sata M. Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: a population-based study in an HCV hyperendemic area. J Gastroenterol. 2013;48:93–100. doi: 10.1007/s00535-012-0610-3. [DOI] [PubMed] [Google Scholar]
- 11.Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502. doi: 10.1016/j.atherosclerosis.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CS, Kao JH, Chao YC, Lin HH, Fan YC, Huang CJ, Tsai PS. Interferon-based therapy reduces risk of stroke in chronic hepatitis C patients: a population-based cohort study in Taiwan. Aliment Pharmacol Ther. 2013;38:415–423. doi: 10.1111/apt.12391. [DOI] [PubMed] [Google Scholar]
- 13.Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200–3205. doi: 10.1111/j.1572-0241.2000.03252.x. [DOI] [PubMed] [Google Scholar]
- 14.Ates F, Topal E, Kosar F, Karincaoglu M, Yildirim B, Aksoy Y, Aladag M, Harputluoglu MM, Demirel U, Alan H, et al. The relationship of heart rate variability with severity and prognosis of cirrhosis. Dig Dis Sci. 2006;51:1614–1618. doi: 10.1007/s10620-006-9073-9. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462–1464. doi: 10.1016/0140-6736(92)92042-E. [DOI] [PubMed] [Google Scholar]
- 16.Milan A, Caserta MA, Del Colle S, Dematteis A, Morello F, Rabbia F, Mulatero P, Pandian NG, Veglio F. Baroreflex sensitivity correlates with left ventricular morphology and diastolic function in essential hypertension. J Hypertens. 2007;25:1655–1664. doi: 10.1097/HJH.0b013e3281ddb0a0. [DOI] [PubMed] [Google Scholar]
- 17.Lantelme P, Khettab F, Custaud MA, Rial MO, Joanny C, Gharib C, Milon H. Spontaneous baroreflex sensitivity: toward an ideal index of cardiovascular risk in hypertension? J Hypertens. 2002;20:935–944. doi: 10.1097/00004872-200205000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Okada N, Takahashi N, Yufu K, Murozono Y, Wakisaka O, Shinohara T, Anan F, Nakagawa M, Hara M, Saikawa T, et al. Baroreflex sensitivity predicts cardiovascular events in patients with type 2 diabetes mellitus without structural heart disease. Circ J. 2010;74:1379–1383. doi: 10.1253/circj.cj-09-0960. [DOI] [PubMed] [Google Scholar]
- 19.Yufu K, Takahashi N, Okada N, Wakisaka O, Shinohara T, Nakagawa M, Hara M, Yoshimatsu H, Saikawa T. Gender difference in baroreflex sensitivity to predict cardiac and cerebrovascular events in type 2 diabetic patients. Circ J. 2011;75:1418–1423. doi: 10.1253/circj.cj-10-1122. [DOI] [PubMed] [Google Scholar]
- 20.Mircoli L, Rivera R, Bonforte G, Fedele L, Genovesi S, Surian M, Ferrari AU. Influence of left ventricular mass, uremia and hypertension on vagal tachycardic reserve. J Hypertens. 2003;21:1547–1553. doi: 10.1097/00004872-200308000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467–1469. doi: 10.1378/chest.107.5.1467. [DOI] [PubMed] [Google Scholar]
- 23.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268–278. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 24.Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804–2811. doi: 10.1093/eurheartj/eht246. [DOI] [PubMed] [Google Scholar]
- 25.Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329–337. doi: 10.1038/ncpgasthep0498. [DOI] [PubMed] [Google Scholar]
- 26.Solà E, Ginès P. Renal and circulatory dysfunction in cirrhosis: current management and future perspectives. J Hepatol. 2010;53:1135–1145. doi: 10.1016/j.jhep.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294–304. doi: 10.1007/s12072-008-9109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong F, Villamil A, Merli M, Romero G, Angeli P, Caraceni P, Steib CJ, Baik SK, Spinzi G, Colombato LA, et al. Prevalence of diastolic dysfunction in cirrhosis and its clinical significance. Hepatology. 2011:54 Suppl 1, A475–A476. [Google Scholar]
- 29.Ruiz-del-Arbol L, Monescillo A, Jimenéz W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 32.Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol. 2012;6:57–66. doi: 10.1586/egh.11.86. [DOI] [PubMed] [Google Scholar]
- 33.Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68–74. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Lizardi-Cervera J, Aguilar-Zapata D. Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol. 2009;8 Suppl 1:S40–S43. [PubMed] [Google Scholar]
- 35.Treeprasertsuk S, Lopez-Jimenez F, Lindor KD. Nonalcoholic fatty liver disease and the coronary artery disease. Dig Dis Sci. 2011;56:35–45. doi: 10.1007/s10620-010-1241-2. [DOI] [PubMed] [Google Scholar]
- 36.Perseghin G. The role of non-alcoholic fatty liver disease in cardiovascular disease. Dig Dis. 2010;28:210–213. doi: 10.1159/000282088. [DOI] [PubMed] [Google Scholar]
- 37.Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, Chiesa C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2013:Epub ahead of print. doi: 10.1002/hep.26610. [DOI] [PubMed] [Google Scholar]
- 38.Jackson H, Solaymani-Dodaran M, Card TR, Aithal GP, Logan R, West J. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology. 2007;46:1131–1137. doi: 10.1002/hep.21795. [DOI] [PubMed] [Google Scholar]
- 39.Prince M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years. Gastroenterology. 2002;123:1044–1051. doi: 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- 40.Howel D, Metcalf JV, Gray J, Newman WL, Jones DE, James OF. Cancer risk in primary biliary cirrhosis: a study in northern England. Gut. 1999;45:756–760. doi: 10.1136/gut.45.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parati G, Di Rienzo M, Mancia G. Dynamic modulation of baroreflex sensitivity in health and disease. Ann N Y Acad Sci. 2001;940:469–487. doi: 10.1111/j.1749-6632.2001.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J. 1998;19 Suppl F:F72–F80. [PubMed] [Google Scholar]
- 43.Hollingsworth KG, Newton JL, Taylor R, McDonald C, Palmer JM, Blamire AM, Jones DE. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol. 2008;6:1041–1048. doi: 10.1016/j.cgh.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Jones DE, Hollingsworth K, Fattakhova G, MacGowan G, Taylor R, Blamire A, Newton JL. Impaired cardiovascular function in primary biliary cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G764–G773. doi: 10.1152/ajpgi.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fickert P, Moustafa T, Trauner M. Primary sclerosing cholangitis--the arteriosclerosis of the bile duct? Lipids Health Dis. 2007;6:3. doi: 10.1186/1476-511X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agelopoulou P, Kapatais A, Varounis C, Grassos C, Kalkandi E, Kouris N, Pierakeas N, Babalis D. Hepatocellular carcinoma with invasion into the right atrium. Report of two cases and review of the literature. Hepatogastroenterology. 2007;54:2106–2108. [PubMed] [Google Scholar]
- 47.Lin TY, Chiu KM, Chien CY, Wang MJ, Chu SH. Case 1. Right ventricular outflow obstruction caused by metastatic hepatocellular carcinoma. J Clin Oncol. 2004;22:1152–1153. doi: 10.1200/JCO.2004.04.151. [DOI] [PubMed] [Google Scholar]
- 48.Saïsse J, Hardwigsen J, Castellani P, Caus T, Le Treut YP. Budd-Chiari syndrome secondary to intracardiac extension of hepatocellular carcinoma. Two cases treated by radical resection. Hepatogastroenterology. 2001;48:836–839. [PubMed] [Google Scholar]
- 49.Liu YC, Ho YL, Huang GT, Chen DS, Sheu JC, Chen CH. Clinical manifestations and survival of patients with hepatocellular carcinoma and cardiac metastasis. J Gastroenterol Hepatol. 2010;25:150–155. doi: 10.1111/j.1440-1746.2009.06036.x. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi N, Yasunori H, Soma I, Fukuchi N, Izawa H, Yoshida T, Ebisui C, Sakita I, Koshino T, Izumiyama K, et al. [Non-surgical treatment of hepatocellular carcinoma with tumor thrombus in the right atrium] Gan To Kagaku Ryoho. 2004;31:1918–1920. [PubMed] [Google Scholar]
- 51.Okuda K, Kage M, Shrestha SM. Proposal of a new nomenclature for Budd-Chiari syndrome: hepatic vein thrombosis versus thrombosis of the inferior vena cava at its hepatic portion. Hepatology. 1998;28:1191–1198. doi: 10.1002/hep.510280505. [DOI] [PubMed] [Google Scholar]
- 52.Zhang QQ, Zu MH, Xu H, Gu YM, Wang WL, Gao ZK. Combining angioplasty with percutaneous microwave ablation for treating primary Budd-Chiari syndrome associated with hepatocellular carcinoma in two patients: A case report. Oncol Lett. 2013;6:612–616. doi: 10.3892/ol.2013.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsui S, Ichida T, Watanabe M, Sugitani S, Suda T, Takahashi T, Asakura H. Clinical features and etiology of hepatocellular carcinoma arising in patients with membranous obstruction of the inferior vena cava: in reference to hepatitis viral infection. J Gastroenterol Hepatol. 2000;15:1205–1211. doi: 10.1046/j.1440-1746.2000.02303.x. [DOI] [PubMed] [Google Scholar]
- 54.Kew MC, McKnight A, Hodkinson J, Bukofzer S, Esser JD. The role of membranous obstruction of the inferior vena cava in the etiology of hepatocellular carcinoma in Southern African blacks. Hepatology. 1989;9:121–125. doi: 10.1002/hep.1840090121. [DOI] [PubMed] [Google Scholar]
- 55.Shrestha SM. Liver cirrhosis and hepatocellular carcinoma in hepatic vena cava disease, a liver disease caused by obstruction of inferior vena cava. Hepatol Int. 2009;3:392–402. doi: 10.1007/s12072-009-9122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD) Eur Respir J. 2004;24:861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 57.Rolla G, Brussino L, Colagrande P, Scappaticci E, Morello M, Bergerone S, Ottobrelli A, Cerutti E, Polizzi S, Bucca C. Exhaled nitric oxide and impaired oxygenation in cirrhotic patients before and after liver transplantation. Ann Intern Med. 1998;129:375–378. doi: 10.7326/0003-4819-129-5-199809010-00005. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J, Yang W, Luo B, Hu B, Maheshwari A, Fallon MB. The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome. J Hepatol. 2012;57:752–758. doi: 10.1016/j.jhep.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palma DT, Philips GM, Arguedas MR, Harding SM, Fallon MB. Oxygen desaturation during sleep in hepatopulmonary syndrome. Hepatology. 2008;47:1257–1263. doi: 10.1002/hep.22143. [DOI] [PubMed] [Google Scholar]
- 60.McAdams HP, Erasmus J, Crockett R, Mitchell J, Godwin JD, McDermott VG. The hepatopulmonary syndrome: radiologic findings in 10 patients. AJR Am J Roentgenol. 1996;166:1379–1385. doi: 10.2214/ajr.166.6.8633451. [DOI] [PubMed] [Google Scholar]
- 61.Martínez GP, Barberà JA, Visa J, Rimola A, Paré JC, Roca J, Navasa M, Rodés J, Rodriguez-Roisin R. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651–657. doi: 10.1016/s0168-8278(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 62.Schenk P, Schöniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Müller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 63.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 64.De BK, Dutta D, Pal SK, Gangopadhyay S, Das Baksi S, Pani A. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol. 2010;24:183–188. doi: 10.1155/2010/349076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodríquez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Highlights of the ERS Task Force on pulmonary-hepatic vascular disorders (PHD) J Hepatol. 2005;42:924–927. doi: 10.1016/j.jhep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502–1510. doi: 10.1002/hep.21431. [DOI] [PubMed] [Google Scholar]
- 67.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dickinson MG, Bartelds B, Borgdorff MA, Berger RM. The role of disturbed blood flow in the development of pulmonary arterial hypertension: lessons from preclinical animal models. Am J Physiol Lung Cell Mol Physiol. 2013;305:L1–14. doi: 10.1152/ajplung.00031.2013. [DOI] [PubMed] [Google Scholar]
- 69.Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492–498. doi: 10.1016/s0735-1097(10)80121-4. [DOI] [PubMed] [Google Scholar]
- 70.Kuo PC, Plotkin JS, Johnson LB, Howell CD, Laurin JM, Bartlett ST, Rubin LJ. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980–986. doi: 10.1378/chest.112.4.980. [DOI] [PubMed] [Google Scholar]
- 71.Fix OK, Bass NM, De Marco T, Merriman RB. Long-term follow-up of portopulmonary hypertension: effect of treatment with epoprostenol. Liver Transpl. 2007;13:875–885. doi: 10.1002/lt.21174. [DOI] [PubMed] [Google Scholar]
- 72.Hoeper MM, Seyfarth HJ, Hoeffken G, Wirtz H, Spiekerkoetter E, Pletz MW, Welte T, Halank M. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096–1102. doi: 10.1183/09031936.00032407. [DOI] [PubMed] [Google Scholar]
- 73.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Sildenafil treatment for portopulmonary hypertension. Eur Respir J. 2006;28:563–567. doi: 10.1183/09031936.06.00030206. [DOI] [PubMed] [Google Scholar]
- 74.Provencher S, Herve P, Jais X, Lebrec D, Humbert M, Simonneau G, Sitbon O. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006;130:120–126. doi: 10.1053/j.gastro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Malagari K, Nikita A, Alexopoulou E, Brountzos E, Papathanasiou M, Mitromaras J, Zakynthinos E, Papiris S, Kelekis DA. Cirrhosis-related intrathoracic disease. Imaging features in 1038 patients. Hepatogastroenterology. 2005;52:558–562. [PubMed] [Google Scholar]
- 76.Benet A, Vidal F, Toda R, Siurana R, De Virgala CM, Richart C. Diagnosis of hepatic hydrothorax in the absence of ascites by intraperitoneal injection of 99m-Tc-Fluor colloid. Postgrad Med J. 1992;68:153. doi: 10.1136/pgmj.68.796.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gurung P, Goldblatt M, Huggins JT, Doelken P, Nietert PJ, Sahn SA. Pleural fluid analysis and radiographic, sonographic, and echocardiographic characteristics of hepatic hydrothorax. Chest. 2011;140:448–453. doi: 10.1378/chest.10-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiol X, Castellví JM, Guardiola J, Sesé E, Castellote J, Perelló A, Cervantes X, Iborra MJ. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719–723. doi: 10.1002/hep.510230410. [DOI] [PubMed] [Google Scholar]
- 79.Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3:582–586. doi: 10.1007/s12072-009-9136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu LU, Haddadin HA, Bodian CA, Sigal SH, Korman JD, Bodenheimer HC, Schiano TD. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004;126:142–148. doi: 10.1378/chest.126.1.142. [DOI] [PubMed] [Google Scholar]
- 81.Siegerstetter V, Deibert P, Ochs A, Olschewski M, Blum HE, Rössle M. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13:529–534. doi: 10.1097/00042737-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 82.Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366–1369. doi: 10.1002/hep.510250611. [DOI] [PubMed] [Google Scholar]
- 83.Milanez de Campos JR, Filho LO, de Campos Werebe E, Sette H, Fernandez A, Filomeno LT, Jatene FB. Thoracoscopy and talc poudrage in the management of hepatic hydrothorax. Chest. 2000;118:13–17. doi: 10.1378/chest.118.1.13. [DOI] [PubMed] [Google Scholar]
- 84.Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, Fix OK, Kay N, Abecassis MI, Gheorghiade M, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58:223–231. doi: 10.1016/j.jacc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 85.Mandell MS, Lindenfeld J, Tsou MY, Zimmerman M. Cardiac evaluation of liver transplant candidates. World J Gastroenterol. 2008;14:3445–3451. doi: 10.3748/wjg.14.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Myers RP, Cerini R, Sayegh R, Moreau R, Degott C, Lebrec D, Lee SS. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37:393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 87.Safran AP, Schaffner F. Chronic passive congestion of the liver in man. Electron microscopic study of cell atrophy and intralobular fibrosis. Am J Pathol. 1967;50:447–463. [PMC free article] [PubMed] [Google Scholar]
- 88.Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265:174–189. doi: 10.1097/00000441-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 89.Cogger VC, Fraser R, Le Couteur DG. Liver dysfunction and heart failure. Am J Cardiol. 2003;91:1399. doi: 10.1016/s0002-9149(03)00370-9. [DOI] [PubMed] [Google Scholar]
- 90.Wanless IR, Liu JJ, Butany J. Role of thrombosis in the pathogenesis of congestive hepatic fibrosis (cardiac cirrhosis) Hepatology. 1995;21:1232–1237. [PubMed] [Google Scholar]
- 91.SHERLOCK S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J. 1951;13:273–293. doi: 10.1136/hrt.13.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lefkowitch JH, Mendez L. Morphologic features of hepatic injury in cardiac disease and shock. J Hepatol. 1986;2:313–327. doi: 10.1016/s0168-8278(86)80043-5. [DOI] [PubMed] [Google Scholar]
- 93.Weisberg IS, Jacobson IM. Cardiovascular diseases and the liver. Clin Liver Dis. 2011;15:1–20. doi: 10.1016/j.cld.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 94.Henrion J, Minette P, Colin L, Schapira M, Delannoy A, Heller FR. Hypoxic hepatitis caused by acute exacerbation of chronic respiratory failure: a case-controlled, hemodynamic study of 17 consecutive cases. Hepatology. 1999;29:427–433. doi: 10.1002/hep.510290202. [DOI] [PubMed] [Google Scholar]
- 95.Mathurin P, Durand F, Ganne N, Mollo JL, Lebrec D, Degott C, Erlinger S, Benhamou JP, Bernuau J. Ischemic hepatitis due to obstructive sleep apnea. Gastroenterology. 1995;109:1682–1684. doi: 10.1016/0016-5085(95)90659-2. [DOI] [PubMed] [Google Scholar]
- 96.Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) 2003;82:392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 97.ELLENBERG M, OSSERMAN KE. The role of shock in the production of central liver cell necrosis. Am J Med. 1951;11:170–178. doi: 10.1016/0002-9343(51)90102-7. [DOI] [PubMed] [Google Scholar]
- 98.KILLIP T, PAYNE MA. High serum transaminase activity in heart disease. Circulatory failure and hepatic necrosis. Circulation. 1960;21:646–660. doi: 10.1161/01.cir.21.5.646. [DOI] [PubMed] [Google Scholar]
- 99.CLARKE WT. Centrilobular hepatic necrosis following cardiac infarction. Am J Pathol. 1950;26:249–255. [PMC free article] [PubMed] [Google Scholar]
- 100.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: clinical presentation and pathogenesis. Am J Med. 2000;109:109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 101.Birgens HS, Henriksen J, Matzen P, Poulsen H. The shock liver. Clinical and biochemical findings in patients with centrilobular liver necrosis following cardiogenic shock. Acta Med Scand. 1978;204:417–421. [PubMed] [Google Scholar]
- 102.Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011;20:135–142. doi: 10.1055/s-0031-1284434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caraceni P, Bianchi C, Domenicali M, Maria Pertosa A, Maiolini E, Parenti Castelli G, Nardo B, Trevisani F, Lenaz G, Bernardi M. Impairment of mitochondrial oxidative phosphorylation in rat fatty liver exposed to preservation-reperfusion injury. J Hepatol. 2004;41:82–88. doi: 10.1016/j.jhep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 104.Gitlin N, Serio KM. Ischemic hepatitis: widening horizons. Am J Gastroenterol. 1992;87:831–836. [PubMed] [Google Scholar]
- 105.Cassidy WM, Reynolds TB. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gastroenterol. 1994;19:118–121. doi: 10.1097/00004836-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 106.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Daskalopoulos G, Pinzani M, Murray N, Hirschberg R, Zipser RD. Effects of captopril on renal function in patients with cirrhosis and ascites. J Hepatol. 1987;4:330–336. doi: 10.1016/s0168-8278(87)80542-1. [DOI] [PubMed] [Google Scholar]
- 108.Brookman LJ, Rolan PE, Benjamin IS, Palmer KR, Wyld PJ, Lloyd P, Flesch G, Waldmeier F, Sioufi A, Mullins F. Pharmacokinetics of valsartan in patients with liver disease. Clin Pharmacol Ther. 1997;62:272–278. doi: 10.1016/S0009-9236(97)90029-1. [DOI] [PubMed] [Google Scholar]
- 109.Gillis JC, Markham A. Irbesartan. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in the management of hypertension. Drugs. 1997;54:885–902. doi: 10.2165/00003495-199754060-00007. [DOI] [PubMed] [Google Scholar]
- 110.Markham A, Goa KL. Valsartan. A review of its pharmacology and therapeutic use in essential hypertension. Drugs. 1997;54:299–311. doi: 10.2165/00003495-199754020-00009. [DOI] [PubMed] [Google Scholar]
- 111.Marino MR, Langenbacher KM, Raymond RH, Ford NF, Lasseter KC. Pharmacokinetics and pharmacodynamics of irbesartan in patients with hepatic cirrhosis. J Clin Pharmacol. 1998;38:347–356. doi: 10.1002/j.1552-4604.1998.tb04434.x. [DOI] [PubMed] [Google Scholar]
- 112.Kirch W, Schäfer-Korting M, Mutschler E, Ohnhaus EE, Braun W. Clinical experience with atenolol in patients with chronic liver disease. J Clin Pharmacol. 1983;23:171–177. doi: 10.1002/j.1552-4604.1983.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 113.Buchi KN, Rollins DE, Tolman KG, Achari R, Drissel D, Hulse JD. Pharmacokinetics of esmolol in hepatic disease. J Clin Pharmacol. 1987;27:880–884. doi: 10.1002/j.1552-4604.1987.tb05583.x. [DOI] [PubMed] [Google Scholar]
- 114.Zaman R, Jack DB, Wilkins MR, Kendall MJ. Lack of effect of liver disease on the pharmacokinetics of acebutolol and diacetolol: a single dose study. Biopharm Drug Dispos. 1985;6:131–137. doi: 10.1002/bdd.2510060204. [DOI] [PubMed] [Google Scholar]
- 115.Fredrick MJ, Pound DC, Hall SD, Brater DC. Furosemide absorption in patients with cirrhosis. Clin Pharmacol Ther. 1991;49:241–247. doi: 10.1038/clpt.1991.23. [DOI] [PubMed] [Google Scholar]
- 116.Garguichevich JJ, Ramos JL, Gambarte A, Gentile A, Hauad S, Scapin O, Sirena J, Tibaldi M, Toplikar J. Effect of amiodarone therapy on mortality in patients with left ventricular dysfunction and asymptomatic complex ventricular arrhythmias: Argentine Pilot Study of Sudden Death and Amiodarone (EPAMSA) Am Heart J. 1995;130:494–500. doi: 10.1016/0002-8703(95)90357-7. [DOI] [PubMed] [Google Scholar]
- 117.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 118.Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM, Klodell CT, Boyle AJ, John R, Chen L, et al. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation. 2009;120:2352–2357. doi: 10.1161/CIRCULATIONAHA.108.814863. [DOI] [PubMed] [Google Scholar]
- 119.Dichtl W, Vogel W, Dunst KM, Grander W, Alber HF, Frick M, Antretter H, Laufer G, Pachinger O, Pölzl G. Cardiac hepatopathy before and after heart transplantation. Transpl Int. 2005;18:697–702. doi: 10.1111/j.1432-2277.2005.00122.x. [DOI] [PubMed] [Google Scholar]
- 120.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR, Heitjan DF, Meier P, Ascheim DD, Levitan RG, et al. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg. 1999;67:723–730. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 121.Wadia Y, Etheridge W, Smart F, Wood RP, Frazier OH. Pathophysiology of hepatic dysfunction and intrahepatic cholestasis in heart failure and after left ventricular assist device support. J Heart Lung Transplant. 2005;24:361–370. doi: 10.1016/j.healun.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 122.Yang JA, Kato TS, Shulman BP, Takayama H, Farr M, Jorde UP, Mancini DM, Naka Y, Schulze PC. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J Heart Lung Transplant. 2012;31:601–610. doi: 10.1016/j.healun.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Crespo-Leiro MG, Robles O, Paniagua MJ, Marzoa R, Naya C, Flores X, Suárez F, Gómez M, Grille Z, Cuenca JJ, et al. Reversal of cardiac cirrhosis following orthotopic heart transplantation. Am J Transplant. 2008;8:1336–1339. doi: 10.1111/j.1600-6143.2008.02227.x. [DOI] [PubMed] [Google Scholar]
- 124.Chokshi A, Cheema FH, Schaefle KJ, Jiang J, Collado E, Shahzad K, Khawaja T, Farr M, Takayama H, Naka Y, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 2012;31:591–600. doi: 10.1016/j.healun.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim EY, Ko HH, Yoshida EM. A concise review of hepatitis C in heart and lung transplantation. Can J Gastroenterol. 2011;25:445–448. doi: 10.1155/2011/947838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Te HS, Anderson AS, Millis JM, Jeevanandam V, Jensen DM. Current state of combined heart-liver transplantation in the United States. J Heart Lung Transplant. 2008;27:753–759. doi: 10.1016/j.healun.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Ciocca M, Alvarez F. [Alagille syndrome] Arch Argent Pediatr. 2012;110:509–515. doi: 10.5546/aap.2012.509. [DOI] [PubMed] [Google Scholar]
- 128.Zubiaurre L, Zapata E, Bujanda L, Castillo M, Oyarzabal I, Gutiérrez-Stampa MA, Cosme A. Cytomegalovirus hepatitis and myopericarditis. World J Gastroenterol. 2007;13:647–648. doi: 10.3748/wjg.v13.i4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elkiran O, Karakurt C, Selimoglu A, Karabiber H, Kocak G, Celik SF, Colak C. Subclinical diastolic dysfunction in children with Wilson’s disease assessed by tissue Doppler echocardiography: a possible early predictor of cardiac involvement. Acta Cardiol. 2013;68:181–187. doi: 10.1080/ac.68.2.2967276. [DOI] [PubMed] [Google Scholar]
- 130.Hlubocká Z, Marecek Z, Linhart A, Kejková E, Pospísilová L, Martásek P, Aschermann M. Cardiac involvement in Wilson disease. J Inherit Metab Dis. 2002;25:269–277. doi: 10.1023/a:1016546223327. [DOI] [PubMed] [Google Scholar]
- 131.Licata A, Brucato V, Di Marco V, Barbaria F, Craxì A. [Iron overload disease: recent findings] Ann Ital Med Int. 2004;19:145–154. [PubMed] [Google Scholar]
- 132.Giakoustidis A, Cherian TP, Antoniadis N, Giakoustidis D. Combined cardiac surgery and liver transplantation: three decades of worldwide results. J Gastrointestin Liver Dis. 2011;20:415–421. [PubMed] [Google Scholar]
- 133.Hegazi MO, Ahmed S. Atypical clinical manifestations of graves’ disease: an analysis in depth. J Thyroid Res. 2012;2012:768019. doi: 10.1155/2012/768019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Estruch R, Fernández-Solá J, Sacanella E, Paré C, Rubin E, Urbano-Márquez A. Relationship between cardiomyopathy and liver disease in chronic alcoholism. Hepatology. 1995;22:532–538. [PubMed] [Google Scholar]
- 135.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]


