Abstract
Sexual selection is responsible for the evolution of male ornaments and armaments, but its role in the evolution of cognition—the ability to process, retain and use information—is largely unexplored. Because successful courtship is likely to involve processing information in complex, competitive sexual environments, we hypothesized that sexual selection contributes to the evolution and maintenance of cognitive abilities in males. To test this, we removed mate choice and mate competition from experimental populations of Drosophila melanogaster by enforcing monogamy for over 100 generations. Males evolved under monogamy became less proficient than polygamous control males at relatively complex cognitive tasks. When faced with one receptive and several unreceptive females, polygamous males quickly focused on receptive females, whereas monogamous males continued to direct substantial courtship effort towards unreceptive females. As a result, monogamous males were less successful in this complex setting, despite being as quick to mate as their polygamous counterparts with only one receptive female. This diminished ability to use past information was not limited to the courtship context: monogamous males (but not females) also showed reduced aversive olfactory learning ability. Our results provide direct experimental evidence that the intensity of sexual selection is an important factor in the evolution of male cognitive ability.
Keywords: sexual selection, cognition, learning, experimental evolution, courtship, Drosophila
1. Introduction
Sexual selection is usually not considered a major force driving the evolution of cognition, in particular in animals with stereotyped, genetically determined courtship [1]. Yet males of many species are faced with a complex and competitive sexual environment containing both male competitors and females of varying quality and receptivity. For example, in Drosophila mating takes place in aggregations on food sources, where flies also feed and lay eggs. Females of several species are often present; only a fraction of conspecific females are receptive at any time, and these receptive females are often greatly outnumbered by males searching for mating opportunities. The ability to locate and focus courtship efforts on receptive and fertile conspecific females is thus a crucial determinant of male reproductive success. These abilities involve processing complex sensory information and are known to rely in part on learning [2–6]. We therefore hypothesized that sexual selection contributes to the maintenance of such cognitive abilities in males.
To test this hypothesis, we imposed strict monogamy on three replicate populations of the fruitfly Drosophila melanogaster for over 100 generations by randomly pairing males and females, thus eliminating all modalities of sexual selection, including competition for mates, mate choice and interlocus sexual conflict. Three polygamous control populations, which experienced sexual selection, were maintained in parallel. Any adaptations in males that aid competitive mating success, including the ability to differentiate between receptive and unreceptive females or persuade females to mate, should be advantageous under polygamy but mostly irrelevant under this monogamy regime. Therefore, if cognitive abilities that contribute to sexual success carry any cost they should decline under monogamy owing to the action of natural selection. Even without costs, a decline is expected due to mutation accumulation and genetic drift. Consistent with these predictions, we show that cognitive performance of males from monogamous populations is reduced relative to polygamous control males, in both sexual and non-sexual contexts. This rapid evolutionary decay points to a fundamental role of sexual selection in the maintenance of cognitive performance.
2. Results
We first determined whether males from monogamous populations have reduced competitive reproductive success relative to polygamous males. Groups of five sexually naive males from either a monogamous or a polygamous population were allowed to compete with five ebony males for mating opportunities with five ebony females. The ebony flies used in our experiment come from an independent population with an uncontrolled polygamous mating system. Because these flies have dark coloration caused by a recessive allele, any wild-type progeny produced by ebony females in this assay must be sired by males from the focal experimental populations. In this competitive setting, males from monogamous populations had greatly reduced reproductive success relative to polygamous males (figure 1; F1,4 = 25.10, p < 0.01).
Figure 1.
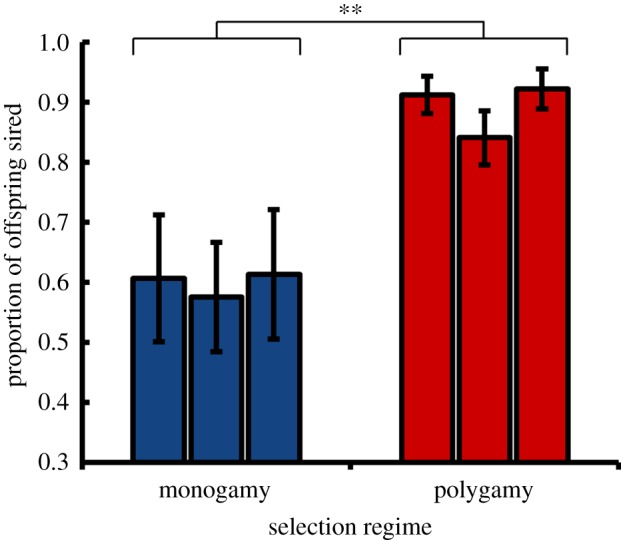
Competitive reproductive success for evolved males. The proportion of offspring (±s.e.) that were phenotypically wild-type, and therefore sired by males from the evolved populations, when males were placed in competition with ebony males for ebony females (n = 22–30 vials per population). (Online version in colour.)
This difference in male sexual success might result from females being more reluctant to mate with monogamous males (e.g. because the males are less attractive or court less vigorously). However, when individual males were allowed to court and mate with a single receptive female, there was no difference between selection regimes in the time to copulation (figure 2; t4 = 0.06, p = 0.95). While this does not necessarily mean that females would exhibit no preference in a choice situation [7], it does indicate no gross difference in male attractiveness to females. Further, males from the two selection regimes did not differ in their locomotor activity in two assays (climbing response to shock: electronic supplementary material, figure S1a; F1,4 = 0.44, p = 0.54; overall locomotion: electronic supplementary material, figure S1b; F1,4 = 0.95, p = 0.38), indicating that monogamous males are not less active or mobile than polygamous males.
Figure 2.
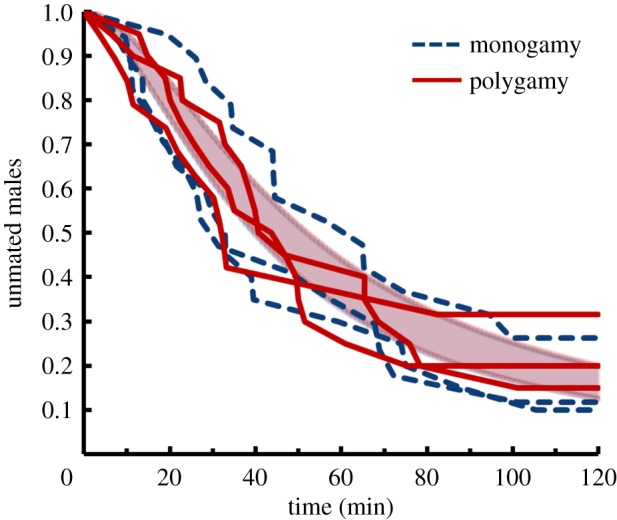
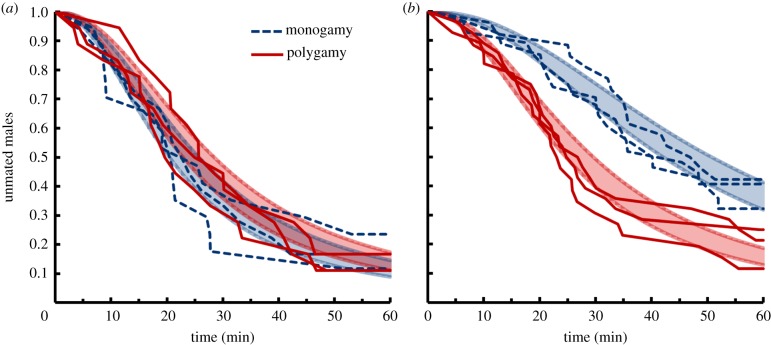
Latency to copulation for males placed with one virgin, receptive female. The proportion of males that have not mated over a 2 h time course; monogamous and polygamous populations are depicted in dashed blue and solid red, respectively, along with overlapping fitted curves and error bands (±s.e.) for each selection regime. (Online version in colour.)
We thus hypothesized that the lower competitive reproductive success of monogamous males is caused in part by the challenge presented by the presence of multiple females of varying levels of receptivity. To test this, we quantified time to copulation of single males faced with one receptive (virgin) female accompanied by either one or five unreceptive, previously mated females. Males from both selection regimes took longer to achieve mating when five rather than one unreceptive females were present (figure 3a,b; t4 = 4.67, p < 0.01). This indicated that the presence of multiple unreceptive females interfered with male success with a receptive female. Furthermore, this interference had a greater effect on males from monogamous populations than it did on males from polygamous populations. Whereas both types of males achieved copulation equally rapidly when only one unreceptive female was present (figure 3a; selection regime effect: t4 = 0.68, p = 0.53), monogamous males were slower than polygamous males when five unreceptive females were present (figure 3b; selection regime effect: t4 = −4.02, p = 0.02). This effect was large—the median monogamous male took 19 min (75%) longer to achieve copulation than the median polygamous male.
Figure 3.
Latency to copulation for males faced with multiple females. The proportion of males that have not mated over a 1 h time course when the environment consists of (a) one receptive and one unreceptive female (n = 17–18 males per population) or (b) one receptive and five unreceptive females (n = 26–28 males per population). The monogamous and polygamous populations are depicted in dashed blue and solid red, respectively, along with fitted curves and error bands (±s.e.) for each selection regime. (Online version in colour.)
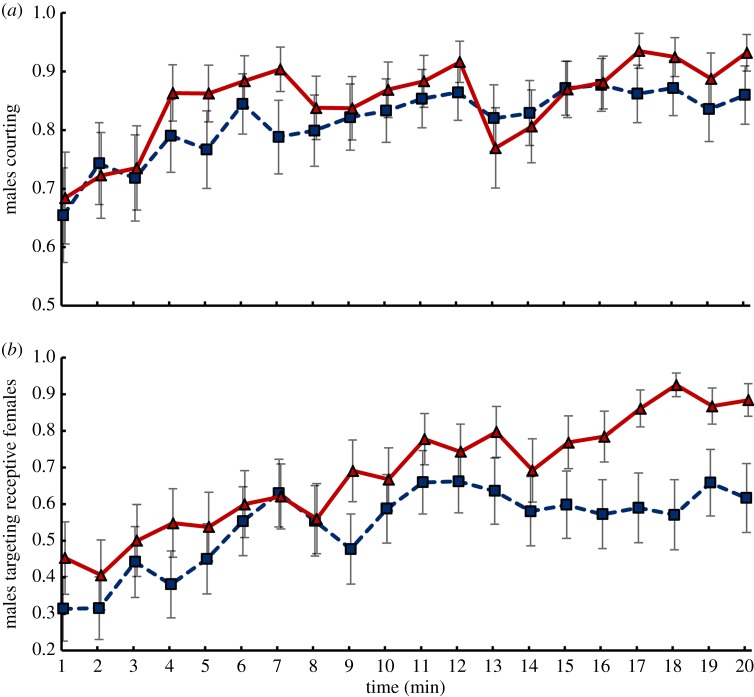
In order to shed light on the mechanism behind this difference, we again confronted single males with one receptive female and five unreceptive females, and observed their behaviour during the first 20 min of interaction. Every minute we recorded whether or not the male was courting and, if so, whether the courtship was directed at the receptive female. Males from both selection regimes courted more as time passed (figure 4a; time effect: F1,181 = 57.75, p < 0.0001), but selection regime did not affect overall courtship intensity (selection regime effect: F1,4 = 0.35, p = 0.59; selection regime × time interaction: F1,181 = 0.20, p = 0.66). However, even though over time males from both selection regimes increasingly focused their courtship effort on the receptive female (figure 4b; monogamous: F1,91 = 12.06, p < 0.001; polygamous: F1,90 = 55.95, p < 0.0001), this improvement in focus was more pronounced in polygamous males than monogamous males (selection regime × time interaction: F1,181 = 8.30, p < 0.01). By the end of the 20 min observational period, 88% of polygamous males were courting the receptive female versus only 62% of monogamous males.
Figure 4.
Courtship intensity and targeting. The proportion of males that are (a) actively courting and, if courting, (b) targeting the receptive female over a 20 min time course when the environment consists of one receptive and five unreceptive females (n = 29–32 males per population). Means (±s.e.) are depicted for monogamous (blue squares) and polygamous (red triangles) selection regimes. (Online version in colour.)
The increasing focus of courtship activity on the receptive female indicates that the ability to discriminate between receptive and unreceptive females improves with experience. This is consistent with previous research, which has shown that male ability to discriminate against unreceptive females relies in part on associative learning, whereby olfactory cues emitted by unreceptive females are associated with failed courtship [3,8–10]. Does the poorer focus of courtship on the receptive female shown by the monogamous males reflect their poorer olfactory learning, and if so, does the difference extend to non-sexual contexts? We addressed this question with a Pavlovian conditioning assay [11] that challenged groups of flies to form an association between an odour and aversive mechanical shock. Because this assay could be applied to flies of either sex, it also allowed us to test if differences in learning ability between the monogamous and polygamous populations are specific to males or extend to females. Same-sex groups of flies were exposed to cycles of one odour presented with shock and the second odour without shock. One hour later, the flies were placed in an elevator maze and allowed to choose between the two odours for 60 s. We found that males from the monogamous regime indeed showed reduced learning performance in this assay relative to males from the polygamous regime (average learning scores of 0.17 versus 0.42, respectively; figure 5a; F1,4 = 26.60, p < 0.01). Importantly, no such difference was observed for females; if anything, monogamous females tended to learn slightly (but not significantly) better than polygamous females (figure 5b; selection regime effect for female data: F1,4 = 3.40, p = 0.14; sex × regime interaction for male and female data combined: F1,133 = 37.12, p < 0.0001). Neither sex differed between the selection regimes in innate responses to the odorants used in the assays (electronic supplementary material, figure S2a–d; males: F1,4 = 0.03, p = 0.86; females: F1,4 = 0.07, p = 0.81), indicating that the difference in male learning performance does not result from a difference in odour perception.
Figure 5.
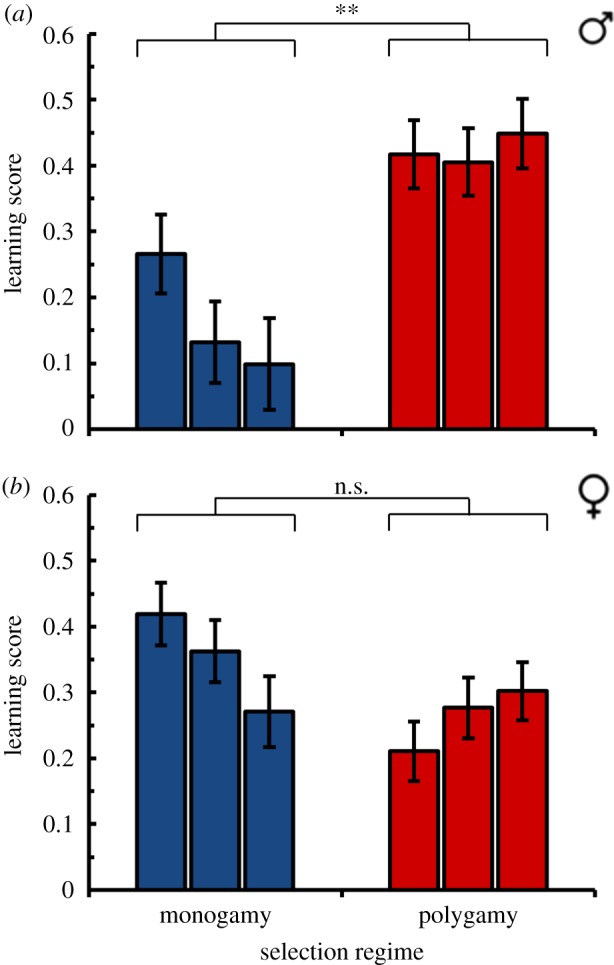
One-hour olfactory memory for evolved fly populations. Learning scores (marginal mean ± s.e.) are shown for each population for (a) males and (b) females (n = 12 measures per sex per population). (Online version in colour.)
3. Discussion
Evolution in the absence of sexual selection led to reduction in the performance of males in two relatively complex cognitive tasks: the ability to focus courtship efforts on a receptive female mixed with several unreceptive females and the ability to avoid an odour previously paired with aversive shock. While the latter task obviously relies on associative learning, the difference in the ability to focus on receptive females is also likely to reflect reduced ability of monogamous males to profit from experience. This is indicated by the faster improvement of courtship focus in polygamous than monogamous males over time, and is consistent with the known role of learning in discrimination between receptive and unreceptive females [3,8–10,12], and between females and immature males [13]. Performance in simpler behavioural tasks—mating with a single receptive female, locomotion and climbing response to shock—was not affected. It is possible that other aspects of cognition, for example the ability of males to discriminate between receptive and unreceptive females based on olfactory, visual or auditory cues, could be different between males from monogamous and polygamous selection regimes. We have no evidence for such a difference, though, as the main effect—a longer time to copulation in males from monogamous populations when housed with many unreceptive females—is not seen when males are paired with one receptive and one unreceptive female. Furthermore, naive monogamous males respond to odours as strongly as naive polygamous males.
These declines in complex cognitive tasks evolved independently in all three replicate populations subjected to monogamy, thus excluding random genetic drift as their sole cause [14]. They are also unlikely to reflect stronger inbreeding of the monogamous populations. Under our selection regimes, monogamous populations have an equivalent or greater effective population size (because of reduced variation in male mating success) than polygamous populations and thus are less vulnerable to the effects of inbreeding. Further, flies from monogamous populations outperform polygamous flies on measures of net reproductive output [15], which would not be expected if they were suffering from stronger inbreeding depression. Lastly, inbreeding should affect both sexes similarly, yet females did not differ between selection regimes in learning performance.
Our monogamous selection regime minimizes conflict between the sexes over mating and female reproductive effort [16], and therefore should favour less antagonistic males, which harass females less. However, we show that monogamous males court as intensely as polygamous males and are also as quick to mate when paired with individual, receptive females. Furthermore, it is not clear why reduction in male harassment would lead to a diminished ability to learn. The evolutionary decline in male performance in our monogamous fly populations is therefore unlikely to have been favoured as a means of reducing sexual antagonism.
The reduced male cognitive performance we see under monogamy is instead likely to be a consequence of its diminished adaptive value in the absence of male competition and female choice. The ability to learn is a costly adaptation [17–21], expected to be maintained only if the costs are exceeded by its benefits for Darwinian fitness. If the benefits diminish owing to environmental change or experimental manipulation, natural selection is expected to favour reduced investment in such costly traits. Alternatively, reduced male cognitive ability could result from antagonistic pleiotropy between the sexes [22]. If alleles reducing male cognitive performance improve some aspect of female fitness, they are expected to increase in frequency once selection on males has been relaxed by enforced monogamy. Furthermore, even without trade-offs, traits that cease to be adaptive are expected to decay due to genetic drift and mutation accumulation. One might speculate that complex cognitive traits should be more prone to such decay because they involve the interaction of many components (and thus present a larger genomic target for mutations) and are more sensitive to deviations from the optimal state of those components. Consistent with this notion, olfactory learning performance in Drosophila is more sensitive to inbreeding than innate responses to odours [23]. We cannot discern to what extent the reduced cognitive performance in our monogamous males is owing to direct selection favouring reduced investment in cognitive traits versus decay by genetic drift or mutation accumulation after selection has been relaxed [24]. In either case, our results reveal that sexual selection is a crucial force maintaining male cognitive performance in Drosophila.
Although our study focuses on males, female choice also involves perception and processing of complex information and, in Drosophila, is known to involve learning from experience [25] as well as following choices made by other females [26]. As the opportunity for female choice is eliminated in our monogamy regime, the adaptive value of mate choice-related cognitive traits might be expected to diminish for females as well as males. It is thus remarkable that, in contrast to males, female olfactory learning performance did not decline after 100 generations under monogamy. This not only demonstrates that the learning abilities of the two sexes can diverge but also suggests that learning brings mate-choice-independent fitness advantages to females even under simple laboratory conditions. Possibly, learning is still important for females in the context of mating under monogamy because it allows a female to learn that no other males are around and thus accept a male perceived to be of low quality. Alternatively, because females were pooled after mating and laid eggs under a high density of 50 females per 16 cm2 of medium surface, it is possible that learning helps females to compete for food and oviposition sites. Finally, we cannot exclude that the costs of learning are simply lower for females than males.
It has been suggested that the complexity of the social environment is a major factor in the evolution of brain size and cognition, and this ‘social brain hypothesis’ has received empirical support (reviewed in [27]). The role of mating systems, however, is more complicated. Work looking specifically at mating systems in non-human primates [28] and bats [29] shows higher brain investment in species with less intense male–male competition for mating opportunities. This has been interpreted as a consequence of the cognitive demands of pair bonding, non-existent in our system, along with the resources freed by reduced investment in metabolically expensive testes. The disparity between that work and our own results, where monogamous males show reduced cognitive performance, highlights the fact that the cognitive challenges imposed by different mating systems are likely to depend on taxon-specific details and differ between the sexes. For example, in some polygynous mammal species males outperform females in spatial learning tasks, while such dimorphism is smaller or absent in related monogamous species [30–32]. These differences have been attributed to differences in home range rather than directly to sexual selection—males in polygynous species typically roam over much larger areas than females, while in monogamous species the home ranges of the two sexes tend to be similar [31]. While this interpretation may be correct, our study provides direct experimental evidence that sexual selection can influence the evolution of cognition independently of differences in spatial behaviour by targeting those cognitive traits that aid individuals in mate competition within complex sexual environments.
4. Material and methods
(a). Experimental evolution design
The fly populations used in the experiment have been described previously [15]. Briefly, a long-term laboratory population (the IV population) that was initiated from wild D. melanogaster captured in 1975 was subdivided into six replicate populations in 2007. In three of these populations, the opportunity for sexual selection was minimized by enforcing monogamy. In the remaining three polygamous populations, flies experienced both female choice and male–male competition every generation. All of the populations were maintained throughout the experiment with a census size of 200 individuals.
In order to enforce monogamy, each generation virgin females were randomly paired with one virgin male each and allowed to spend 2 days mating in vials. By contrast, in polygamous populations groups of five virgin females were combined with groups of five virgin males in vials and also allowed to spend 2 days mating. After 2 days in these vials, males from both selection regimes were discarded and females from each replicate population were placed into two bottles, 50 females per bottle. The mated females spent the next 3 days laying eggs in these bottles before also being discarded. These bottles were the source of the next generation's flies, which were passed back through the experimental treatment.
(b). General assay methods
All assays were performed between 88 and 114 generations of evolution, after allowing one generation of common garden rearing in order to control for parental effects. The flies used in these assays were 4–5 days old, with ages matched to the day within all individual assays. The assays were performed in standard culture vials, always with standard 2% yeast food (water, agar [Milian CH], brewer's yeast [Migros CH], cornmeal, sucrose and Nipagin [Sigma-Aldrich CH]) present. When assays spanned more than 1 day, the assays were performed in balanced blocks so that the same number of measures were taken for all populations each day.
Courtship assays all took place during the morning hours between lights on at 8.00 and 12.00. The males used were unmated except where noted. Receptive females came from the base IV population and so are equally related to all of the evolved populations.
The IVe population, established in 1992 from a spontaneous recessive ebony mutant repeatedly backcrossed into the IV background [33], was used as a standardized competitor in tests of male reproductive success and also as the source for unreceptive females. These ebony females are easily distinguished from wild-type flies by body coloration, making the assays technically manageable, but are otherwise behaviourally unimpaired. In order to generate unreceptive IVe females, groups of 15 ebony males and five ebony female virgins were placed in vials during the evening before each experiment for mating. Females classified as unreceptive rarely mated with males in assays performed the following morning (five times total) and the rate of occurrence did not differ between selection regimes, so when this occurred the vials were discarded and no observations were retained for analyses. Likewise, if an individual fly died or escaped during handling these vials were discarded and no observations were recorded.
(c). Male competitive reproductive success
Male reproductive success of the evolved populations was measured by letting five males from the focal population compete with five ebony males for five ebony virgin females. After 2 days, the flies were discarded but the vials were retained. All offspring that emerged from these vials were collected and the number of flies from each brood that were wild-type or ebony was scored.
(d). Latency to copulation for naive males with a single receptive female
In order to determine whether flies from the evolved populations took relatively more or less time to mate with virgin, receptive females, individual males were placed into a standard vial with a receptive female in the afternoon, separated by a divider. The next morning, the dividers were removed and the latency to copulation scored for all males. Flies that did not mate in 120 min were treated as right-censored observations in the analyses.
(e). Latency to copulation in the presence of unreceptive females
To measure the proficiency of individual males at mating with a virgin, receptive female in a complex social environment, groups of either one receptive and one unreceptive female or one receptive and five unreceptive females were shaken into vials containing one naive male. Latency to copulation was recorded. Flies that did not mate in 60 min were treated as right-censored observations in the subsequent analyses.
(f). Behavioural tracking in the presence of unreceptive females
As in the complex social environment assay outlined above, five unreceptive females and one receptive female were shaken into vials with individual males. Vials were scored every minute for 20 min for whether or not the male was courting and, if so, which class of female the male was courting. For males that successfully mated during the 20 min window (16% in monogamy and 18% in polygamy, not significantly different between selection regimes), data were retained only for those minutes up to and including the onset of copulation.
(g). Olfactory learning
The olfactory learning paradigm [19] involves challenging flies to form an association between an odour (the conditioned stimulus, CS+) and an aversive mechanical shock (unconditioned stimulus, US). We measured the sexes independently by exposing same-sex groups of approximately 60 flies to three cycles of conditioning. In each cycle, flies were first exposed for 30 s to one odour (CS+) and subjected to shock (1 s of shaking every 5 s), followed by 60 s of air, another 30 s of a second odour alone, and finally 60 more seconds of air. The two odours used in the learning assay were octanol and 4-methyl-cyclohexanol dissolved in paraffin (0.6 ml per litre), each used equally as CS+.
One hour later, the flies were placed in a T-maze and allowed to choose between the two odours for 60 s. The number of flies in each arm of the T-maze was counted. Flies remaining in the central chamber were counted but did not differ numerically between selection regimes and were not included in the analysis.
Both odours used in the learning assay are known to be aversive to naive flies. As a control for innate differences in how aversive the odours are to different populations, we also measured naive flies in the T-maze.
(h). Activity levels
Two different measures of locomotor activity were obtained in order to test whether any differences between selection regimes in other assays might be attributable to activity levels. First, we used an assay to measure climbing response to shock (described in [34]). Groups of twenty flies were tapped to the bottom of an apparatus consisting of two connected vials. The percentage of the flies that had climbed 8 cm within 10 s (the ‘climbing pass rate’) was recorded.
Next, we recorded the movements of individual males in transparent cylindrical chambers (1.2 cm diameter × 0.8 cm high) with webcams placed above the chamber. Males were first transferred to these chambers and allowed to recover for 10 min, then recorded for 5 min. We used the software CvMob (http://www.cvmob.ufba.br) to track the movement of the individual males and quantified activity as the total number of pixels traversed by the flies.
(i). Statistical analysis
All statistical analysis was performed in SAS v. 9.2 [35] using either PROC GLIMMIX for generalized linear mixed models (pseudo-likelihood estimation of parameters and Wald F-tests for effect significance with degrees of freedoms computed by the containment method) or PROC NLMIXED for proportional hazard frailty models. Block effects were included in the linear mixed models as random effects when experiments were run across multiple days.
Competitive mating was analysed with a generalized linear mixed model where the binomial response (offspring either wild-type or ebony) was modelled with selection regime as a fixed effect and replicate population nested within selection regime as a random effect. Olfactory learning was modelled in the same way, separately for each sex, with the response variable the direction in which the fly moved in the T-maze (odour either correct or incorrect) and with the addition of odorant as a fixed effect. Following a convention [11,36], we express learning performance as a learning score equal to 2P – 1, where P is the proportion of flies choosing correctly.
The behavioural assays where latency to copulation was obtained for each male were analysed using a time-to-failure/survival analysis framework. We used proportional hazards frailty models with an underlying log-logistic-distributed baseline hazard, accounting for right-censored data (males that never mated). Latency to copulation was modelled with selection regime as a fixed effect and replicate population nested with selection regime as a random (or ‘frailty’) effect. The assays involving one or five unreceptive females were modelled in the same way, with additional fixed effects for the number of unreceptive females present (one or five), and an interaction between selection regime and the number of unreceptive females.
The behavioural time-series data were analysed with a repeated-measures generalized linear mixed model. Here, whether or not a male was courting (or whether he was courting the correct female) was a binomially distributed response variable modelled with selection regime, time and the selection regime × time interaction as fixed effects, and replicate population nested within selection regime as a random effect. Because each fly was observed every minute for 20 min, the identity of each fly was included in the model as a random effect with a first-order autoregressive covariance structure (TYPE = AR(1) in SAS PROC GLIMMIX RANDOM statement) to account for the decay in covariance as distance between neighbouring time points increases.
Acknowledgements
We thank Dorin Pirogan for assistance with the activity assays and Michel Chapuisat for helpful comments on the manuscript.
Data accessibility
All data: dryad doi:10.5061/dryad.k58bd.
Funding statement
This study has been supported by the Swiss National Science Foundation.
References
- 1.Shettleworth SJ. 2010. Cognition, evolution, and behavior, 2nd edn. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Dukas R. 2006. Learning in the context of sexual behaviour in insects. Anim. Biol. 56, 125–141 (doi:10.1163/157075606777304258) [Google Scholar]
- 3.Dukas R. 2005. Experience improves courtship in male fruit flies. Anim. Behav. 69, 1203–1209 (doi:10.1016/j.anbehav.2004.08.012) [Google Scholar]
- 4.Byrne PG, Rice WR. 2006. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. B 273, 917–922 (doi:10.1098/rspb.2005.3372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reif M, Linsenmair KE, Heisenberg M. 2002. Evolutionary significance of courtship conditioning in Drosophila melanogaster. Anim. Behav. 63, 143–155 (doi:10.1006/anbe.2001.1876) [Google Scholar]
- 6.Griffith LC, Ejima A. 2009. Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn. Mem. 16, 743–750 (doi:10.1101/lm.956309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allison JD, Cardé RT. 2008. Male pheromone blend preference function measured in choice and no-choice wind tunnel trials with almond moths, Cadra cautella. Anim. Behav. 75, 259–266 (doi:10.1016/j.anbehav.2007.04.033) [Google Scholar]
- 8.Siegel RW, Hall JC. 1979. Conditioned-responses in courtship behavior of normal and mutant Drosophila. Proc. Natl Acad. Sci. USA 76, 3430–3434 (doi:10.1073/pnas.76.7.3430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejima A, Smith BPC, Lucas C, Van Naters WV, Miller CJ, Carlson JR, Levine JD, Griffith LC. 2007. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 17, 599–605 (doi:10.1016/j.cub.2007.01.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejima A, Smith BPC, Lucas C, Levine JD, Griffith LC. 2005. Sequential learning of pheromonal cues modulates memory consolidation in trainer-specific associative courtship conditioning. Curr. Biol. 15, 194–206 (doi:10.1016/j.cub.2005.01.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mery F, Pont J, Preat T, Kawecki TJ. 2007. Experimental evolution of olfactory memory in Drosophila melanogaster. Physiol. Biochem. Zool. 80, 399–405 (doi:10.1086/518014) [DOI] [PubMed] [Google Scholar]
- 12.Dukas R. 2004. Male fruit flies learn to avoid interspecific courtship. Behav. Ecol. 15, 695–698 (doi:10.1093/beheco/arh068) [Google Scholar]
- 13.Gailey DA, Hall JC, Siegel RW. 1985. Reduced reproductive success for a conditioning mutant in experimental populations of Drosophila melanogaster. Genetics 111, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC. 2012. Experimental evolution. Trends Ecol. Evol. 27, 547–560 (doi:10.1016/j.tree.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 15.Hollis B, Houle D. 2011. Populations with elevated mutation load do not benefit from the operation of sexual selection. J. Evol. Biol. 24, 1918–1926 (doi:10.1111/j.1420-9101.2011.02323.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088 (doi:10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469 (doi:10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mery F, Kawecki TJ. 2004. An operating cost of learning in Drosophila melanogaster. Anim. Behav. 68, 589–598 (doi:10.1016/j.anbehav.2003.12.005) [Google Scholar]
- 19.Mery F, Kawecki TJ. 2005. A cost of long-term memory in Drosophila. Science 308, 1148 (doi:10.1126/science.1111331) [DOI] [PubMed] [Google Scholar]
- 20.Snell-Rood EC, Davidowitz G, Papaj DR. 2011. Reproductive tradeoffs of learning in a butterfly. Behav. Ecol. 22, 291–302 (doi:10.1093/beheco/arq169) [Google Scholar]
- 21.Snell-Rood EC, Papaj DR, Gronenberg W. 2009. Brain size: a global or induced cost of learning? Brain Behav . Evol. 73, 111–128 (doi:10.1159/000213647) [DOI] [PubMed] [Google Scholar]
- 22.Rice WR. 1984. Sex-chromosomes and the evolution of sexual dimorphism. Evol. Int. J. Org. Evol. 38, 735–742 (doi:10.2307/2408385) [DOI] [PubMed] [Google Scholar]
- 23.Nepoux V, Haag CR, Kawecki TJ. 2010. Effects of inbreeding on aversive learning in Drosophila. J. Evol. Biol. 23, 2333–2345 (doi:10.1111/j.1420-9101.2010.02094.x) [DOI] [PubMed] [Google Scholar]
- 24.Maughan H, Masel J, Birky CW, Nicholson WL. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177, 937–948 (doi:10.1534/genetics.107.075663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dukas R. 2005. Learning affects mate choice in female fruit flies. Behav. Ecol. 16, 800–804 (doi:10.1093/beheco/ari057) [Google Scholar]
- 26.Mery F, Varela SAM, Danchin E, Blanchet S, Parejo D, Coolen I, Wagner RH. 2009. Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734 (doi:10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- 27.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 28.Schillaci MA. 2008. Primate mating systems and the evolution of neocortex size. J. Mammal. 89, 58–63 (doi:10.1644/06-Mamm-a-417.1) [Google Scholar]
- 29.Pitnick S, Jones KE, Wilkinson GS. 2006. Mating system and brain size in bats. Proc. R. Soc. B 273, 719–724 (doi:10.1098/rspb.2005.3367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaulin SJC, Fitzgerald RW. 1989. Sexual selection for spatial-learning ability. Anim. Behav. 37, 322–331 (doi:10.1016/0003-3472(89)90121-8) [Google Scholar]
- 31.Jones CM, Braithwaite VA, Healy SD. 2003. The evolution of sex differences in spatial ability. Behav. Neurosci. 117, 403–411 (doi:10.1037/0735-7044.117.3.403) [DOI] [PubMed] [Google Scholar]
- 32.Perdue BM, Snyder RJ, Zhihe Z, Marr J, Maple TL. 2011. Sex differences in spatial ability: a test of the range size hypothesis in the order Carnivora. Biol. Lett. 7, 380–383 (doi:10.1098/rsbl.2010.1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houle D, Rowe L. 2003. Natural selection in a bottle. Am. Nat. 161, 50–67 (doi:10.1086/345480) [DOI] [PubMed] [Google Scholar]
- 34.Ali YO, Escala W, Ruan K, Zhai RG. 2011. Assaying locomotor, learning, and memory deficits in Drosophila models of neurodegeneration. J. Vis. Exp. 49, e2504 (doi:10.3791/2504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute S. 2011. The SAS system for windows, release 9.2. Cary, NC: SAS Institute [Google Scholar]
- 36.Waddell S, Quinn WG. 2001. What can we teach Drosophila? What can they teach us? Trends Genet. 17, 719–726 (doi:10.1016/S0168-9525(01)02526-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data: dryad doi:10.5061/dryad.k58bd.