Abstract
Marine mammal mass strandings have occurred for millions of years, but their origins defy singular explanations. Beyond human causes, mass strandings have been attributed to herding behaviour, large-scale oceanographic fronts and harmful algal blooms (HABs). Because algal toxins cause organ failure in marine mammals, HABs are the most common mass stranding agent with broad geographical and widespread taxonomic impact. Toxin-mediated mortalities in marine food webs have the potential to occur over geological timescales, but direct evidence for their antiquity has been lacking. Here, we describe an unusually dense accumulation of fossil marine vertebrates from Cerro Ballena, a Late Miocene locality in Atacama Region of Chile, preserving over 40 skeletons of rorqual whales, sperm whales, seals, aquatic sloths, walrus-whales and predatory bony fish. Marine mammal skeletons are distributed in four discrete horizons at the site, representing a recurring accumulation mechanism. Taphonomic analysis points to strong spatial focusing with a rapid death mechanism at sea, before being buried on a barrier-protected supratidal flat. In modern settings, HABs are the only known natural cause for such repeated, multispecies accumulations. This proposed agent suggests that upwelling zones elsewhere in the world should preserve fossil marine vertebrate accumulations in similar modes and densities.
Keywords: taphonomy, strandings, fossil record, harmful algal blooms
1. Introduction
During the past approximately 50 Myr, marine mammals evolved in ocean ecosystems that have undergone global changes in sea level, temperature, productivity and ocean circulation [1–5]. Within this time frame, multiple marine mammal lineages evolved from trophic obscurity (i.e. terrestrial ancestry, with little influence on ocean ecosystems) to ecological dominance in marine food webs [6–10]. Understanding how marine mammals, such as cetaceans, pinnipeds and sirenians, ascended to become apex consumers in marine food webs [11] requires data from the fossil record. Palaeobiologists have used counts of fossil species [2,4,5,12] to outline evolutionary changes in richness at the scale of geologic time [13], especially during episodes of major climatic changes [3,4]. These latter studies investigated evolutionary causes and responses over protracted, diachronic time frames. However, testing ecological interactions requires diversity datasets from synchronic snapshots at specific scales that account for time-averaging, sampling density and other metrics of diversity, such as abundance [14]. This latter goal is a challenge because the marine mammal fossil record consists mostly of singleton occurrences [13] and not dense accumulations.
Obtaining ecological snapshots of large mobile predators such as marine mammals is logistically difficult because their life-history traits (e.g. long life, low fecundity, large range) have broad temporal and geographical parameters [6–9]. Palaeoecologists working with terrestrial mammal and marine invertebrate communities have discovered that sampling diversity with increased temporal- and spatial-averaging generates death assemblage datasets that compare well with living communities [15–18]. Recently, Pyenson [19,20] demonstrated that death assemblages of modern cetaceans (e.g. strandings) faithfully record ecological snapshots of living communities at temporal and spatial scales commensurate with their macroecology, which suggests that certain fossil assemblages might retain similarly faithful ecological data. Marine mammal mass strandings are well recorded in historical times [19,20], but the putative cases from the fossil record cannot be linked to a particular causal mechanism [21,22], especially without a better understanding of the taphonomic mechanisms that both preserve and prevent fossil marine mammal material from entering the sedimentary record [23]. Here, we describe an unusual accumulation of fossil marine vertebrates from the Late Miocene of Chile that provides unique insights into the mechanisms that preserve dense deposits of marine mammal material, and the oceanographic processes responsible for their origin.
2. Material and methods
(a). Locality
From 2010 to 2012, road expansion along the Pan-American Highway in Atacama Region of Chile (figure 1) opened a 20 × 250 m quarry at a site, called Cerro Ballena (27°02′31.51″ S, 70°47′42.18″ W), which revealed over 40 complete and partial marine mammal skeletons, along with isolated remains of other marine vertebrates. A road-cut stratigraphic profile of the site exposes approximately 9 m of fine to very fine-grained sandstones belonging to the Bahía Inglesa Formation [24–27], unconformably overlain by a Pleistocene transgressive–regressive marine terrace sequence [28]. Cerro Ballena is located too far north for stratigraphic correlation with other reported localities of the Bahía Inglesa Formation [28], or any recognized members that have been proposed previously ([26] and see the electronic supplementary material). Within approximately 8 m of the formation at Cerro Ballena, four different bone-bearing levels (Bone Levels 1–4; BL1–BL4) produced articulated and associated marine mammal fossils (figures 1 and 2). The quarry, which is now paved over, represents only a small portion of the fossiliferous levels, with geologic maps of the unit indicating a local aerial extent of approximately 2 km2 (see the electronic supplementary material).
Figure 1.
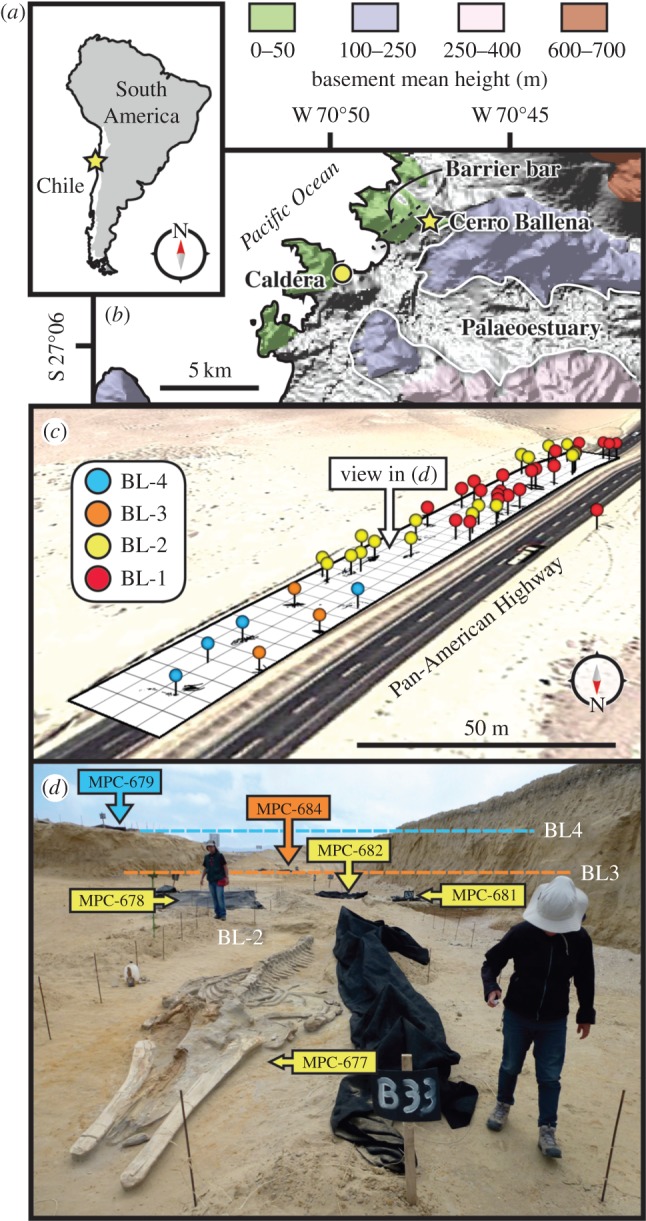
Locality and geographical information for Cerro Ballena showing (a) South America with (b) the palaeocoastline of the Caldera Basin outlined over NASA Shuttle Radar Topography Mission data; (c) quarry map showing specimen positions and colour-coded stratigraphic layer, created in Google Earth; (d) view of the quarry and the top three bone-bearing layers (BL2–4), facing northeast, from MPC 677 in situ. See http://cerroballena.si.edu and the electronic supplementary material for more details.
Figure 2.
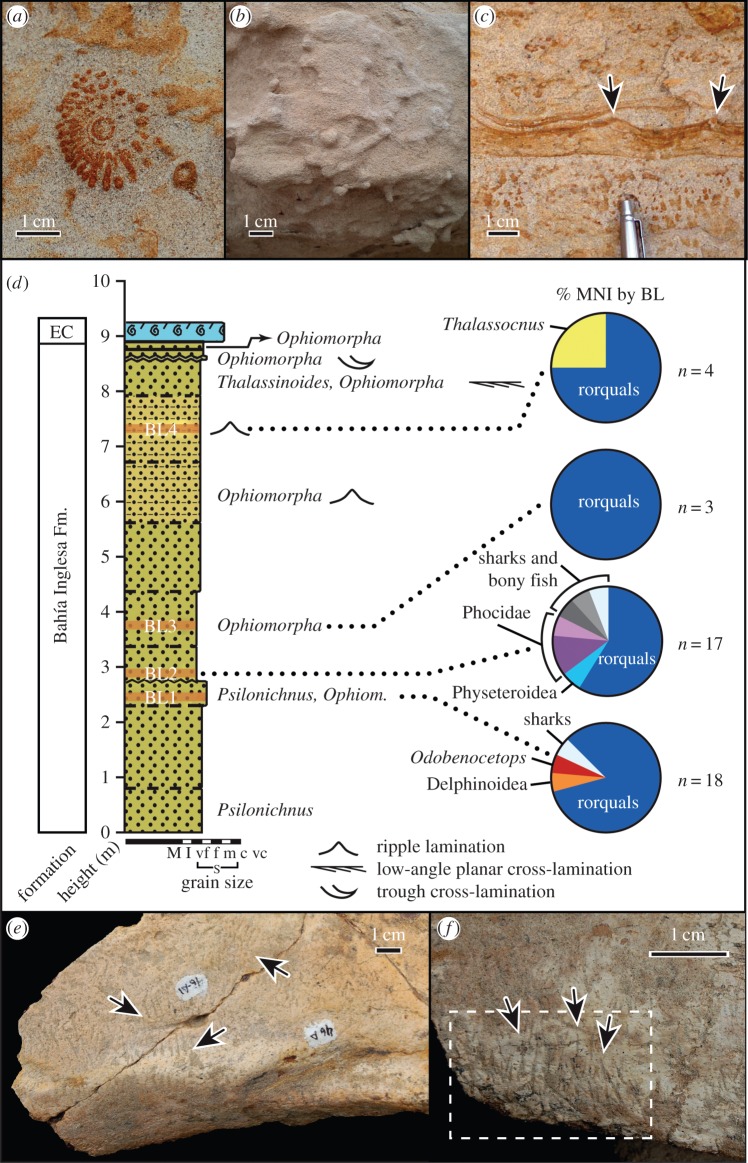
Stratigraphic and sedimentological data from Cerro Ballena. (a) Unidentified, iron-stained traces resembling algal growth structures; (b) Psilonichnus, a supratidal trace fossil; (c) iron-stained tuft-like forms resembling algae, and possible algal mats covering wave ripples (indicated with arrows), with pen for scale and (d) stratigraphic column, with vertebrate diversity data expressed as percentage of the MNI from each bone-bearing layer (BL1–4). (e,f) Crab feeding traces on the skull bones of MPC 662, from BL-1. c, coarse grained; EC, Estratos de Caldera; f, fine grained; I, siltstone; M, mudstone; m, medium grained; S, sandstone; vc, very coarse grained; vf, very fine grained.
(b). Geologic age
The general region surrounding Caldera contains fossiliferous marine sediments belonging to the Mio-Pliocene age Bahía Inglesa Formation [25], which has produced an extensive list of fossil marine vertebrates [26–28]. We did not find any biostratigraphically relevant invertebrate and microfossil indicators in strata at Cerro Ballena, although there were two biostratigraphically useful vertebrate fossils from Cerro Ballena that also occur in the Mio-Pliocene Pisco Formation of Peru: aquatic sloths (Thalassocnus) and sharks (Carcharodon). Isolated elements of aquatic sloths from Cerro Ballena are referred to the species Thalassocnus natans [29]. Both T. natans and Carcharodon hastalis from Cerro Ballena are correlated with the El Jahuay (ELJ) and Montemar Horizon (MTM) horizons in the Sacaco Basin of Peru [30]. In turn, this yields upper and lower bounds on the age of this unit of the Bahía Inglesa Formation at Cerro Ballena as 9.03–6.45 Ma, following [31] (see the electronic supplementary material). The overlap in stratigraphic range between these two taxa implies that the strata in Cerro Ballena were deposited at a time period in between the deposition of the ELJ and MTM horizons of the Pisco Formation. Thus, we infer a Late Miocene age (or Late Tortonian to Early Messinian stage) for the Bahía Inglesa Formation part of the section at Cerro Ballena, which coincided with a rise in sea level caused by transgressive–regressive cycling and tectonic subsidence along this part of the coastline [32].
(c). Depositional environment and sedimentology
In the road-cut section of the Bahía Inglesa Formation at Cerro Ballena, we measured a stratigraphic section, noted sedimentary structures and observed invertebrate trace fossils (figure 2a–c).
Sediment samples collected from BL1–4 were freshly excavated and covered in optically clear resin (Epo-Tek 301, Epoxy Technology, Billerica, MA, USA). Embedded samples were then cut and thin-sectioned for scanning electron microscopy and electron spectroscopy using an FEI Nova NanoSEM 600 under low vacuum with the gaseous analytical detector for imaging and an energy dispersive X-ray spectroscopy detector (ThermoFisher) for geochemical analysis. Samples were either placed directly on carbon-tape or embedded in epoxy and thin-sectioned, and left uncoated for SEM and EDS characterization (see the electronic supplementary material, figure S1). Light microscopy was performed using an Olympus BX51 microscope with a Chameleon digital video camera.
(d). Capturing, processing and rendering three-dimensional digital datasets
Under time-sensitive and salvage circumstances, we documented in situ skeletal remains using three-dimensional digital tools, before they were collected for study and care at their repositories (see the electronic supplementary material). Photogrammetry and computer vision datasets for fossil rorquals were captured with 20 and 30 cm aluminium scale bars and metal markers (to assess line of sight and control for coverage quality) on a Canon 5D with multiple lenses, and geotagged using a Garmin Etrex GPS. We also used Munsell colour charts for colour calibration, accuracy and downstream correction in photography editing software packages. Raw digital datasets were processed into coherent models by aligning datasets, cleaning up noise and removing redundant data using Geomagic v. 2012, Polyworks v. 12.0 and Zbrush v. 4R3 for the very large dataset of MPC (Museo Paleontologico de Caldera) 677. Direct Dimensions, Inc. (Owings Mills, MD, USA) aligned the laser arm dataset using Geomagic, and the model was then retopologized using Zbrush for MPC 677, creating an orthogonal digital rendering from three-dimensional polygon data (figure 3). URC Ventures (Redmond, WA, USA) created orthogonal renderings from three-dimensional point cloud datasets (figure 4) by aligning and retopologizing point cloud data (see the electronic supplementary material, figure S2). The resultant three-dimensional datasets provided sub-centimetre accuracy, and full resolution texture-mapped imagery is available at http://cerroballena.si.edu.
Figure 3.
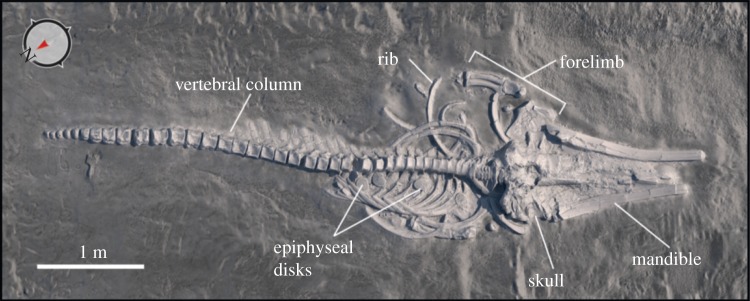
Orthogonal digital three-dimensional polygon model of the most complete fossil rorqual specimen at Cerro Ballena, MPC 677. True north indicated by arrow. See http://cerroballena.si.edu and the electronic supplementary material for more details.
Figure 4.
High dynamic range images of orthogonal three-dimensional point clouds capturing adult and juvenile fossil rorqual skeletons from Cerro Ballena. (a) MPC 678; (b) MPC 684; (c) over-lapping adult and juvenile specimens, clockwise MPC 666, 665 and 667; (d) MPC 685 and (e) MPC 675. Small-scale bars 20 cm, large-scale bars 30 cm. True north indicated by arrow, and stratigraphic layer noted by bone-bearing level number. See http://cerroballena.si.edu and the electronic supplementary information for more details and source data.
3. Results and discussion
Taphonomic analysis of the site reveals several features that are directly comparable to modern marine mammal mass strandings. First, the site preserves multiple species of marine mammals, dominated by abundant skeletons (MNI = 31; table 1) of large baleen whales (clade Balaenopteridae or rorquals) that are likely all from the same species (see the electronic supplementary materials), and encompass a range of ontogenetic stages, from calves to mature individuals (figures 3 and 4). Other marine mammal species include: (i) at least two different phocid seals (Acrophoca, and a new morphotype); (ii) an extinct species of sperm whale (Scaldicetus morphotype); (iii) a walrus-like toothed whale (Odobenocetops) and (iv) an aquatic sloth (T. natans) (tables 1 and 2; see the electronic supplementary material, figures S3–S7). Second, we devised a simple, three-stage categorization to capture the range of marine vertebrate taphonomy at Cerro Ballena: (Stage 1) articulated, either completely or mostly; (Stage 2) disarticulated, but associated elements and (Stage 3) isolated, separated elements. Non-cetacean vertebrates consist of associated, semi-articulated and/or disarticulated skeletal material (Stages 2 and 3). By contrast, rorqual skeletons included many fully articulated, intact and nearly complete skeletons (Stage 1), along with disarticulated skeletons with low skeletal scatter (i.e. less than the distance of their body length; figure 3; table 2; and see the electronic supplementary material, tables S1–S11). Third, rose diagrams of the rorquals’ skeletons long axis orientation (i.e. vertebral column) reveal that they are orthogonal to current flow in each level, analogous to body orientation patterns observed for some modern mass strandings ([33]; see the electronic supplementary material, figure S8 and table S4). Lastly, rorquals occur mostly ventral up, across all BLs (table 2). The dominance of ventral up carcasses, combined with their high articulation and long axis orientation, is a strong sign that they washed in dying or dead and were then buried [34,35].
Table 1.
Diversity of fossil marine vertebrates at Cerro Ballena, with minimum number of individuals (MNI) by bone-bearing level (BL) and with range of skeletal articulation stages. This tabulation does not include 11 additional, unidentified large cetacean skeletons (see the electronic supplementary material, figures S3–S7 and S9).
| clade | taxon | BL occurrence | total MNI | articulation |
|---|---|---|---|---|
| Mysticeti | Balaenopteridae | BL 1–4 | 31 | Stages 1–3 |
| Phocidae | Acrophoca sp. | BL 2 | 2 | Stages 2 and 3 |
| Elasmobranchii | Carcharodon hastalis | BL 1, 2 | 2 | Stage 3 |
| Odontoceti | Delphinoidea | BL 1 | 1 | Stage 3 |
| Odontoceti | Physeteroidea | BL 2 | 1 | Stages 2 and 3 |
| Odontoceti | Odobenocetops sp. | BL 1 | 1 | Stage 2 |
| Phocidae | Phocidae n. gen. | BL 2 | 1 | Stage 3 |
| Nothrotheriidae | Thalassocnus natans | BL 4 | 1 | Stage 3 |
| Osteichythes | Istiophoridae | BL 2 | 1 | Stage 3 |
| Osteichythes | Xiphiidae | BL 2 | 1 | Stage 3 |
Table 2.
Taphonomic attributes of fossil rorqual skeletons at Cerro Ballena, ranked stratigraphically by BL. Number of individual specimens (NISP) is scored for percentage oriented ventral up, skeletal articulation and scatter and total length (TL). See the electronic supplementary material, tables S1–11.
| BL level | % ventral up | NISP | dominant mode(s) of articulation | average scatter (m) | NISP for scatter | average TL (m) | NISP for TL |
|---|---|---|---|---|---|---|---|
| BL-4 | 33 | 3 | Stage 2 | 2.77 | 3 | 8.62 | 3 |
| BL-3 | 67 | 3 | Stage 1 | 2.21 | 3 | 7.63 | 2 |
| BL-2 | 67 | 6 | Stages 1 and 3 | 2.80 | 7 | 7.43 | 7 |
| BL-1 | 92 | 12 | Stage 1 | 3.45 | 13 | 7.97 | 9 |
| average | 75 | 2.83 | 7.91 |
The dominance of fossil rorqual skeletons at Cerro Ballena, across all bone-bearing levels, evokes a modern stranding event, whereby many individual cetaceans are beachcast, either dead or alive. Mass stranding events for socially gregarious species of toothed whales are well documented [36], but mass strandings for rorquals are rare. The most compelling analogue is a rorqual mass stranding of 14 humpback whales (Megaptera novaeangliae) over the course of five weeks along approximately 50 km of coastline around Cape Cod, MA, USA in 1987–1988 [35]. This assemblage included males, females and one calf, whose necropsies showed no signs of trauma or predation. Tissue assays of Atlantic mackerel (Scomber scombrus), from stomach contents, revealed high concentrations of saxitoxins, which are dinoflagellate neurotoxins. This evidence, along with the documented aberrant behaviour of one of the dying whales, and the geographical and temporal spans of the event, pointed to the previously unrecognized trophic transfer of major algal toxins. Since then, other cases of harmful algal blooms (HABs) involving marine mammals have been reported at similar geographical and temporal scales [37–41].
The assemblage at Cerro Ballena shares specific features with HAB-mediated mass strandings. These similarities help delimit the cause of death and the factors that have driven their concentration and preservation at this site. The presence of repeated, multispecies assemblages argues for a taxonomically broad death mechanism, such as HABs. The proximity of many specimens, including juvenile and adult rorqual skeletons in direct contact or few metres apart (figure 4c), along with different marine vertebrate taxa approximately 10 m apart, suggests strong post-mortem spatial focusing, prior to burial at each level (figure 1c). Intraspecific and interspecific taphonomic variation does not eliminate this possibility, as actualistic studies of catastrophic cetacean death assemblages show a wide variety of decay stages [42]. HAB-mediated mortalities at Cerro Ballena would also partly explain the absence of vertebrate scavenging and the absence of skeletal trauma [43]. The general completeness of rorqual skeletons, in contrast to the disarticulation of other marine vertebrates, reflects a size bias or temporal delay in scavenging, permitting more disarticulation and abrasion. Sharks represented by isolated teeth suggest attritional input or potential scavenging by-products (see the electronic supplementary material, figure S9). However, billfish remains (Xiphiidae and Istiophoridae) suggest that these large predatory consumers are similarly susceptible to HABs, a finding that has been reported in the modern world (see the electronic supplementary material). Isolated remains of aquatic sloths (T. natans) may reflect incidental, attritional input or actual HAB-mediated mortality based on extant HAB toxin transfers (i.e. inhalation) for modern herbivorous marine mammals [40]. Collectively, the taphonomy of Cerro Ballena indicates that repeated marine mammal mortalities were relatively rapid (hours to weeks in duration), geographically widespread and allochthonous (i.e. at sea). These latter traits are all consistent with HAB-related mortalities in the modern world, which show taphonomic signals that are temporally delayed and physically remote from their source [38,40,41,44].
The depositional environment in which vertebrate carcasses were buried was supratidal, based on ichnological and sedimentological evidence. We observed abundant traces of Psilonichnus, which typically occurs on supratidal flats, and Skolithos and Ophiomorpha, belonging to the Skolithos ichnofacies, also common to tidal flats (figure 2; see the electronic supplementary material, figure S10). In other parts of the fossil record, Psilonichnus has been interpreted as a crab trace fossil [45]. Given the unique food resource provided by marine mammal carcasses, it is not surprising to find scavenging traces on individual balaenopterid bones that we attribute to crabs (figure 2e,f). These short (approx. 1 cm), sharp and closely associated traces on the skull bones of MPC 662 are similar in trace morphology to those described for penguin bones from the Miocene of Argentina [46]. The lack of variation in grain size and scarcity of erosional surfaces in the section at Cerro Ballena further indicate a more or less constant sedimentation encompassing approximately 10–16 kyr of deposition, based on rates for modern tidal flats (see the electronic supplementary material). The preservation of delicate features resembling tufted algal mats (figure 2c, see [47] and the electronic supplementary material, figure S11) reflects rapid post-mortem replacement by iron oxides, and it also indicates the contemporaneous presence of algae and high iron concentrations, which promote algal growth in this depositional environment.
In terms of concentration mechanism, dead or dying marine vertebrates were delivered to south-facing embayment, protected from normal wave action by basement rocks and a barrier bar to the west (figure 1b). This interpretation is supported by the absence of north–south-oriented wave ripples (which should be present if this area had been directly exposed to Pacific Ocean waves) and the presence of low-angle planar cross-bedded sandstone at the top of the sequence, typical of a beach or berm (figure 2). Storm surges flooding the supratidal flats to a depth of about 1.5 m, as calculated from estimates of wind velocity, duration and fetch (see the electronic supplementary material), would have been sufficient to float the largest carcasses from the south, allowing hydraulic sorting to modally orient them at each level [48]. The absence of major disarticulation and limited skeletal scatter for any marine mammal skeleton further supports limited initial scavenging of floating carcasses, and rapid transit time (hours to days) between death at sea and coming to rest on a protected, supratidal flat. Such a shallow and mostly subaerial environment also excluded large marine scavengers. Equally, the surrounding desert environment (which already existed at the time) lacked sufficiently large terrestrial predators [49] that could dismember the largest of the carcasses.
Thus, the supratidal flat worked as a taphonomic trap [19], preserving carcasses that arrived during storms or spring tides in an excellent environment for decay in situ, mostly free of scavengers. As carcasses were buried under a relatively continuous rate of fine sediments, each mass stranding horizon yielded a discrete layer of skeletal remains. Sediment samples under light and scanning electron microscopy lacked distinct algal cell fragments (e.g. diatom frustules), but there were widespread approximately 5–10 μm spherical apatite grains encrusted in iron oxides (see the electronic supplementary material, figure S1), which could result from mineral replacement of non-siliceous algae (e.g. cyanobacteria) from coastal HABs. However, we cannot definitively confirm their biogenic source nor discriminate between their pre-depositional or post-depositional origins.
Alternative mass stranding death mechanisms lack modern analogues or fail to explain the full range of evidence at Cerro Ballena. For example, taxon-specific herding, breeding or stranding behaviours do not explain the full range of taxa at the site, which includes both pelagic and coastal species that do not inhabit supratidal environments (table 1). Tsunamis would have generated death assemblages lacking large body size selectivity (tables 1 and 2) and would result in high-energy sedimentary structures that are not present. Pandemic causes, such as morbillivirus, are not taxonomically broad, nor would it be parsimonious as a recurring mechanism over 10–16 kyr. Thus, all other alternative death mechanisms besides HABs fail to explain the iterative preservation of four bone-bearing levels.
The excavation quarry at Cerro Ballena yielded a density of associated, fossil marine mammal skeletons unrivaled elsewhere in the world. The density of individual cetacean specimens at Cerro Ballena, for example, is greater than other attritional deposits in the cetacean fossil record, including the Sharktooth Hill bonebed from the Middle Miocene of California [23] and the Eocene lagoonal deposits of Wadi Al-Hitan in Egypt [50]. The density of rorqual skeletons in BL1 (see the electronic supplementary material, table S12) alone is 10 times greater than associated, individual densities reported from the Mio-Pliocene Pisco Basin of southern Peru [51], which are preserved in distal, marine shelf environments, and notably lack multiple marine mammal species in close association (less than 10 m). Cerro Ballena is also surprising in its abundance of complete rorqual skeletons because baleen whale strandings are comparatively rare in the modern world. We propose that this ecological asymmetry arises from the shifted baseline of baleen whale abundances. In remote areas today, such as the Southern Ocean, there are examples of super-aggregations that are unusual by today's standards, but match historical and anecdotal accounts of baleen whale abundances prior to industrial whaling [52].
Evidence for HAB-mediated death assemblages of marine vertebrates in the fossil record is limited to only a few cases because of the difficulty in attributing HABs as a causal agent [53,54]. Modern analogues of marine mammal deaths caused by HABs outline a likely pathway that occurred repeatedly at this site during the Late Miocene. We propose that toxins, generated by HABs, poisoned multiple species of marine vertebrates, through ingestion of contaminated prey and/or inhalation, causing relatively rapid death at sea. Carcasses then floated towards the coastline, where they entered the estuary and were transported by locally generated, northward propagating storm waves into a restricted supratidal flat, where they were buried to the exclusion of major scavenging and disarticulation. This sequence was recorded four times during the deposition of sediment (approx. 10–16 kyr) at Cerro Ballena. The conditions that lead to this repeated phenomenon are tied to upwelling systems along westerly margins of continental coastlines [55]. Along the coast of western South America, ferruginous runoff from the Andes leads to increased iron in the ocean, which boosts productivity where iron is a limiting nutrient for phytoplankton growth [55], while also promoting HABs (e.g. cyanobacteria and dinoflagellates [44]). The antiquity of these processes probably pre-dates modern tectonic configurations, although it has not been documented in the fossil record until now. We propose that upwelling systems, fuelled by iron-rich runoff, in other regions of the world will have similar, repeated accumulations of marine consumers [56].
Acknowledgements
We thank two anonymous reviewers for comments that improved the manuscript. For discussion and access to unpublished data, we thank P. D. Gingerich, R. E. Fordyce, D. E. G. Briggs, J. R. Geraci, G. A. Early, L. Mazzuca, J. Castle, J. H. Rodger Jr, J. G. Mead, C. W. Potter, D. J. Bohaska, D. R. Lindberg, J. A. Goldbogen, M. T. Clementz, S. L. Wing and A. K. Behrensmeyer. For assistance in the field, we thank C. P. Figueroa-Bravo, R. E. Yury-Yañez, S. Fuentes Tamblay, S. Hillebrandt, D. Tornini, R. Terreros, J. Arevalo, D. del Monte and B. Tornini. We are indebted to G. Kovak-Lewis, B. Semerjian and D. Boardman at URC Ventures for analytical support. Also, we thank D. Cole (Smithsonian GIS Coordinator), J. Cope (N Formation Design), E. L. Montaño for assistance with NASA elevation datasets, T. Dzambazova (Autodesk), A. Velevski and R. Wiedemeier for website assistance, P. Fu (3D Systems), C. Gomez (Museo Nacional de Historia Natural in Santiago), L. López and the Consejo de Monumentos Nacionales (CMN), and G. Waibel, J. Blundell, and L. Lewis of the Smithsonian's Digitization Program Office for their support. This paper is Caldera Paleontology Project contribution no. 4.
Funding statement
Excavation work was conducted under CMN permit no. 5979 to M.E.S. and was financed by Sacyr Chile S.A. Funding from CONICYT, Becas Chile, Departamento de Postgrado y Postítulo of the Vicerrectoría de Asuntos Académicos of Universidad de Chile supported C.S.G. This work was also funded by a National Museum of Natural History (NMNH) Small Grant Award, discretionary funding from NMNH Office of the Director, the Smithsonian Institution's Remington Kellogg Fund, two National Geographic Society Committee on Research Exploration grants (8903-11, 9019-11) to N.D.P. and by U-REDES (Domeyko II UR-C12/1, Universidad de Chile) to A. O. Vargas.
References
- 1.Norris RD, Kirtland Turner S, Hull PM, Ridgwell A. 2013. Marine ecosystem responses to Cenozoic global change. Science 341, 492–498 (doi:10.1126/science.1240543) [DOI] [PubMed] [Google Scholar]
- 2.Lipps JH, Mitchell ED., Jr 1976. Trophic model for the adaptive radiations and extinctions of pelagic marine mammals. Paleobiology 2, 147–155 [Google Scholar]
- 3.Fordyce RE. 2003. Cetacea evolution and Eocene–Oligocene oceans revisited. In From greenhouse to icehouse. The marine Eocene–Oligocene transition (eds Prothero DR, Ivany LC, Nesbitt EA.), pp. 154–170 New York, NY: Columbia University Press [Google Scholar]
- 4.Marx FG, Uhen MD. 2010. Climate, critters, and cetaceans: Cenozoic drivers of the evolution of modern whales. Science 327, 993–996 (doi:10.1126/science.1185581) [DOI] [PubMed] [Google Scholar]
- 5.Suto I, Kawamura K, Hagimoto S, Teraishi A, Tanaka Y. 2012. Changes in upwelling mechanisms drove the evolution of marine organisms. Palaeogeogr. Palaeoclimatol. Palaeoecol. 339–341, 39–51 (doi:10.1016/j.palaeo.2012.04.014) [Google Scholar]
- 6.Schwarz LK, Goebel ME, Costa DP, Kilpatrick AM. 2013. Top-down and bottom-up influences on demographic rates of Antarctic fur seals Arctocephalus gazella. J. Animal Ecol. 82, 903–911 (doi:10.1111/1365-2656.12059) [DOI] [PubMed] [Google Scholar]
- 7.Springer AM, Estes JA, van Vliet GB, Williams TM, Doak DF, Danner EM, Forney KA, Pfister B. 2003. Sequential megafaunal collapse in the North Pacific Ocean: an ongoing legacy of industrial whaling? Proc. Natl Acad. Sci. USA 100, 12 223–12 228 (doi:10.1073/pnas.1635156100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ainley DG, et al. 2010. Impacts of cetaceans on the structure of Southern Ocean food webs. Mar. Mamm. Sci. 26, 482–498 (doi:10.1111/j.1748-7692.2009.00337.x) [Google Scholar]
- 9.Croll DA, Marinovic B, Benson S, Chavez FP, Black N, Ternullo R, Tershy BR. 2005. From wind to whales: trophic links in a coastal upwelling system. Mar. Ecol. Prog. Ser. 289, 117–130 (doi:10.3354/meps289117) [Google Scholar]
- 10.Velez-Juarbe J, Domning DP, Pyenson ND. 2012. Iterative evolution of sympatric seacow (Dugongidae, Sirenia) assemblages during the past ∼26 million years. PLoS ONE 7, e31294 (doi:10.1371/journal.pone.0031294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terbough J, Estes JA. (eds). 2010. Trophic cascades: predators, prey, and the changing dynamics of nature. Washington, DC: Island Press [Google Scholar]
- 12.Marx FG. 2009. Marine mammals through time: when less is more in the study of palaeodiversity. Proc. R. Soc. B 276, 887–892 (doi:10.1098/rspb.2008.1473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhen MD, Pyenson ND. 2007. Diversity estimates, biases, and historiographic effects: resolving cetacean diversity in the Tertiary. Palaeontol. Electronica. 10, 11A [Google Scholar]
- 14.Pyenson ND, Irmis RB, Lipps JH. 2010. Comment on ‘Climate, critters, and cetaceans: Cenozoic drivers of the evolution of modern whales’. Science 330, 178 (doi:10.1126/science.1189866) [DOI] [PubMed] [Google Scholar]
- 15.Terry RC. 2009. The dead do not lie: using skeletal remains for rapid assessment of historical small-mammal community baselines. Proc. R. Soc. B 277, 1193–1201 (doi:10.1098/rspb.2009.1984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JH. 2011. Ghosts of Yellowstone: multi-decadal histories of wildlife populations captured by bones on a modern landscape. PLoS ONE 6, e18057 (doi:10.1371/journal.pone.0018057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidwell SM. 2001. Preservation of species abundance in marine death assemblages. Science 294, 1091–1094 (doi:10.1126/science.1064539) [DOI] [PubMed] [Google Scholar]
- 18.Kidwell SM. 2002. Time-averaged molluscan death assemblages: palimpsests of richness, snapshots of abundance. Geology 30, 803–806 (doi:10.1130/0091-7613(2002)030<0803:TAMDAP>2.0.CO;2) [Google Scholar]
- 19.Pyenson ND. 2010. Carcasses on the coast: measuring the ecological fidelity of the cetacean stranding record in eastern North Pacific Ocean. Paleobiology 36, 453–480 (doi:10.1666/09018.1) [Google Scholar]
- 20.Pyenson ND. 2011. The high fidelity of the cetacean stranding record: insights into measuring diversity by integrating taphonomy and macroecology. Proc. R. Soc. B 278, 3608–3616 (doi:10.1098/rspb.2011.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemper CM, Pledge N, Ling JK. 1997. Subfossil evidence of strandings of the sperm whale Physeter macrocephalus in Gulf St Vincent, South Australia. Rec. South Australia Mus. 29, 41–53 [Google Scholar]
- 22.Baldanza A, Bizzarri R, Famiani F, Monaco P, Pellegrino R, Sassi P. 2013. Enigmatic, biogenically induced structures in Pleistocene marine deposits: a first record of fossil ambergris. Geology 41, 1075–1078 (doi:10.1130/G34731.1) [Google Scholar]
- 23.Pyenson ND, Irmis RB, Barnes LG, Mitchell ED, Jr, McLeod SA, Lipps JH. 2009. Origin of a widespread marine bonebed deposited during the middle Miocene Climatic Optimum. Geology 37, 519–522 (doi:10.1130/G25509A.1) [Google Scholar]
- 24.Marquardt C, Lavenu A, Ortlieb L, Godoy E, Comte D. 2004. Coastal neotectonics in Southern Central Andes: uplift and deformation of marine terraces in Northern Chile (27°S). Tectonophysics 394, 193–219 (doi:10.1016/j.tecto.2004.07.059) [Google Scholar]
- 25.Achurra LE, Lacassie JP, Le Roux JP, Marquardt C, Belmar M, Ruiz-del-Solar J, Ishman SE. 2009. Manganese nodules in the Miocene Bahía Inglesa Formation, north-central Chile: petrography, geochemistry, genesis and palaeoceanographic significance. Sed. Geol. 217, 128–139 (doi:10.1016/j.sedgeo.2009.03.016) [Google Scholar]
- 26.Walsh SA, Suárez ME. 2006. New penguin remains from the Pliocene of northern Chile. Hist. Biol. 18, 115–126 (doi:10.1080/08912960600640796) [Google Scholar]
- 27.Gutstein CS, Cozzuol MA, Vargas AO, Suárez ME, Schultz CL, Rubilar-Rogers D. 2009. Patterns of skull variation of Brachydelphis (Cetacea, Odontoceti) from the Neogene of the Southeastern Pacific. J. Mamm. 90, 504–519 (doi:10.1644/07-MAMM-A-081.1) [Google Scholar]
- 28.Valenzuela-Toro AM, Gutstein CS, Varas-Malca RM, Suárez ME, Pyenson ND. 2013. Pinniped turnover in the South Pacific Ocean: new evidence from the Plio-Pleistocene of the Atacama Desert, Chile. J. Vert. Paleontol. 33, 216–233 (doi:10.1080/02724634.2012.710282) [Google Scholar]
- 29.Muizon C, de McDonald HG. 1995. An aquatic sloth from the Pliocene of Peru. Nature 375, 224–227 (doi:10.1038/375224a0) [Google Scholar]
- 30.Muizon C, McDonald HG, Salas R, Urbina M. 2004. The youngest species of the aquatic sloth Thalassocnus and a reassesment of the relationships of the nothrothere sloths (Mammalia: Xenarthra). J. Vert. Paleontol. 24, 387–397 (doi:10.1671/2429a) [Google Scholar]
- 31.Ehret DJ, Macfadden BJ, Jones DS, Devries TJ, Foster DA, Salas-Gismondi R. 2012. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the Upper Neogene Pisco Formation of Peru. Palaeontology 55, 1139–1153 [Google Scholar]
- 32.Le Roux JP, et al. 2005. Neogene-Quaternary coastal and offshore sedimentation in north-central Chile: record of sea-level changes and implications for Andean tectonism. J. South Am. Earth Sci. 19, 83–98 (doi:10.1016/j.jsames.2003.11.003) [Google Scholar]
- 33.Geraci JR, St. Aubin DJ. 1977. Mass stranding of the long-finned pilot whale, Globicephala melaena, on Sable Island, Nova Scotia. J. Fish. Res. Board Can. 34, 2193–2196 (doi:10.1139/f77-288) [Google Scholar]
- 34.Schäfer W. 1972. Ecology and palaeoecology of marine environments. Chicago, IL: University of Chicago Press [Google Scholar]
- 35.Geraci JR, Anderson DM, Timperi RJ, St. Aubin DJ, Early GA, Prescott JH, Mayo CA. 1989. Humpback whales (Megaptera novaeangliae) fatally poisoned by dinoflagellate toxin. Can. J. Fish. Aquat. Sci. 46, 1895–1898 (doi:10.1139/f89-238) [Google Scholar]
- 36.Sergeant DE. 1982. Mass strandings of toothed whales (Odontoceti) as a population phenomenon. Sci. Rep. Whales Res. Inst. Tokyo 34, 1–47 [Google Scholar]
- 37.Van Dolah FM, Doucette GJ, Gulland F, Bossart G, Rowles T. 2003. Impacts of algal toxins on marine mammals. In Toxicology of marine mammals (eds Vos J, Bossart GD, Fournier M, O'Shea T.), pp. 247–269 London, UK: Taylor & Francis [Google Scholar]
- 38.de la Riva GT, Johnson CK, Gulland FM, Langlois GW, Heyning JE, Rowles TK, Mazet JA. 2009. Association of an unusual marine mammal mortality event with Pseudo-nitzschia spp. blooms along the southern California coastline. J. Wildl. Dis. 45, 109–121 (doi:10.7589/0090-3558-45.1.109) [DOI] [PubMed] [Google Scholar]
- 39.Scholin CA, et al. 2000. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 403, 80–84 (doi:10.1038/47481) [DOI] [PubMed] [Google Scholar]
- 40.Flewelling LJ, et al. 2005. Red tides and marine mammal mortalities. Nature 435, 755–756 (doi:10.1038/nature435755a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fire SE, Wang Z, Berman M, Langlois GW, Morton SL, Sekula-Wood E, Benitez-Nelson CR. 2010. Trophic transfer of the harmful algal toxin domoic acid as a cause of death in a minke whale (Balaenoptera acutorostrata) stranding in southern California. Aquat. Mamm. 36, 342–350 (doi:10.1578/AM.36.4.2010.342) [Google Scholar]
- 42.Liebig PM, Flessa KW, Taylor SA. 2007. Taphonomic variation despite catastrophic mortality: analysis of a mass stranding of false killer whales (Pseudorca crassidens), Gulf of California, Mexico. Palaios 22, 384–391 (doi:10.2110/palo.2005.p05-052r) [Google Scholar]
- 43.Fallows C, Gallagher AJ, Hammerschlag N. 2013. White sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PLoS ONE 8, e60797 (doi:10.1371/journal.pone.0060797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver MW, Bargu S, Coale SL, Benitez-Nelson CR, Garcia AC, Roberts KJ, Sekula-Wood E, Bruland KW, Coale KH. 2010. Toxic diatoms and domoic acid in natural and iron enriched waters of the oceanic Pacific. Proc. Natl Acad. Sci. USA 109, 20 762–20 767 (doi:10.1073/pnas.1006968107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frey RW, Curran HA, Pemberton SG. 1994. Tracemaking activities of crabs and their environmental significance: the ichnogenus Psilonichnus. J. Paleontol. 58, 333–350 [Google Scholar]
- 46.Cione AL, Hospitaleche CA, Perez LM, Laza JH, Cesar I. 2010. Trace fossils on penguin bones from the Miocene of Chubut, southern Argentina. Alcheringa 34, 433–454 (doi:10.1080/03115511003793470) [Google Scholar]
- 47.Mayall M, Wright VP. 1981. Algal tuft structures from the upper Triassic of southwest England. Palaeontology 24, 655–660 [Google Scholar]
- 48.Rogers RR, Kidwell SM. 2007. The origin and interpretation of bonebeds. In Bonebeds: genesis, analysis, and paleobiological significance (eds Rogers RR, Eberth DA, Fiorillo AR.), pp. 1–64 Chicago, IL: University of Chicago Press [Google Scholar]
- 49.Prevosti FJ, Forasiepi AM, Zimicz N. 2013. The evolution of the Cenozoic terrestrial mammalian predator guild in South America: competition or replacement? J. Mamm. Evol. 20, 3–21 (doi:10.1007/s10914-011-9175-9) [Google Scholar]
- 50.Gingerich PD. 1992. Marine mammals (Cetacea and Sirenia) from the Eocene of Gebel Mokattam and Fayum, Egypt: stratigraphy, age, and paleoenvironments. Univ. Mich. Papers Paleont. 30, 1–84 [Google Scholar]
- 51.Brand LR, Esperante R, Chadwick AV, Poma O, Alomia M. 2004. Fossil whale preservation implies high diatom accumulation rate in the Miocene–Pliocene Pisco Formation of Peru. Geology 32, 165–168 (doi:10.1130/G20079.1) [Google Scholar]
- 52.Nowacek DP, Friedlaender AS, Halpin PN, Hazen EL, Johnston DW, Read AJ, Espinasse B, Zhou M, Zhu Y. 2011. Super-aggregations of krill and humpback whales in Wilhelmina Bay, Antarctic Peninsula. PLoS ONE 6, e19173 (doi:10.1371/journal.pone.0019173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emslie SD, Morgan GS. 1994. A catastrophic death assemblage and paleoclimatic implications of Pliocene seabirds of Florida. Science 264, 684–685 (doi:10.1126/science.264.5159.684) [DOI] [PubMed] [Google Scholar]
- 54.Castle JW, Rodgers JH., Jr 2009. Hypothesis for the role of toxin-producing algae in Phanerozoic mass extinctions based on evidence from the geologic record and modern environments. Environ. Geosci. 16, 1–23 (doi:10.1306/eg.08110808003) [Google Scholar]
- 55.Dezileau L, Ulloa O, Hebbeln D, Lamy F, Reyss J-L, Fontugne M. 2004. Iron control of past productivity in the coastal upwelling system off the Atacama Desert, Chile. Paleoceanography 19, PA3012 (doi:10.1029/2004PA001006) [Google Scholar]
- 56.Brongersma-Sanders M. 1957. Mass mortality in the sea. Geol. Soc. Am. Mem. 1 67, 941–1010 [Google Scholar]