Abstract
AIM: To investigate the diagnosis, pathogenesis, natural history, and management of nodular regenerative hyperplasia (NRH) in patients with human immunodeficiency virus (HIV).
METHODS: We performed a systematic review of the medical literature regarding NRH in patients with HIV. Inclusion criteria include reports with biopsy proven NRH. We studied the clinical features of NRH, in particular, related to its presenting manifestation and laboratory values. Combinations of the following keywords were implemented: “nodular regenerative hyperplasia”, “human immunodeficiency virus”, “noncirrhotic portal hypertension”, “idiopathic portal hypertension”, “cryptogenic liver disease”, “highly active antiretroviral therapy” and “didanosine”. The bibliographies of these studies were subsequently searched for any additional relevant publications.
RESULTS: The clinical presentation of patients with NRH varies from patients being completely asymptomatic to the development of portal hypertension – namely esophageal variceal bleeding and ascites. Liver associated enzymes are generally normal and synthetic function well preserved. There is a strong association between the occurrence of NRH and the use of antiviral therapies such as didanosine. The management of NRH revolves around treating the manifestations of portal hypertension. The prognosis of NRH is generally good since liver function is preserved. A high index of suspicion is required to make a identify NRH.
CONCLUSION: The appropriate management of HIV-infected persons with suspected NRH is yet to be outlined. However, NRH is a clinically subtle condition that is difficult to diagnose, and it is important to be able to manage it according to the best available evidence.
Keywords: Human immunodeficiency virus, Nodular regenerative hyperplasia, Ascites, Systematic review, Liver complications
Core tip: Liver complications in patients with human immunodeficiency virus (HIV) is emerging as a public health concern. The appropriate management of HIV-infected persons with suspected nodular regenerative hyperplasia (NRH) is yet to be outlined. However, NRH is a clinically subtle condition that is difficult to diagnose, and it is important to be able to manage it according to the best available evidence. We believe the implications of our manuscript will have immediate clinical implications.
INTRODUCTION
Nodular regenerative hyperplasia (NRH) is the diffuse transformation of the liver parenchyma into micronodules without intervening fibrosis[1]. NRH is associated with a number systemic diseases, including human immunodeficiency virus (HIV) infection, and over the past several years, it has become an increasingly recognized entity that causes noncirrhotic portal hypertension (NCPH)[2].
The pathogenesis of NRH is thought to be vascular in origin, initiated by endothelial damage and causing an uneven distribution of obliteration of the small portal venules throughout the liver parenchyma[3]. In addition to endothelial damage, recurrent micro-thrombosis of the portal vasculature is also thought to contribute to the obliterative phenomenon. These micronodules tend to form in areas of preserved blood flow and are thought to represent a compensatory hypertrophic response to the neighboring acini with impaired blood flow[2,4]. The diagnosis of NRH is definitively made with the histological presence of micronodules not greater than 3 mm thick and without intervening fibrosis[1].
The pathogenesis of NRH in HIV infection remains unclear. Several theories have been proposed, including a “two-hit” model in which recurrent gut bacterial translocation to the portal tract in combination with vascular endothelial damage ultimately results in portal hypertension[5]. Other theories suggest that direct viral or immune-mediated damage contribute to the obliterative venopathy[6]. However drug-induced hepatotoxicity has become the prevailing theory. The prolonged exposure to highly active antiretroviral therapy (HAART), namely didanosine (DDI) has been strongly correlated, and in all cases of proposed HAART-associated NRH, DDI has been present in each[5-15].
The significance of the association between NRH and HIV is underscored by the evolving patterns of disease in HIV-infected persons. Approximately 34 million people are currently infected with the virus worldwide. In developed nations, where individuals have ready access to HAART the mortality of HIV is decreasing[5]. The causes of death are shifting and studies have shown that liver disease accounts for as high as 18% of deaths in the post-HAART era[6]. These findings place liver disease in the top three causes of mortality in HIV-infected persons, out ranking opportunistic infections and acquired immune deficiency syndrome (AIDS)-defining illnesses. While hepatic viral co-infection accounts for a majority of the liver disease in HIV patients, both alcoholic and non-alcoholic liver diseases are also important. Of emerging relevance, is the burden of NCPH, namely NRH.
The purpose of this paper is to perform a systematic review of the clinical syndrome and outcomes in patients with NRH specifically related to HIV. The manifestations of NRH in HIV patients are similar to those that have previously been well defined in patients with NRH of other etiologies. We will focus on the clinical manifestations reported and suggest a methodical approach to the HIV patient with known or suspected NRH.
MATERIALS AND METHODS
We performed a search of the MEDLINE database for all published studies in all available languages on NRH in HIV-infected patients. Combinations of the following keywords were implemented: “nodular regenerative hyperplasia”, “human immunodeficiency virus”, “noncirrhotic portal hypertension”, “idiopathic portal hypertension”, “cryptogenic liver disease”, “highly active antiretroviral therapy” and “didanosine”. The bibliographies of these studies were subsequently searched for any additional relevant publications.
Our search yielded 68 unique hits (Figure 1). These publications were reviewed for relevance to the topic of interest. A total of 49 publications pertaining to the phenomenon of NCPH in the context of HIV-infection and its treatment were found. The papers were then reviewed in further detail to identify cases where the specific diagnosis of NRH was made, and cases were only included if diagnosis was based on histologic identification. A total of 95 cases that met our inclusion criteria were found. We excluded any cases where the diagnosis of NCPH was attributed to conditions other than NRH, therefore cases of hepatoportal sclerosis (HPS) and other obilterative portal venopathies not meeting diagnostic criteria for NRH were excluded. Of note, we did include cases where both HPS and NRH were found in the same liver. We excluded any cases where liver biopsy was not done and therefore no definitive diagnosis of NRH could be made.
Figure 1.
Flow diagram outlining methods of search. NRH: Nodular regenerative hyperplasia; HIV: Human immunodeficiency virus.
RESULTS
Epidemiology
NRH is a rare condition, and although it has become increasingly recognized, its epidemiology remains poorly understood. The incidence in the general population is approximately 2.6%, an estimate that comes from a large autopsy study done in 1990[1]. The majority of the existing data is based from case reports and case series, and the incidence appears to be increasing as NRH is becoming more widely recognized[7]. The diagnosis is frequently missed as liver biopsy is usually only undertaken in symptomatic patients and NRH often exists sub-clinically. Moreover, diagnosis requires adequate specimen, appropriate use of reticulin staining and evaluation by a skilled pathologist, making histologic confirmation difficult[8].
The first published case of a patient with HIV found to have NRH was reported from Spain in 1993 in a patient with HIV infection and visceral leishmaniasis[9]. We identified a total of 94 additional cases of biopsy-confirmed NRH in HIV-positive patients, most of which were reported within the last decade (Table 1). Given that the same limitations in diagnosis apply to HIV-infected persons, the incidence is likely grossly underestimated in this patient population as well. Epidemiological studies geared toward select patient populations are needed and may shed more light on the actual incidence of NRH in HIV patients as well as in the general population.
Table 1.
Literature review of nodular regenerative hyperplasia in human immunodeficiency virus positive patients (biopsy confirmed)
| Ref. | Study type | Number of patients | Clinical presentation |
| Arey et al[21] | Case report | 1 | Abdominal distention, abdominal, pain, EV |
| Bihl et al[31] | Case report | 1 | Abdominal pain, ALP, ascites, EV, GIB, splenomegaly |
| Bissonnette et al[11] | Case report | 2 | Ascites, EV, GIB |
| Cachay et al[15] | Case series | 1 | ALP |
| Saifee et al[52] | Case series | 11 | Ascites, EV, GIB |
| Cesari et al[53] | Case control | 5 | Ascites, EV, GIB, splenomegaly |
| Cotte et al[12] | Case control | 13 | Abdominal pain, ALP, ascites, EV, GIB, splenomegaly |
| Alvarez Díaz et al[33] | Case report | 2 | ALP, ascites, EV, GIB |
| Ding et al[54] | Case report | 1 | ALP, EV, GIB, ascites |
| Dinh et al[55] | Case control | 3 | Ascites, encephalopathy, EV |
| Fernandez-Miranda et al[9] | Case report | 1 | Unknown |
| Garvey et al[19] | Case report | 2 | EV, splenomegaly |
| Hofmaenner et al[27] | Case report | 1 | Epigastric pain, ALP |
| Kochin et al[56] | Case report | 1 | EV, GIB |
| Kovari et al[23] | Case control | 1 | EV |
| Maida et al[8] | Case series | 2 | Splenomegaly |
| Mallet et al[4] | Case control | 13 | ALP, EV, GIB |
| Mallet et al[57] | Case report | 1 | Portal hypertension |
| Mallet et al[30] | Case series | 8 | ALP, ascites, EV, splenomegaly |
| Mendizabal et al[58] | Case control | 5 | EV, GIB |
| Podevin et al[59] | Case report | 1 | Ascites, EV, splenomegaly |
| Sandrine et al[20] | Case report | 1 | ALP, ascites, EV, splenomegaly |
| Santiago et al[28] | Case report | 1 | Abdominal distention, splenomegaly |
| Schiano et al[22] | Case report | 1 | ALP, EV, splenomegaly |
| Scourfield et al[60] | Retrospective cohort | 4 | Unknown |
| Schouten et al[61] | Case report | 3 | EV, GIB |
| Stebbing et al[62] | Retrospective cohort | 2 | ALP |
| Tateo et al[13] | Case report | 3 | ALP, ascites, EV, GIB |
| Vispo et al[29] | Case report | 3 | ALP, ascites, EV, GIB |
ALP: Abnormal liver panel; EV: Esophageal varices; GIB: Gastrointestinal bleed.
Clinical presentation
The clinical presentation of the cases found was highly variable. The diagnosis was prompted by incidental lab abnormalities in asymptomatic patients, and in other cases, patients presented with manifestations of portal hypertension such as esophageal varices, ascites, and hypersplenism (Table 1). Jaundice was universally not reported. According to our findings, the most common manifestation of NRH in HIV-positive patients was esophageal varices, which were identified in at least 66 of 95 cases. Only 28 patients developed gastrointestinal bleeding in the setting of esophageal varices (Table 2). In many cases, once the clinical syndrome of portal hypertension was identified, patients underwent screening esophagogastroduodenoscopy and were placed on prophylactic non-selective beta-blockers (NSBB) if varices were found. Patients were reported to be on NSBB in almost all cases.
Table 2.
Manifestations of portal hypertension in patients with nodular regenerative hyperplasia and human immunodeficiency virus n (%)
| Manifestation | Patients identified | References |
| Ascites | 30 (32) | [4,12,19,20,28,33,52,53,58,60] |
| Esophageal varices | 61 (66) | [4,12,19,20,28,33,52,53,58,60] |
| Hepatic encephalopathy | 1 (1.1) | [54] |
| Splenomegaly | 25 (27) | [4,12,19,20,31,52,53,54,58,60] |
| Portal thrombosis | 11 (12) | [4,12,19,20,31,52,53,54,58] |
Liver synthetic function as indicated by INR and albumin is well preserved across all cases in which it was reported. Liver associated enzymes may be only mildly elevated (Table 3). Patients were also not uncommonly found to have thrombophilias including protein C and protein S deficiencies, which may be associated with the pathogenesis of NRH and the development of portal vein thrombosis in these patients (Table 3).
Table 3.
Select laboratory tests associated with nodular regenerative hyperplasia
| Laboratory test | Range | References |
| Albumin (g/dL) | 1.9-4.5 | [22,28,52,54,55] |
| Aspartate transaminase (IU/L) | 15-139 | [4,8,20-22,30,31,38,54,55,59,60] |
| Alanine transaminase (IU/L) | 13-196 | [3,7,19-21,30,31,52,54,55,59,60] |
| Alkaline Phosphatase (IU/L) | 92-541 | [4,20-23,28,31,52,53,55,59,60] |
| Total bilrubin (mg/dL) | 0.6-3.6 | [21,22,31,52,54,55,59,60] |
| Gamma glutamyl transpeptidase (IU/L) | 12-771 | [4,20,22,28,31,53,55,59,60] |
| White blood cell count (cells/mm3) | 1090-4800 | [4,21,22,28,30,31,33,53-55,60] |
| Hemoglobin (g/dL) | 6.6-13 | [22,55,60] |
| Platelet (1000/mm3) | 61-273 | [4,22,23,52,54,55,59,60] |
When compared to the clinical presentation of patients with NRH without HIV, similar findings have been reported. In one recent series including 42 patients, the most common presenting abnormality was an abnormal liver profile, existing in 76% of cases. Varices were detected in 26% of patients. None of these patients had synthetic liver dysfunction as implicated by normal INR[10]. In another series of 24 patients, similar rates of various clinical features of NRH were reported[11]. These findings mimic those of other similar case reports and are also similar to the findings presented in patient’s specifically with NRH and HIV.
Diagnosis
Tthe diagnosis of NRH is a histologic one, requiring liver biopsy. Histologic features are shown in Figure 2. The use of a reticulin stain is usually necessary to make the diagnosis. Important features on the reticulin stain include: nodular apperance, characterized by alternating hypertrophic and atrophic hepatocytes. Highlighting the frequent delay in diagnosis, in one report of 13 patients on HAART who developed NRH, the mean time from presentation to diagnosis of NRH was approximately 38 mo[12]. This point highlights the sub-optimal diagnosis of NRH, leading to its under-appreciation as an important clinical entity in the HIV population. Diagnosis is further limited by the presence of a clear workup bias in that it is usually either the symptomatic patient, or the patient with long-term DDI exposure who undergoes diagnostic testing for NRH. Furthermore, consideration of NRH is certainly more common in the academic setting[13].
Figure 2.
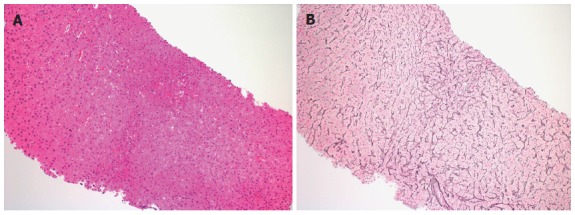
Needle liver biopsy of male infected with human immunodeficiency virus. A: His risk factor for the development of Nodular Regenerative Hyperplasia was long term use of didanosine. Hepatocytes size varies zonally, occasional areas of small hepatocytes with increased nuclear cytoplasmic ratio alternate with areas of a more normal appearing morphology in the hematoxylin-eosin stain (original magnification × 100); B: The reticulin stain highlights and confirms nodular regeneration throughout the specimen with nodular areas of regenerative hepatocytes as characterized by widened hepatocellular plates alternating with areas of normal appearing lobular architecture and then areas of narrowed, attenuated hepatocytes (original magnification × 100).
Radiologically, the diagnosis of NRH is also difficult. Findings are variable and range from none to diffuse hypoechoic nodules. On ultrasound, findings may include widespread nodularity of the liver can mimic cirrhosis[14]. On computed tomography, the nodules are usually hypodense and typically do not enhance with contrast. Finally on magnetic resonance imaging, suface nodularity and nodules of similar signal intensity to the liver may be noted[14]. Because the findings on imaging are non-specific and non-diagnostic, clinical correlation is key in determining the next best step in diagnosis.
Natural history
The natural history of NRH is poorly understood. There is likely an inherent bias to diagnose and report symptomatic cases, and NRH is likely more indolent than appreciated. This notion is supported by the large autopsy study by Wanless in which only one of 64 patients had been diagnosed with NRH prior to death. Few of these patients had developed manifestations of portal hypertension prior to death[1]. These findings are likely explained by the preservation of hepatic synthetic function observed in patients with NRH. From the available data, the presentation of NRH is variable and ranges from subtle findings on serum liver tests, to vague abdominal symptoms, to overt gastrointestinal hemorrhage and other severe manifestations of portal hypertension. To date, the overall prognosis has not been well defined, as there have not been substantial outcomes data reported in the literature. In one long-term follow up study of a cohort of eight HIV-infected patients with NCPH, there was only one death, which was attributed to non-NCPH related causes. The remainder of the patients underwent regular screening EGD and was placed on NSBB therapy. If the patients were found to have NCPH prior to the development of variceal bleeding, they tended to remain minimally symptomatic with supportive and preventative care. On the other hand patients who were not treated in a timely manner tended to develop refractory bleeding. One patient with severe NRH required repeated TIPS and was placed on the liver transplant waiting list while another underwent surgical hepatorenal shunt[15].
Associated conditions and medications
Nodular regenerative hyperplasia has long been associated with rheumatologic, autoimmune, hematologic and myeloproliferative disorders[7]. Of particular clinical consequence is the link between NRH and thrombophilias. The association is theoretically attributed to the increased chronic micro-thrombosis of the small portal venules, leading to their constriction, obliteration and eventually to intrahepatic portal hypertension[16]. Many of these patients are also predisposed to the development of portal vein thrombosis, which can lead to end-stage disease. In one study, 12 out of 2600 persons with HIV infection were found to have NCPH, and NRH was a common histologic diagnosis. Of the 12 patients with NCPH, half were found to have portal thrombosis[17]. Among all cases, the percentage of patients with portal thrombosis was close to 15% (Table 2).
The association between NRH and antiretroviral therapy has been identified mostly through case reports and small case-control studies. In particular, specific associations have been made with DDI. One of the earliest associations with DDI and NCPH and NRH was from a case-control analysis[18]. Their findings were supported in a number of succeeding publications[14,15,17,19-23]. More recently, the results of another analysis suggested that patients with NRH had a longer exposure not only to DDI, but stavudine, tenofovir, and a combination of DDI and stavudine and DDI and tenofovir as well[12].
Aside from HAART, other medications have also been linked to NRH. Its association with azathioprine (AZA), 6-mercaptopurine, 6-thioguanine, busulphan, cyclophosphamide and most recently, oxaliplatin-based therapies have also been reported[24,25]. A presumed mechanism is damage of the endothelium in the small hepatic veins leading to the phenomenon of obliterative portal venopathy. Interestingly, in one report of AZA-induced NRH, some patients demonstrated normalization of liver enzyme markers and histological regression of NRH after withdrawal of AZA[26]. Such histological regression has never been reported in the case of HAART and HIV-associated NRH.
Treatment
There is no definitive treatment for NRH. The mainstay of management is removal of the offending agent and prevention and supportive care of disease manifestations. It is important to note that in many of the cases in which NRH was linked to DDI, the exposure had been long-since stopped prior to the time of NRH diagnosis. Cessation of DDI or other implicated HAART agents may improve outcomes, however it has not been shown to result in reversal of the disease process[27]. In part because of its associated hepatotoxicity, the use of DDI has declined substantially and it is now regarded as a lower-tier anti-retroviral medication[12]. Although it is clear that DDI-induced NRH does persist for years after cessation of the medication; interruption has still been associated with an overall better prognosis[27-29].
Treatment of the clinical manifestations of NRH revolves around the primary prophylaxis of esophageal variceal bleeding in asymptomatic patients. For cases in which details were provided, we observed that in almost all asymptomatic cases of esophageal varices, primary prophylaxis with NSBB was started, and propranolol was the usual drug of choice. Many patients also underwent endoscopic band ligation (EBL), either as primary prophylaxis or after manifestation with bleeding. There were several cases of failure of NSBB or EBL, for which patients underwent transjugular intrahepatic portosystemic shunt (TIPS). At least one of these cases was documented as refractory and TIPS was done as bridge to transplant[15]. In contrast to non-HIV related NRH, ascites was not uncommonly reported in our review of HIV patients with NRH. At least 12 cases of ascites were reported, however the need for paracentesis was not reported. Ascites is infrequently reported in NRH with patients without HIV[10].
The utility of anticoagulation in patients with NRH is a burgeoning concept. Mallet et al[30] described a series of 21 patients with clinical evidence of prothrombotic state in the setting of NCPH. The median protein S level in this group was nearly half of normal. The coagulation abnormalities in patients with NRH have particular significance as suggested by Bihl et al[31], who reported a case in which the patient’s clinical course improved dramatically after initiation of anticoagulation. It appears that the benefit of anticoagulation in these patients may be multifactorial. One theory is that it slows disease progression by preventing the micro-thrombosis thought to play a pathogenic role in NRH. In addition, given the noted thrombophilias in these patients, anticoagulation prevents portal vein thrombosis, which can lead to refractory manifestations of portal hypertension and end-stage liver disease[30-32]. These findings suggest potential benefit of anticoagulation therapy and warrant further studies specifically in the context of NRH.
Patients with severe liver disease and intractable symptoms of portal hypertension should be considered for liver transplant, which has been successfully described in 7 cases of patients with HIV and NRH[13,30]. In most of these cases, the indication for liver transplant was severe and intractable symptoms portal hypertension, with or without complete portal vein thrombosis. In one case, the indication to consider transplant was hepatic encephalopathy secondary to TIPS placement[33]. All 7 patients who underwent liver transplant reportedly had excellent survival. However, it is important to note that there have been cases in which NRH reappears in the transplanted liver[34], although this has yet to be described in a patient with NRH in the setting of HIV. Regardless, for this reason, continued anticoagulant therapy even after transplant may be warranted and necessitates further investigation.
DISCUSSION
The burden of liver disease in HIV-infected persons is substantial and a comprehensive and interdisciplinary approach to management is crucial. All HIV-positive individuals should undergo regular liver function testing at least biannually as they are routinely exposed to hepatotoxic medications and are generally at increased risk for both viral and non-viral hepatitides[35]. NRH should be considered in all HIV-positive patients with unexplained signs and symptoms of portal hypertension. A thorough history of past and current antiretroviral drug regimens should be obtained as it has been shown that the effects of NRH persist long after cessation of the offending agent.
Diagnosis of NRH requires a high index of suspicion given its uncommon incidence and varied presentation of portal hypertension. Imaging techniques, such as abdominal ultrasound or computed tomography (CT) are frequently used to further evaluate patients with liver disease. Hepatic nodularity may be appreciated on liver ultrasound, and when combined with a clinical context of relatively preserved hepatic function, NRH should remain high on the differential. The hepatic nodularity on ultrasound is often followed up with CT imaging, however findings of diffuse micronodularity can also mimic cirrhosis[14,36].
NRH is one of the potential causes of non-cirrhotic portal hypertension in patients with HIV. Other potential causes of non-cirrhotic intrahepatic portal hypertension include schistosomiasis, sinusoidal obstruction syndrome, and idiopathic portal hypertension (hepatoportal sclerosis)[7,22,37]. In particular, antiretroviral therapy has been associated with both NRH and idiopathic portal hypertension. It has been hypothesized that idiopathic portal hypertension can lead to both NRH and portal vein thrombosis[2].
Accurate diagnosis of NRH requires the histological assessment[7]. Since the nodularity is often heterogeneously distributed, liver biopsy requires an ample sized tissue. The transjugular route is often used to minimize bleeding complications[38]. However, there have been cases where diagnosis was initially missed and later found only via wedge biopsy[39]. Classification of non-neoplastic, diffuse parenchymal liver disease necessitates a sample of at least 2-3 cm in length and at least a 16-gauge caliber needle[40]. The hepatocyte atrophy, a key histologic feature is best identified with reticulin staining which must be employed to make the diagnosis[41].
The pathogenesis of NRH is believe to be related to the differential of blood supply to the liver that leaves some areas ischemic, and other with compensatory hypertrophy which leads to the formation of hepatic nodules. There are multiple diseases, conditions, and medications associated with its development[7]. In contrast to non-HIV patients, patients with HIV and NRH tend to have a higher incidence of asictes. It is unclear why the incidence differs but the variance may be potentially related to the underlying cause of NRH in HIV patients being largely pharmacologic from antiretroviral drugs.
DDI which has been most strongly linked to the development of NRH, belongs to a class of medications known as nucleoside reverse transcriptase inhibitors (NRTI). It functions as a purine analogue and interferes with the transcription of DNA and RNA[27]. Azathioprine, 6-mercaptopurine 6-thioguanine and other chemotherapeutic agents that have been previously linked to NRH have a similar mechanism of action[42]. Although current knowledge suggests that NRH is not a reversible condition, several studies have shown improved outcomes with cessation of causative medications.
Treatment of NRH generally revolves around manifestations of portal hypertension. Once the diagnosis is confirmed, patients should undergo screening for esophageal varices with esophagogastroduodenoscopy (EGD)[43]. Patients with NRH should be considered for screening for HCC. Although this remains a relatively controversial topic, a possible pathogenetic relationship between NRH and HCC has been described[1,25,44-50]. The inability to conclusively establish a propensity of NRH to develop into HCC may be a result of its underestimated incidence, rather than a lack of association between the two conditions. Liver transplantation is an uncommon treatment for NRH since liver function is usually preserved. In a recently published systematic review, the authors found severe portal hypertension as the most common indication for liver transplantation[51].
The importance of NRH in HIV-infected individuals is growing, especially as patients are experiencing increased longevity and longer exposure to medications. High index of suspicion is required to make a diagnosis since there are patients with HIV who develop manifestations of portal hypertension such as ascites and variceal bleeding from cirrhotic and non-cirrhotic causes. Treatment generally revolves around treating the manifestation of portal hypertension.
COMMENTS
Background
Nodular regenerative hyperplasia (NRH) causes portal hypertension in patients with human immunodeficiency virus (HIV). Unlike cirrhosis, NRH is not associated with liver synthetic function. On imaging, NRH and cirrhosis appear similar with nodular liver contours. Patients with NRH can present with ascites and variceal bleeding.
Research frontiers
Further research in needed in the utility of non-invasive methods of making the diagnosis of NRH. Studies are essential to determine the long term consequences of NRH. More research is needed to understand the pathogenesis of NRH.
Innovations and breakthroughs
There is increasing appreciation of the epidemiology and association of NRH with HIV. There is also enhanced awareness of the association of certain antiretroviral medications such as didanosine and NRH.
Applications
The article adds to the literature the current understanding of the diagnosis, risk factors, natural history and, management of NRH.
Terminology
The diagnosis of NRH requires a liver biopsy. Liver parenchymal cells are clustered in nodules. Unlike cirrhosis, there are fibrotic bands encompassing the nodule.
Peer review
One of the most important issues raised during peer review was that there may be differences in the presentation in patients affected by NRH with and without HIV. Patients with NRH and HIV are more likely to have ascites than those patients with NRH without HIV.
Footnotes
P- Reviewers: Berzigotti A, Devarbhavi H, Reddy DN S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- 2.Chang PE, Miquel R, Blanco JL, Laguno M, Bruguera M, Abraldes JG, Bosch J, Garcia-Pagan JC. Idiopathic portal hypertension in patients with HIV infection treated with highly active antiretroviral therapy. Am J Gastroenterol. 2009;104:1707–1714. doi: 10.1038/ajg.2009.165. [DOI] [PubMed] [Google Scholar]
- 3.Shimamatsu K, Wanless IR. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343–350. doi: 10.1002/hep.510260214. [DOI] [PubMed] [Google Scholar]
- 4.Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187–192. doi: 10.1097/QAD.0b013e3280119e47. [DOI] [PubMed] [Google Scholar]
- 5.Demberg T, Robert-Guroff M. Controlling the HIV/AIDS epidemic: current status and global challenges. Front Immunol. 2012;3:250. doi: 10.3389/fimmu.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Semin Liver Dis. 2012;32:103–113. doi: 10.1055/s-0032-1316473. [DOI] [PubMed] [Google Scholar]
- 7.Hartleb M, Gutkowski K, Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400–1409. doi: 10.3748/wjg.v17.i11.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S, et al. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103–107. [PubMed] [Google Scholar]
- 9.Fernandez-Miranda C, Colina F, Delgado JM, Lopez-Carreira M. Diffuse nodular regenerative hyperplasia of the liver associated with human immunodeficiency virus and visceral leishmaniasis. Am J Gastroenterol. 1993;88:433–435. [PubMed] [Google Scholar]
- 10.Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, Stanley AJ, Campbell S. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010;22:1001–1005. doi: 10.1097/MEG.0b013e3283360021. [DOI] [PubMed] [Google Scholar]
- 11.Bissonnette J, Généreux A, Côté J, Nguyen B, Perreault P, Bouchard L, Pomier-Layrargues G. Hepatic hemodynamics in 24 patients with nodular regenerative hyperplasia and symptomatic portal hypertension. J Gastroenterol Hepatol. 2012;27:1336–1340. doi: 10.1111/j.1440-1746.2012.07168.x. [DOI] [PubMed] [Google Scholar]
- 12.Cotte L, Bénet T, Billioud C, Miailhes P, Scoazec JY, Ferry T, Brochier C, Boibieux A, Vanhems P, Chevallier M, et al. The role of nucleoside and nucleotide analogues in nodular regenerative hyperplasia in HIV-infected patients: a case control study. J Hepatol. 2011;54:489–496. doi: 10.1016/j.jhep.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Tateo M, Sebagh M, Bralet MP, Teicher E, Azoulay D, Mallet V, Pol S, Castaing D, Samuel D, Duclos-Vallée JC. A new indication for liver transplantation: nodular regenerative hyperplasia in human immunodeficiency virus-infected patients. Liver Transpl. 2008;14:1194–1198. doi: 10.1002/lt.21493. [DOI] [PubMed] [Google Scholar]
- 14.Jha P, Poder L, Wang ZJ, Westphalen AC, Yeh BM, Coakley FV. Radiologic mimics of cirrhosis. AJR Am J Roentgenol. 2010;194:993–999. doi: 10.2214/AJR.09.3409. [DOI] [PubMed] [Google Scholar]
- 15.Cachay ER, Peterson MR, Goicoechea M, Mathews WC. Didanosine Exposure and Noncirrhotic Portal Hypertension in a HIV Clinic in North America: a Follow-up Study. Br J Med Med Res. 2011;1:346–355. doi: 10.9734/bjmmr/2011/554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auerbach E, Aboulafia DM. Venous and arterial thromboembolic complications associated with HIV infection and highly active antiretroviral therapy. Semin Thromb Hemost. 2012;38:830–838. doi: 10.1055/s-0032-1328887. [DOI] [PubMed] [Google Scholar]
- 17.Vispo E, Moreno A, Maida I, Barreiro P, Cuevas A, Albertos S, Soriano V. Noncirrhotic portal hypertension in HIV-infected patients: unique clinical and pathological findings. AIDS. 2010;24:1171–1176. doi: 10.1097/QAD.0b013e3283389e26. [DOI] [PubMed] [Google Scholar]
- 18.Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, Barreiro P, Mura MS, Babudieri S, et al. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177–182. doi: 10.1097/01.qai.0000221683.44940.62. [DOI] [PubMed] [Google Scholar]
- 19.Garvey LJ, Thomson EC, Lloyd J, Cooke GS, Goldin RD, Main J. Response to Mallet et al., ‘Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients’. AIDS. 2007;21:1494–1495. doi: 10.1097/QAD.0b013e3281e7ed64. [DOI] [PubMed] [Google Scholar]
- 20.Sandrine PF, Sylvie A, André E, Abdoulaye D, Bernard L, André C. Nodular regenerative hyperplasia: a new serious antiretroviral drugs side effect? AIDS. 2007;21:1498–1499. doi: 10.1097/QAD.0b013e328235a54c. [DOI] [PubMed] [Google Scholar]
- 21.Arey B, Markov M, Ravi J, Prevette E, Batts K, Nadir A. Nodular regenerative hyperplasia of liver as a consequence of ART. AIDS. 2007;21:1066–1068. doi: 10.1097/QAD.0b013e3280fa81cb. [DOI] [PubMed] [Google Scholar]
- 22.Schiano TD, Uriel A, Dieterich DT, Fiel MI. The development of hepatoportal sclerosis and portal hypertension due to didanosine use in HIV. Virchows Arch. 2011;458:231–235. doi: 10.1007/s00428-010-1004-7. [DOI] [PubMed] [Google Scholar]
- 23.Kovari H, Ledergerber B, Peter U, Flepp M, Jost J, Schmid P, Calmy A, Mueller NJ, Muellhaupt B, Weber R. Association of noncirrhotic portal hypertension in HIV-infected persons and antiretroviral therapy with didanosine: a nested case-control study. Clin Infect Dis. 2009;49:626–635. doi: 10.1086/603559. [DOI] [PubMed] [Google Scholar]
- 24.Arnott ID, Ghosh S. Portal hypertension in the presence of minimal liver damage in Crohn’s disease on long-term azathioprine: possible endothelial cell injury. Eur J Gastroenterol Hepatol. 2000;12:569–573. doi: 10.1097/00042737-200012050-00016. [DOI] [PubMed] [Google Scholar]
- 25.Russmann S, Zimmermann A, Krähenbühl S, Kern B, Reichen J. Veno-occlusive disease, nodular regenerative hyperplasia and hepatocellular carcinoma after azathioprine treatment in a patient with ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:287–290. doi: 10.1097/00042737-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Seiderer J, Zech CJ, Diebold J, Schoenberg SO, Brand S, Tillack C, Göke B, Ochsenkühn T. Nodular regenerative hyperplasia: a reversible entity associated with azathioprine therapy. Eur J Gastroenterol Hepatol. 2006;18:553–555. doi: 10.1097/00042737-200605000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Hofmaenner D, Kovari H, Weber A, Weishaupt D, Speck RF. Nodular regenerative hyperplasia of the liver associated with didanosine persists for years even after its interruption. BMJ Case Rep. 2011;2011:pii: bcr0320113928. doi: 10.1136/bcr.03.2011.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santiago I, Vilgrain V, Cipriano MA, Oliveira C, Ferreira M, Reis D. Case 183: Obliterative portal venopathy. Radiology. 2012;264:297–302. doi: 10.1148/radiol.12110585. [DOI] [PubMed] [Google Scholar]
- 29.Vispo E, Morello J, Rodriguez-Novoa S, Soriano V. Noncirrhotic portal hypertension in HIV infection. Curr Opin Infect Dis. 2011;24:12–18. doi: 10.1097/QCO.0b013e3283420f08. [DOI] [PubMed] [Google Scholar]
- 30.Mallet VO, Varthaman A, Lasne D, Viard JP, Gouya H, Borgel D, Lacroix-Desmazes S, Pol S. Acquired protein S deficiency leads to obliterative portal venopathy and to compensatory nodular regenerative hyperplasia in HIV-infected patients. AIDS. 2009;23:1511–1518. doi: 10.1097/QAD.0b013e32832bfa51. [DOI] [PubMed] [Google Scholar]
- 31.Bihl F, Janssens F, Boehlen F, Rubbia-Brandt L, Hadengue A, Spahr L. Anticoagulant therapy for nodular regenerative hyperplasia in a HIV-infected patient. BMC Gastroenterol. 2010;10:6. doi: 10.1186/1471-230X-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012;56 Suppl 1:S25–S38. doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez Díaz H, Mariño Callejo A, García Rodríguez JF. Non-cirrhotic portal hypertension in human immunodeficiency virus-infected patients: a new challenge in antiretroviral therapy era. Open AIDS J. 2011;5:59–61. doi: 10.2174/1874613601105010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devarbhavi H, Abraham S, Kamath PS. Significance of nodular regenerative hyperplasia occurring de novo following liver transplantation. Liver Transpl. 2007;13:1552–1556. doi: 10.1002/lt.21142. [DOI] [PubMed] [Google Scholar]
- 35.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available from: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 36.Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology. 1997;202:1–16. doi: 10.1148/radiology.202.1.8988182. [DOI] [PubMed] [Google Scholar]
- 37.Jackson BD, Doyle JS, Hoy JF, Roberts SK, Colman J, Hellard ME, Sasadeusz JJ, Iser DM. Non-cirrhotic portal hypertension in HIV mono-infected patients. J Gastroenterol Hepatol. 2012;27:1512–1519. doi: 10.1111/j.1440-1746.2012.07148.x. [DOI] [PubMed] [Google Scholar]
- 38.Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol. 2004;75:225–230. doi: 10.1002/ajh.20024. [DOI] [PubMed] [Google Scholar]
- 39.Louwers LM, Bortman J, Koffron A, Stecevic V, Cohn S, Raofi V. Noncirrhotic Portal Hypertension due to Nodular Regenerative Hyperplasia Treated with Surgical Portacaval Shunt. Case Rep Med. 2012;2012:965304. doi: 10.1155/2012/965304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 41.Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7–14. doi: 10.1002/hep.21258. [DOI] [PubMed] [Google Scholar]
- 42.Núñez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology. 2010;52:1143–1155. doi: 10.1002/hep.23716. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 44.Nzeako UC, Goodman ZD, Ishak KG. Hepatocellular carcinoma and nodular regenerative hyperplasia: possible pathogenetic relationship. Am J Gastroenterol. 1996;91:879–884. [PubMed] [Google Scholar]
- 45.Søgaard PE. Nodular transformation of the liver, alpha-fetoprotein, and hepatocellular carcinoma. Hum Pathol. 1981;12:1052. doi: 10.1016/s0046-8177(81)80268-7. [DOI] [PubMed] [Google Scholar]
- 46.Stromeyer FW, Ishak KG. Nodular transformation (nodular “regenerative” hyperplasia) of the liver. A clinicopathologic study of 30 cases. Hum Pathol. 1981;12:60–71. doi: 10.1016/s0046-8177(81)80242-0. [DOI] [PubMed] [Google Scholar]
- 47.Colina F, Alberti N, Solis JA, Martinez-Tello FJ. Diffuse nodular regenerative hyperplasia of the liver (DNRH). A clinicopathologic study of 24 cases. Liver. 1989;9:253–265. doi: 10.1111/j.1600-0676.1989.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi S, Saito K, Nakanuma Y. Nodular regenerative hyperplasia of the liver in hepatocellular carcinoma. An autopsy study. J Clin Gastroenterol. 1993;16:155–159. doi: 10.1097/00004836-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki M, Hodo Y, Nakanuma Y. Hepatocellular carcinoma arising in primary biliary cirrhosis presenting with nodular regenerative hyperplasia: report of an autopsy case. J Gastroenterol Hepatol. 2006;21:1866–1868. doi: 10.1111/j.1440-1746.2006.04289.x. [DOI] [PubMed] [Google Scholar]
- 50.Kataoka TR, Tsukamoto Y, Kanazawa N, Izumi T, Awata N, Nishizawa Y, Ohsawa M, Ishiguro S. Concomitant hepatocellular carcinoma and non-Hodgkin’s lymphoma in a patient with nodular regenerative hyperplasia. Pathol Int. 2006;56:279–282. doi: 10.1111/j.1440-1827.2006.01956.x. [DOI] [PubMed] [Google Scholar]
- 51.Manzia TM, Gravante G, Di Paolo D, Orlando G, Toti L, Bellini MI, Ciano P, Angelico M, Tisone G. Liver transplantation for the treatment of nodular regenerative hyperplasia. Dig Liver Dis. 2011;43:929–934. doi: 10.1016/j.dld.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, Galambos MR, Rubin RA. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol. 2008;6:1167–1169. doi: 10.1016/j.cgh.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 53.Cesari M, Schiavini M, Marchetti G, Caramma I, Ortu M, Franzetti F, Galli M, Antinori S, Milazzo L. Noncirrhotic portal hypertension in HIV-infected patients: a case control evaluation and review of the literature. AIDS Patient Care STDS. 2010;24:697–703. doi: 10.1089/apc.2010.0160. [DOI] [PubMed] [Google Scholar]
- 54.Ding A, Lee A, Callender M, Loughrey M, Quah SP, Dinsmore WW. Hepatic encephalopathy as an unusual late complication of transjugular intrahepatic portosystemic shunt insertion for non-cirrhotic portal hypertension caused by nodular regenerative hyperplasia in an HIV-positive patient on highly active antiretroviral therapy. Int J STD AIDS. 2010;21:71–72. doi: 10.1258/ijsa.2009.009038. [DOI] [PubMed] [Google Scholar]
- 55.Dinh MH, Stosor V, Rao SM, Miller FH, Green RM. Cryptogenic liver disease in HIV-seropositive men. HIV Med. 2009;10:447–453. doi: 10.1111/j.1468-1293.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 56.Kochin I, Magid M, Arnon R, Glasscock A, Kerkar N, Miloh T. Variceal bleeding in an adolescent with HIV diagnosed with hepatoportal sclerosis and nodular regenerative hyperplasia. J Pediatr Gastroenterol Nutr. 2010;50:340–343. doi: 10.1097/MPG.0b013e3181a70f63. [DOI] [PubMed] [Google Scholar]
- 57.Mallet V, Pol S. [A puzzling case of portal hypertension in a patient with human immunodeficiency and hepatitis C virus co-infection] Gastroenterol Clin Biol. 2007;31:878–880. doi: 10.1016/s0399-8320(07)73984-2. [DOI] [PubMed] [Google Scholar]
- 58.Mendizabal M, Craviotto S, Chen T, Silva MO, Reddy KR. Noncirrhotic portal hypertension: another cause of liver disease in HIV patients. Ann Hepatol. 2009;8:390–395. [PubMed] [Google Scholar]
- 59.Podevin P, Spiridon G, Terris B, Chauvelot-Moachon L, Guillevin L, Chaussade S, Sogni P, Salmon-Ceron D. Nodular regenerative hyperplasia of the liver after IL-2 therapy in an HIV-infected patient. AIDS. 2006;20:313–315. doi: 10.1097/01.aids.0000198084.62701.dc. [DOI] [PubMed] [Google Scholar]
- 60.Scourfield A, Waters L, Holmes P, Panos G, Randell P, Jackson A, Mandalia S, Gazzard B, Nelson M. Non-cirrhotic portal hypertension in HIV-infected individuals. Int J STD AIDS. 2011;22:324–328. doi: 10.1258/ijsa.2010.010396. [DOI] [PubMed] [Google Scholar]
- 61.Schouten JN, Verheij J, Janssen HL. [Non-cirrhotic portal hypertension: rare cause of upper gastrointestinal bleeding] Ned Tijdschr Geneeskd. 2010;154:A1276. [PubMed] [Google Scholar]
- 62.Stebbing J, Wong N, Tan L, Scourfield A, Jiao LR, Shousha S, Grover D, Bower M, Nelson M. The relationship between prolonged antiretroviral therapy and cryptogenic liver disease. J Acquir Immune Defic Syndr. 2009;50:554–556. doi: 10.1097/QAI.0b013e31819c338f. [DOI] [PubMed] [Google Scholar]


