Abstract
Sialoadhesin is a macrophage-restricted adhesion molecule of 185 kDa that mediates sialic acid-dependent binding to cells. It is expressed strongly by macrophages in lymphoid and haemopoietic tissues where it is likely to mediate cell-cell interactions. Here we report the molecular cloning of murine sialoadhesin and show that it is a new member of the immunoglobulin (Ig) superfamily with 17 Ig-like domains. COS cells transfected with a cDNA encoding full-length sialoadhesin bound mouse bone marrow cells in a sialic acid-dependent manner. Alternatively spliced cDNAs, predicting soluble forms of sialoadhesin containing the first three or 16 Ig-like domains of sialoadhesin, were expressed in COS cells and the respective proteins purified. When immobilized on plastic, the 16-domain form bound cells in a sialic acid-dependent manner, suggesting that sialoadhesin can function in both secreted and membrane-bound forms. The most similar proteins in the database were CD22, myelin-associated glycoprotein, Schwann cell myelin protein and CD33. Like sialoadhesin, CD22 mediates sialic acid-dependent cell adhesion. The sequence similarity of sialoadhesin to CD22 and related members of the Ig superfamily indicates the existence of a novel family of sialic acid binding proteins involved in cell-cell interactions.
Full text
PDF




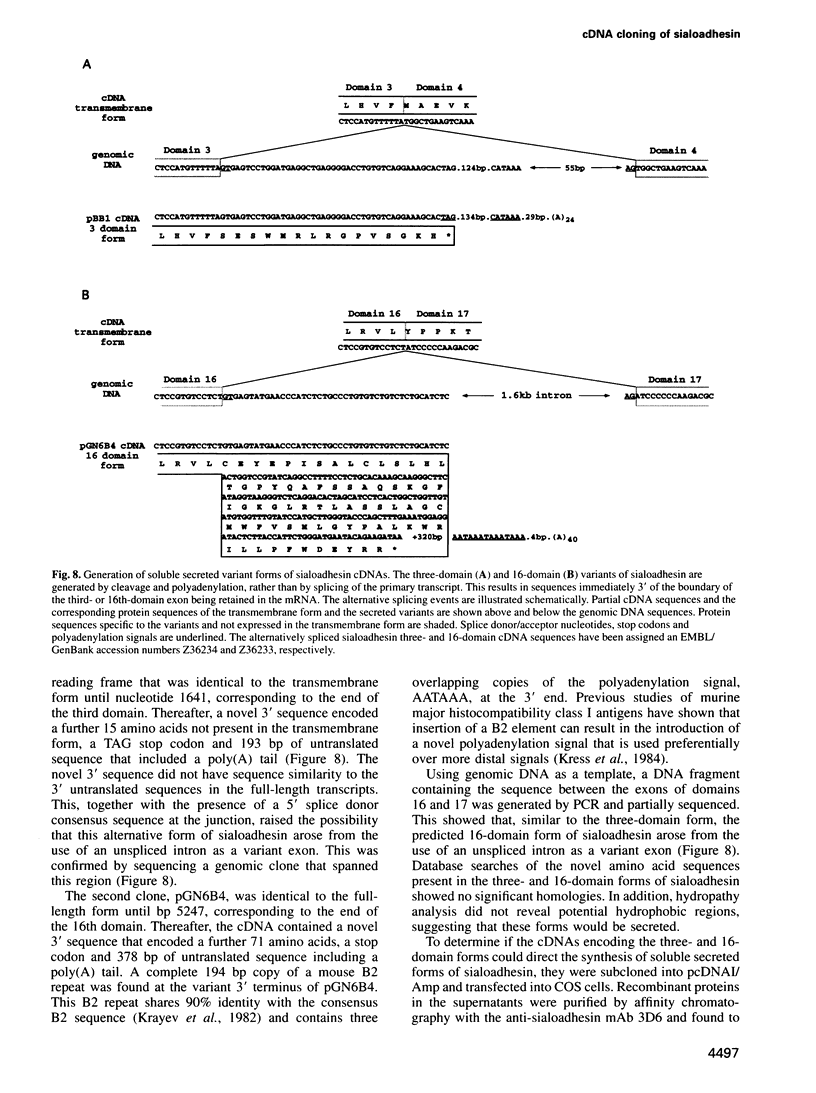
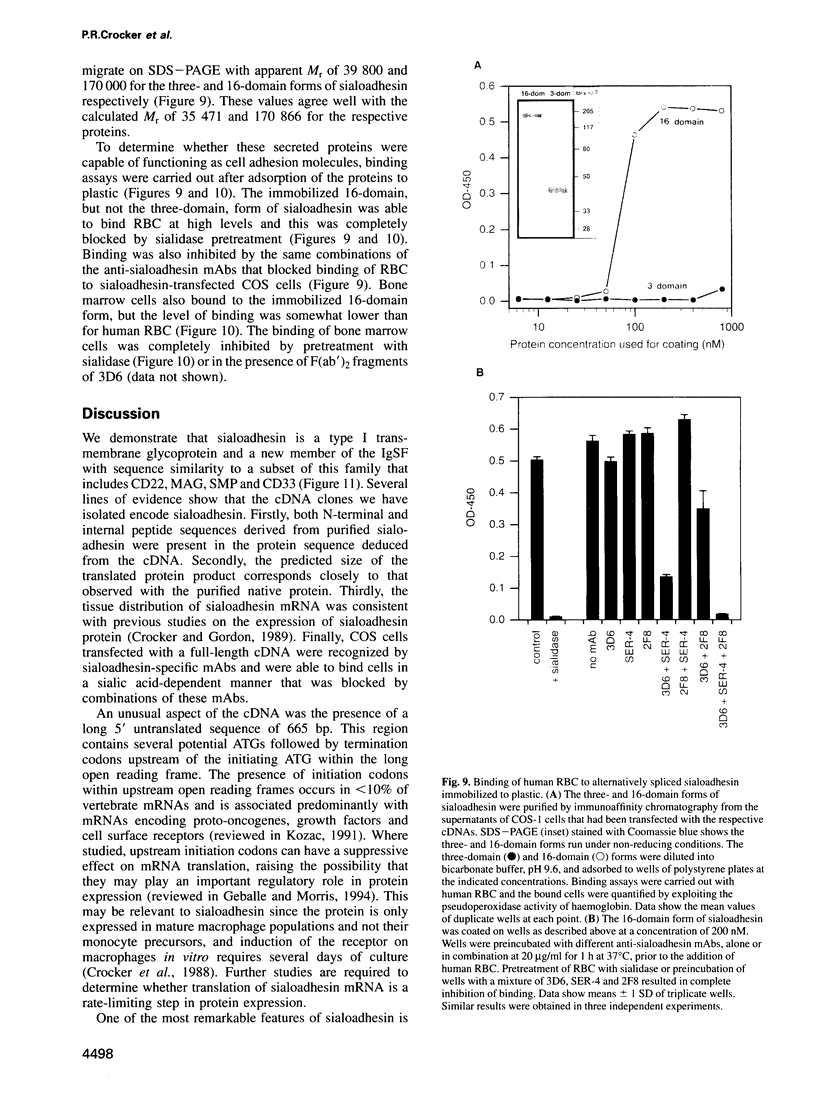





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alzari P. M., Lascombe M. B., Poljak R. J. Three-dimensional structure of antibodies. Annu Rev Immunol. 1988;6:555–580. doi: 10.1146/annurev.iy.06.040188.003011. [DOI] [PubMed] [Google Scholar]
- Barton D. E., Arquint M., Roder J., Dunn R., Francke U. The myelin-associated glycoprotein gene: mapping to human chromosome 19 and mouse chromosome 7 and expression in quivering mice. Genomics. 1987 Oct;1(2):107–112. doi: 10.1016/0888-7543(87)90002-4. [DOI] [PubMed] [Google Scholar]
- Baumheter S., Singer M. S., Henzel W., Hemmerich S., Renz M., Rosen S. D., Lasky L. A. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993 Oct 15;262(5132):436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Berg E. L., McEvoy L. M., Berlin C., Bargatze R. F., Butcher E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993 Dec 16;366(6456):695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Braesch-Andersen S., Stamenkovic I. Sialylation of the B lymphocyte molecule CD22 by alpha 2,6-sialyltransferase is implicated in the regulation of CD22-mediated adhesion. J Biol Chem. 1994 Apr 22;269(16):11783–11786. [PubMed] [Google Scholar]
- Brandriff B. F., Gordon L. A., Tynan K. T., Olsen A. S., Mohrenweiser H. W., Fertitta A., Carrano A. V., Trask B. J. Order and genomic distances among members of the carcinoembryonic antigen (CEA) gene family determined by fluorescence in situ hybridization. Genomics. 1992 Apr;12(4):773–779. doi: 10.1016/0888-7543(92)90308-f. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985 Sep 1;162(3):993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986 Dec 1;164(6):1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Hill M., Gordon S. Regulation of a murine macrophage haemagglutinin (sheep erythrocyte receptor) by a species-restricted serum factor. Immunology. 1988 Dec;65(4):515–522. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Kelm S., Dubois C., Martin B., McWilliam A. S., Shotton D. M., Paulson J. C., Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991 Jul;10(7):1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Werb Z., Gordon S., Bainton D. F. Ultrastructural localization of a macrophage-restricted sialic acid binding hemagglutinin, SER, in macrophage-hematopoietic cell clusters. Blood. 1990 Sep 15;76(6):1131–1138. [PubMed] [Google Scholar]
- Dulac C., Cameron-Curry P., Ziller C., Le Douarin N. M. A surface protein expressed by avian myelinating and nonmyelinating Schwann cells but not by satellite or enteric glial cells. Neuron. 1988 May;1(3):211–220. doi: 10.1016/0896-6273(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Dulac C., Tropak M. B., Cameron-Curry P., Rossier J., Marshak D. R., Roder J., Le Douarin N. M. Molecular characterization of the Schwann cell myelin protein, SMP: structural similarities within the immunoglobulin superfamily. Neuron. 1992 Feb;8(2):323–334. doi: 10.1016/0896-6273(92)90298-r. [DOI] [PubMed] [Google Scholar]
- Dörken B., Moldenhauer G., Pezzutto A., Schwartz R., Feller A., Kiesel S., Nadler L. M. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986 Jun 15;136(12):4470–4479. [PubMed] [Google Scholar]
- Engel P., Nojima Y., Rothstein D., Zhou L. J., Wilson G. L., Kehrl J. H., Tedder T. F. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993 Jun 1;150(11):4719–4732. [PubMed] [Google Scholar]
- Fujita N., Sato S., Kurihara T., Kuwano R., Sakimura K., Inuzuka T., Takahashi Y., Miyatake T. cDNA cloning of mouse myelin-associated glycoprotein: a novel alternative splicing pattern. Biochem Biophys Res Commun. 1989 Dec 29;165(3):1162–1169. doi: 10.1016/0006-291x(89)92724-1. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Morris D. R. Initiation codons within 5'-leaders of mRNAs as regulators of translation. Trends Biochem Sci. 1994 Apr;19(4):159–164. doi: 10.1016/0968-0004(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W. N., Teglund S., Bremer K., Hammarström S. The pregnancy-specific glycoprotein family of the immunoglobulin superfamily: identification of new members and estimation of family size. Genomics. 1992 Apr;12(4):780–787. doi: 10.1016/0888-7543(92)90309-g. [DOI] [PubMed] [Google Scholar]
- Kimber S. J. Glycoconjugates and cell surface interactions in pre- and peri-implantation mammalian embryonic development. Int Rev Cytol. 1990;120:53–167. doi: 10.1016/s0074-7696(08)61599-5. [DOI] [PubMed] [Google Scholar]
- Kincade P. W. Molecular interactions between stromal cells and B lymphocyte precursors. Semin Immunol. 1991 Nov;3(6):379–390. [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Barra Y., Seidman J. G., Khoury G., Jay G. Functional insertion of an Alu type 2 (B2 SINE) repetitive sequence in murine class I genes. Science. 1984 Nov 23;226(4677):974–977. doi: 10.1126/science.6095445. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. A 'roll' in acute inflammation. Curr Biol. 1993 Oct 1;3(10):680–682. doi: 10.1016/0960-9822(93)90067-x. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Law C. L., Torres R. M., Sundberg H. A., Parkhouse R. M., Brannan C. I., Copeland N. G., Jenkins N. A., Clark E. A. Organization of the murine Cd22 locus. Mapping to chromosome 7 and characterization of two alleles. J Immunol. 1993 Jul 1;151(1):175–187. [PubMed] [Google Scholar]
- Leprince C., Draves K. E., Geahlen R. L., Ledbetter J. A., Clark E. A. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W. Blood cell cytoadhesion molecules. Exp Hematol. 1992 Mar;20(3):288–301. [PubMed] [Google Scholar]
- McEver R. P. Selectins. Curr Opin Immunol. 1994 Feb;6(1):75–84. doi: 10.1016/0952-7915(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Morris L., Crocker P. R., Fraser I., Hill M., Gordon S. Expression of a divalent cation-dependent erythroblast adhesion receptor by stromal macrophages from murine bone marrow. J Cell Sci. 1991 May;99(Pt 1):141–147. doi: 10.1242/jcs.99.1.141. [DOI] [PubMed] [Google Scholar]
- Morris L., Crocker P. R., Gordon S. Murine fetal liver macrophages bind developing erythroblasts by a divalent cation-dependent hemagglutinin. J Cell Biol. 1988 Mar;106(3):649–656. doi: 10.1083/jcb.106.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Strubel N. A., Bischoff J. A role for sialyl Lewis-X/A glycoconjugates in capillary morphogenesis. Nature. 1993 Sep 16;365(6443):267–269. doi: 10.1038/365267a0. [DOI] [PubMed] [Google Scholar]
- Noonan D. M., Fulle A., Valente P., Cai S., Horigan E., Sasaki M., Yamada Y., Hassell J. R. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991 Dec 5;266(34):22939–22947. [PubMed] [Google Scholar]
- Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993 Jun 15;268(17):12764–12774. [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Arakawa F., Matsuoka Y., Kosaki G., Nakazato H. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. 1991 May 5;266(13):7995–8001. [PubMed] [Google Scholar]
- Peaker C. J., Neuberger M. S. Association of CD22 with the B cell antigen receptor. Eur J Immunol. 1993 Jun;23(6):1358–1363. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- Pedraza L., Owens G. C., Green L. A., Salzer J. L. The myelin-associated glycoproteins: membrane disposition, evidence of a novel disulfide linkage between immunoglobulin-like domains, and posttranslational palmitylation. J Cell Biol. 1990 Dec;111(6 Pt 1):2651–2661. doi: 10.1083/jcb.111.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper S. C., Ashmun R. A., Look A. T. Molecular cloning, expression, and chromosomal localization of a human gene encoding the CD33 myeloid differentiation antigen. Blood. 1988 Jul;72(1):314–321. [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak M., Sadoul R., Keilhauer G., Landa C., Fahrig T., Schachner M. Myelin-associated glycoprotein, a member of the L2/HNK-1 family of neural cell adhesion molecules, is involved in neuron-oligodendrocyte and oligodendrocyte-oligodendrocyte interaction. J Cell Biol. 1987 Oct;105(4):1893–1899. doi: 10.1083/jcb.105.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. D., Sgroi D., Sjoberg E. R., Stamenkovic I., Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993 Apr 5;268(10):7019–7027. [PubMed] [Google Scholar]
- Powell L. D., Varki A. The oligosaccharide binding specificities of CD22 beta, a sialic acid-specific lectin of B cells. J Biol Chem. 1994 Apr 8;269(14):10628–10636. [PubMed] [Google Scholar]
- Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- Rosen S. D. Robert Feulgen Lecture 1993. L-selectin and its biological ligands. Histochemistry. 1993 Sep;100(3):185–191. doi: 10.1007/BF00269091. [DOI] [PubMed] [Google Scholar]
- Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993 Dec 17;75(6):1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Sgroi D., Varki A., Braesch-Andersen S., Stamenkovic I. CD22, a B cell-specific immunoglobulin superfamily member, is a sialic acid-binding lectin. J Biol Chem. 1993 Apr 5;268(10):7011–7018. [PubMed] [Google Scholar]
- Simmons D., Seed B. Isolation of a cDNA encoding CD33, a differentiation antigen of myeloid progenitor cells. J Immunol. 1988 Oct 15;141(8):2797–2800. [PubMed] [Google Scholar]
- Stamenkovic I., Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990 May 3;345(6270):74–77. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Quarles R. H., Itoyama Y., Webster H. D. Myelin-associated glycoprotein demonstrated immunocytochemically in myelin and myelin-forming cells of developing rat. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1510–1514. doi: 10.1073/pnas.76.3.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H., Sato S., Yagi T., Aizawa S., Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994 Feb 10;367(6463):572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Role of carbohydrates in receptor-mediated fertilization in mammals. Ciba Found Symp. 1989;145:135–155. doi: 10.1002/9780470513828.ch9. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Davis S. J., He Q., Barclay A. N. Structural diversity in domains of the immunoglobulin superfamily. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):637–647. doi: 10.1101/sqb.1989.054.01.075. [DOI] [PubMed] [Google Scholar]
- Wilson G. L., Fox C. H., Fauci A. S., Kehrl J. H. cDNA cloning of the B cell membrane protein CD22: a mediator of B-B cell interactions. J Exp Med. 1991 Jan 1;173(1):137–146. doi: 10.1084/jem.173.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. L., Najfeld V., Kozlow E., Menniger J., Ward D., Kehrl J. H. Genomic structure and chromosomal mapping of the human CD22 gene. J Immunol. 1993 Jun 1;150(11):5013–5024. [PubMed] [Google Scholar]
- Zetter B. R. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993 Aug;4(4):219–229. [PubMed] [Google Scholar]
- van den Berg T. K., Brevé J. J., Damoiseaux J. G., Döpp E. A., Kelm S., Crocker P. R., Dijkstra C. D., Kraal G. Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J Exp Med. 1992 Sep 1;176(3):647–655. doi: 10.1084/jem.176.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P. A., Brown M. H., Davis S. J., Barclay A. N. Affinity and kinetic analysis of the interaction of the cell adhesion molecules rat CD2 and CD48. EMBO J. 1993 Dec 15;12(13):4945–4954. doi: 10.1002/j.1460-2075.1993.tb06188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]