Abstract
Extracorporeal membrane oxygenation (ECMO) is currently used to support patients of all ages with acute severe respiratory failure non-responsive to conventional treatments, and although initial use was almost exclusively in neonates, use for this age group is decreasing while use in older children remains stable (300-500 cases annually) and support for adults is increasing. Recent advances in technology include: refinement of double lumen veno-venous (VV) cannulas to support a large range of patient size, pumps with lower prime volumes, more efficient oxygenators, changes in circuit configuration to decrease turbulent flow and hemolysis. Veno-arterial (VA) mode of support remains the predominant type used; however, VV support has lower risk of central nervous injury and mortality. Key to successful survival is implementation of ECMO before irreversible organ injury develops, unless support with ECMO is used as a bridge to transplant. Among pediatric patients treated with ECMO mortality varies by pulmonary diagnosis, underlying condition, other non-pulmonary organ dysfunction as well as patient age, but has remained relatively unchanged overall (43%) over the past several decades. Additional risk factors associated with death include prolonged use of mechanical ventilation (> 2 wk) prior to ECMO, use of VA ECMO, older patient age, prolonged ECMO support as well as complications during ECMO. Medical evidence regarding daily patient management specifically related to ECMO is scant, it usually mirrors care recommended for similar patients treated without ECMO. Linkage of the Extracorporeal Life Support Organization dataset with other databases and collaborative research networks will be required to address this knowledge deficit as most centers treat only a few pediatric respiratory failure patients each year.
Keywords: Respiratory failure, Pediatrics, Extracorporeal life support, Veno-arterial, Veno-venous
Core tip: Extracorporeal membrane oxygenation (ECMO) is a very important mode of support for patients of all ages with acute severe respiratory failure, non-responsive to conventional treatments. Goal of this review is to describe evolution of ECMO support for respiratory failure, changes and advances in technology, epidemiology, outcomes and care of pediatric respiratory failure patients. Also, we would like to describe changes in modes of support and although veno-arterial (VA) mode of support remains the predominant type used, veno-venous (VV) support is increasingly used especially in older children and adults. We described advantages and limitations of VV ECMO comparing to VA support.
BACKGROUND
Extracorporeal membrane oxygenation (ECMO), a form of prolonged cardiopulmonary bypass (CPB), has been used to “rescue” patients suffering from severe cardiopulmonary failure unresponsive to conventional therapies for over 30 years. ECMO development has been guided by the Extracorporeal Life Support Organization (ELSO)[1], an International Consortium of Health Care Centers that voluntarily contribute detailed data to a registry supporting the vast majority of ECMO clinical research. ELSO also develops and disseminates standards and guidelines for the member programs and sponsors medical education[2,3]. In addition to supporting patients with acute, severe respiratory failure failing conventional management, there are new applications for ECMO recently reported. These include support of patients with chronic respiratory failure as a bridge to transplant[4,5] as well as cardiopulmonary support for organ donation after circulatory determination of death both in vivo[6] and ex vivo[7] Although ECMO is currently used in both adults and children, the pioneering initial application of ECMO was for neonates with severe respiratory failure.
This focus on newborns was because initial applications in patients with respiratory failure[8] found that veno-arterial (VA) bypass dramatically improved survival in “moribund” infants, while the first randomized trial in adults with severe hypoxic respiratory failure reported dismal overall survival (9%) that was not improved by ECMO support[9]. The initial success in newborns was because ECMO support could interrupt the spiral of unrelenting hypoxia and acidosis from severe pulmonary hypertension, while adults with hypoxic respiratory failure treated in the original study reported by Hardart et al[10] had already suffered severe ventilator induced lung injury (VILI) that was not reversible. Early recognition that ECMO support is only potentially effective, when implemented before irreversible organ injury develops, became the key to successful ECMO patient selection. Another lesson learned from early reports was that potential candidates should not have high risk of severe bleeding complications. Premature newborns with gestational age < 35 wk suffered high rates of severe of intraventricular hemorrhage that continues to limit ECMO use for premature infants.
EPIDEMIOLOGY OF NEONATAL RESPIRATORY FAILURE AND ECMO
In the 2013 ELSO international summary 26205 of 53190 (49%) of all patients treated with ECMO reported to ELSO were neonates with respiratory failure, and patients less than a month of age comprised 60 percent of patients treated with ECMO if neonates with cardiac failure (9%) or failed cardiopulmonary resuscitation (3%) are also considered[3].
However, ECMO use to treat severe neonatal respiratory failure peaked in 1992 and steadily declined since, as advances in other less invasive therapies occurred[11,12] such as high frequency oscillatory ventilation[13-15], inhaled nitric oxide[15-20], surfactant[21,22] and maternal antibiotic therapy[23,24]. In 2011, neonatal respiratory failure accounted for 24% of cases reported to ELSO. The neonatal respiratory failure population for whom the annual cases treated with ECMO have remained relatively unchanged over time are infants with congenital diaphragmatic hernia and unfortunately approximately 40%-50% mortality persists among these patients[3,25-27]. Understanding the contribution of reversible pulmonary hypertension relative to lung hypoplasia in these newborns continue to complicate prognosis assessment.
EPIDEMIOLOGY OF PEDIATRIC RESPIRATORY AND ECMO
ELSO annual reports of ECMO use to support pediatric patients with respiratory failure have remained fairly stable over last 5 years (300-500 cases/year) with some transient increases during years of severe influenza outbreaks[3,28-31]. Compared to neonates much less ECMO experience involves pediatric respiratory failure and no clinical trials have established efficacy in this patient group. A United States study led by James Fackler[32] was initiated, but concurrent changes in critical care practices resulted in lower pediatric mortality from acute hypoxic respiratory failure and providers were largely unwilling to enroll patients in an ECMO trial. However, studies of pediatric hypoxic respiratory failure found that centers with ECMO available had lower mortality among patients with respiratory failure compared to centers treating patients without ECMO available for select cases[32]. Furthermore, recent randomized trial in adult ARDS patients reported by Peek et al[33] found that adults transferred to a centralized ECMO center died less frequently when both standardized conventional care and “rescue” ECMO was available compared to patients treated at multiple centers with only conventional care as a treatment option.
Trials in the United Kingdom in both neonates[34,35] and adults[33] have shown that ECMO is an effective treatment for severe respiratory failure compared to conventional support with acceptable cognitive and functional status. The remaining studies in this review refer to children treated with ECMO for respiratory failure but lack key information including: the number of children with a given pulmonary process at risk for severe respiratory failure compared to the number treated with ECMO. The unanswered questions are would a child with a unique set of clinical and demographic features survive without ECMO yesterday, today and tomorrow?
ORIGINAL TECHNOLOGY
Initial ECMO support relied exclusively on VA support typically with arterial (carotid) and venous (internal jugular) cannula in the neck with the distal end of the carotid artery permanently ligated (Figure 1). The cannula drained venous blood by gravity into a reservoir (bladder) and a roller pump provided non-pulsatile propelled blood flow through an oxygenator and returned blood to the infant’s aorta. With this configuration, venous drainage can be augmented by elevating the patient on multiple cushions to increase the pressure gradient between the patient and the ECMO bladder enhancing circulating volume. Systemic anti-coagulation was primarily monitored with activated clotting times. The blood moved through a circuit, and diffusion of gases occurred across a semi-permeable membrane with oxygenation limited by time in the oxygenator membrane, so larger membranes are required to support larger patients. A blood primed bridge existed in the circuit so that flow could be diverted away from the patient to preserve the circuit from clotting when testing to see if the patient was ready for ECMO support to be removed.
Figure 1.
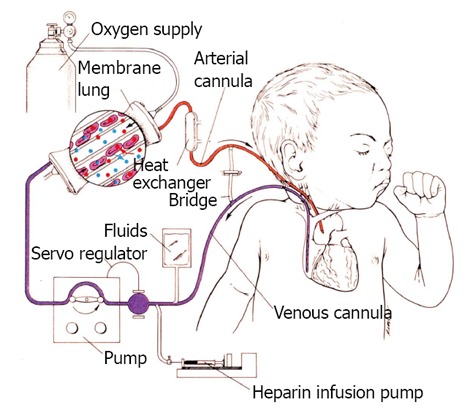
Classic veno arterial extracorporeal membrane oxygenation in an infant with cannulation of the right internal jugular vein draining blood by gravity to a roller pump that generates non pulsatile flow to a membrane oxygenator. Oxygenated blood is returned to the aorta by a catheter inserted in the right internal carotid artery.
TECHNICAL PROBLEMS
The technical limitations to early ECMO primarily revolved around complications due to inadequate flow and oxygen delivery, hemolysis, and anti-coagulation (clot and bleeding complications). Arterial cannulation for infants is primarily limited to either accessing the heart and great vessels either via a sternotomy or open cannulation of the carotid because extremity vessels are inadequate until the child is ambulatory at which time the femoral artery is an option. Infants usually tolerate loss of a unilateral carotid artery, but adults are at greater risk of hemispheric stroke complicating carotid artery cannulation and ligation. Like other neonates treated with CPB[36-39], the majority of critically ill neonates treated with ECMO survive with subtle cognitive deficits, but approximately 10%-15% of survivors manifest more severe neurologic complications and central nervous system complications also increase mortality risk[40-47].
CHANGES AND ADVANCEMENTS IN TECHNOLOGY IN ECMO FOR RESPIRATORY FAILURE
As care for patients with respiratory failure evolved during last 30 years, technological advances in ECMO have also changed to enhance safety, accessibility and facilitate its use, contributing to decreased morbidity and improved survival of patients requiring ECMO support[48].
Major advances in ECMO deployment paralleled advances in conventional CPB including cannulas, pumps, oxygenator bubble detectors and heparin bonded circuits which decrease platelet activation, circuit clotting and prolong the circuit life. There is an overall tendency to use smaller circuit size decreasing priming volume, and exposure to foreign material and blood products.
CANNULAE
Double lumen venous cannulae were initially developed for use in neonates and small infants for veno -venous (VV) support, while multiple single lumen venous cannulas were used for VV support in toddlers, older children, adolescent and adults with respiratory failure. Newer double lumen cannulas with better flow profiles and less blood recirculation have increased ease of VV use in older children.
VV double lumen catheter provides drainage of SVC and IVC and more directed “arterial flow” towards tricuspid valve but require echocardiography or fluoroscopy for initial placemen[49,50]. Insertion of these cannulas can be done percutaneously, theoretically decrease risk of infection and bleeding. In adult patients, use of these cannulas is associated with less sedation use and active rehabilitation of patients during ECMO support[51,52]. Finally manufacture of double lumen catheters for older patients made this technique possible for all size patients[53,54]. However rigorous evaluation of these catheters compared to VV ECMO accomplished with use of multiple venous catheters is needed. Although the catheters appear to decrease reperfusion and provide adequate flow, reported complications include right heart perforation during insertion[55].
PUMPS
The majority of programs traditionally used roller-head (semi-occlusive) pumps, but use of smaller, low-friction centrifugal pump has increased over last several years. These pumps have potential advantages compared to roller-head pumps where circuit flow is dependent on gravity drainage and a reservoir or “bladder” is required. These mechanical features increase turbulent flow, and tubing rupture can occur so longer circuit tubing is needed to “walk the raceway”. Thanks to magnetic drives centrifugal pumps enable use of shorter tubing and smaller priming volume. Hemolysis and renal injury appear to be more common with centrifugal pumps especially in neonates. However, hemolysis can occur with both pump types[56-59].
OXYGENATORS
Initial silicon oxygenators had very large surface areas, which were more difficult to prime and debubble and furthermore their use was associated with a large inflammatory response. Polypropylene, hollow fiber devices were developed and appeared to elicit less inflammation but have a tendency to leak plasma, which decreased oxygenator life span. Some ECMO programs used the hollow fiber devices for rapid deployment and reserved the silicon oxygenators for replacement after ECMO initiation. The newest polymethyl pentene, nonporous hollow fiber oxygenators, are now widely used, provide very efficient gas exchange, with low resistance to flow and use smaller priming volumes[59-61].
CIRCUIT CONFIGURATION AND ANTICOAGULATION
Attention to flow monitoring and pressure changes across the circuit prompted modification to decrease clotting and hemolysis. Removal of the bridge blood prime during ECMO initiation potentially could lower risk of embolic events during trials off when the circuit flow is decreased and the bridge “flashed” periodically. Heparin coated tubing often decreases requirement for anticoagulation use during the initial hours after ECMO initiation, which can be especially important for patients with bleeding complications and requiring surgical procedures.
Anticoagulation strategies to prevent circuit clotting vary widely between institutions. Currently no consensus exists regarding the best way to manage anticoagulation and blood product administration for patients supported on ECMO[62-64]. All centers use unfractionated heparin as the primary anticoagulant, while few centers reported use of alternative anticoagulation agents (direct thrombin inhibitors: Argatroban and Lepirudin or Bivalirudin) when heparin-induced thrombocytopenia is suspected.
Likewise practice varies regarding anticoagulation monitoring. ACT remains the predominant diagnostic test utilized to monitor and adjust anticoagulation, but more specific tests including anti-factor Xa level, antithrombin III (AT III) as well as thromboelastograms are used with increasing frequency at many centers. No studies have demonstrated advantages of one test over another either in complications or cost of monitoring.
VENO VENOUS ECMO VS VENO ARTERIAL
Recognition that profound respiratory failure frequently could be supported with VV ECMO (Table 1), a mode where deoxygenated blood from the patient and oxygenated blood from the ECMO circuit mix in the venous circulation and any venous clots are “filtered” by the patient’s native lungs. Thus for this approach to succeed in pediatric patients, native cardiac output must be adequate and in order to limit VILI, medical providers must accept relative hypoxia in some patients as a treatment goal (saturations 75%-85%).
Table 1.
Extracorporeal membrane oxygenation limitations and advantages comparing veno venous to veno arterial support
| Factors | Veno venous | Veno arterial |
| Systemic emboli | Lower rate unless intra cardiac shunt present | Increased rate of stroke and seizures with carotid cannulation, risk increases with patient age |
| Limb ischemia with femoral arterial cannulation | ||
| Cardiopulmonary support | Does not provide direct hemodynamic support | Provides full hemodynamic support |
| Lower systemic oxygenation | High systemic oxygenation | |
| Increased rate of hypertension during ECMO | Non pulsatile flow | |
| Usually requires some degree of pulmonary gas exchange and lung recruitment | More commonly used with severe air leak | |
| Indirect support with more oxygenated blood provided to pulmonary circulation | ||
| Organ injury | Less acute kidney injury- preserved pulsatile blood flow | More acute kidney injury |
| Less central nervous system injury risk | More central nervous system injury risk | |
| Monitoring | Mixed venous oxygen saturation less reliable due to recirculation | Reliable mixed venous saturation measurements |
| Bleeding | Increased cannula site bleeding | More bleeding with multiple site cannulation and femoral arterial cannulation compared to carotid |
| Infection | Less risk with percutaneous and single cannula use | Greater rates of infection |
| Rehabilitation | Less sedation use if adequate oxygen delivery possible | |
| Mobilization of patients more feasible with single catheter neck catheter |
ECMO: Extracorporeal membrane oxygenation.
VV ECMO can be accomplished using two separate venous catheters or a double lumen catheter. However, recirculation of blood can complicate both blood saturation monitoring and limits effective blood flow. As described above, double lumen catheter improvements decreases recirculation and improved patient arterial oxygenation when optimally positioned.
While extracorporeal support for patients with severe respiratory failure is implemented as a life saving and lung protective measure, it also carries significant risks of complications. Most commonly described and feared are neurological complications: intracranial hemorrhage, thromboembolic or ischemic strokes and seizures as they can have a profound impact on the patient’s overall outcome[42-47]. These complications are related to mode of support, with VV ECMO thought to be safer from thromboembolic stand point. Also sparing of the carotid artery, which remains the most common arterial cannulation site in children with respiratory failure, could contribute to less neurological injury.
In a single center report comparing neonates who survived ECMO, infants treated with VV had significantly lower risk evidence of embolic brain lesions compared to those supported with VA when patients were routinely imaged with MRI[43]. Similarly analysis of ELSO data for pediatric patients with respiratory failure compared to patients found lower rates of central nervous system radiological injury and seizures for children treated with VV compared to VA, however, reports to ELSO, review clinical data and ascertainment of these complications likely vary by reporting center and asymptomatic patients are not routinely evaluated[65]. These findings are also similar to reports of patients supported with VA ECMO to treated failed CPR in adults[66] and children[67-69].
Surveillance of brain function among pediatric patients supported on ECMO varies. Although most centers routinely follow serial cranial ultrasound studies in infants with an open fontanel, imaging of the brain among older patients is often reserved for patients with new neurological deficits. Thus avoidance of prolonged neuromuscular blockage and minimal sedation when possible enhances neurologic monitoring for frequent patient assessment. However assessment of sedated patients if often difficult, and a quarter of pediatric patients with intracranial pathology detected by CT did not have clinical evidence of neurological compromise[70].
Rates of other commonly reported complications during ECMO support including renal failure; bleeding and infection differ by support mode. Development of acute kidney injury (AKI) and fluid overload are common among patients supported on ECMO and especially renal failure is seen more commonly during VA support[3]. It is impossible to assess how much ECMO support contributes to renal failure distinct from injury related to pre-ECMO events. Overzealous fluid removal with diuretics or continuous renal replacement therapy (CRRT), lack of pulsatile flow with VA support and hemolysis all may contribute to development of renal insufficiency[71-75]. A recent report by Wolf et al[76] of patients with congenital heart disease supported on ECMO, highlighted that early implementation of CRRT and too aggressive fluid removal may lead to intravascular volume depletion, aggravate AKI and worsen outcome.
As with other modes of mechanical support, ECMO also carries risk of acquired infection during the bypass run. In 2010 the ELSO international summary data reported the incidence of culture-proven bacterial infection increased with age: 6.1 % in neonates, 18.7% in children and 20.5% in adults and was associated with increased mortality[77,78]. Duration of catheter use, ECMO to support CPR, VA mode were all associated with increased infection risk[79,80].
Bleeding on ECMO is the most frequently described complication resulting from necessity of continuous anti-coagulation to prevent circuit clotting and thromboembolic events. Surgical cannulation site and other procedural incisions are the most commonly reported bleeding sites. Rollins et al[65] evaluated bleeding from surgical cannula site among pediatric patients receiving ECMO for respiratory failure and reported hemorrhage rates were significantly greater for VV (19%) than VA (15%) but among arterial cannula site rates of hemorrhage were greater in the femoral artery (28%) compared to carotid artery (15%).
Trials off VV support are simplified compared to VA because the oxygenator can be “isolated” from the patient at full flow by simply removing the gas source. Trials off VA support increase risk of clot development and emboli due to both stasis with lower pump flows, changes in blood volume relative to heparin dosing and flushing the bridge, which leads to turbulent flow. Finally, among patients treated with VA ECMO for respiratory failure, infants, children and adults have higher mortality compared to patients supported with VV[3,48]; however, this observation is likely confounded at least in part by severity of illness as high need for vasoactive medications and cardiopulmonary arrest immediately preceding initiation of ECMO are viewed by many critical care physicians as a contraindication for VV ECMO use.
OUTCOME/MORTALITY
Because the ELSO registry and other administrative data sets do not systematically collect information regarding disability, hospital mortality is most commonly reported. Among patients treated with ECMO mortality varies by pulmonary diagnosis, underlying condition, other non-pulmonary organ dysfunction as well as patient age[48,81], but has remained relatively unchanged overall (43%) over the past several decades. Although survival among patients without concurrent non-pulmonary disease has improved, ECMO has increasingly been reserved for medically complex patients with acute other organ failure and/or underlying chronic diseases (Table 2). This is logical as ECMO continues to be a “rescue” therapy and mortality from acute respiratory failure in children has declined concurrent with recognition of successful strategies to decrease VILI as well as other improvements in critical care[82-84]. Indirect evidence that “gentle” ventilation has been embraced by the pediatric critical care community is provided by the most recent ELSO registry analysis, in which the duration of pre-ECMO mechanical ventilation was no longer associated with lower survival until ECMO deployment occurred at 2 wk or more after initiation of mechanical ventilation[48,85].
Table 2.
Factors associated with hospital mortality among pediatric patients receiving extracorporeal membrane oxygenation for respiratory failure
| Factors | Increased survival | Increased death |
| Age | Younger | Age > 10 yr |
| Pulmonary process | Asthma | Pertussis |
| Viral pneumonia/bronchiolitis | Sepsis | |
| Aspiration pneumonia | Opportunistic infections | |
| Organ dysfunction | Renal failure/dialysis | |
| Liver injury | ||
| Immune impairment/deficiency | ||
| Cardiac arrest prior to ECMO | ||
| Severity of ventilator associated lung injury prior to ECMO | Severe acidosis | |
| High mean airway pressure | ||
| Duration ventilation > 14 d | ||
| Mode | VV | VA |
| Complications during ECMO | Infection | |
| Stroke | ||
| Cardiac arrest | ||
| Organ failure |
VV: Veno venous; VA: Veno arterial; ECMO: Extracorporeal membrane oxygenation.
ECMO use and prognosis in pediatric patients with intractable respiratory failure differs from use in newborns in several obvious (e.g., range of patient size, age, survival) and less obvious ways (reversibility of disease process). Like neonates, use of VV ECMO is increasing but remains less common than VA support. Recent advances in availability of double lumen catheters that decrease recirculation for use in patients of all sizes may continue to enhance use of VV; however, VV cannulation is technically more difficult and associated with increased cannulation site bleeding and flow issues[65]. VV support should be the default choice for pediatric respiratory failure because central nervous system injury is less common, and recent ELSO analysis did not find increased mortality from a “failed” trial of VV requiring conversion to VA support. Among pediatric patients who require VA, firm recommendations cannot be made regarding arterial site. Although carotid cannulation is avoided in adults due to concern for hemispheric stroke, insufficient data are available in older children to make firm recommendations when children requiring VA support should transition to femoral artery use rather than carotid[65]. When using the femoral artery for VA ECMO, a bypass graft or reperfusion catheter to maintain adequate limb perfusion is often required.
Other factors associated with increased survival among pediatric ECMO patients with respiratory failure include: young age, obstructive lung diseases compared to restrictive processes (e.g., asthma or bronchiolitis vs acute lung injury), and primary lung injury compared to secondary lung injury (e.g., pneumonia vs sepsis)[48,86,87]. Risk of mortality increases for potential ECMO patients with acute respiratory failure if they have either pre-existing chronic non-pulmonary organ failure or develop additional acute organ failure before or after institution of ECMO. Patients who can tolerate net fluid loss while receiving ECMO support have lower risk of death[88], while development of renal failure during ECMO has repeatedly been associated with increased mortality[71-75]. It remains unclear if early fluid removal on ECMO improves outcome. Recently Selewski et al[75] examined the association between fluid overload and outcomes in pediatric ECMO patients receiving continuous renal replacement therapy (CRRT). Among patients treated with ECMO, 28% received concurrent CRRT and survival was significantly lower (34% vs 58%) compared to those not treated with CRRT. However, it is likely that those receiving CRRT had greater severity of illness and greater risk of death.
As expected patients with underlying immune disorders or an active malignancy have lower survival compared to patients with intact immune function[48]. Although profound immunosuppression was once considered an absolute contraindication, now decisions regarding whether a child is an ECMO candidate include nuisances such as the anticipated duration of neutropenia, other organ failure and ultimate cancer prognosis. Although numbers of patients treated with ECMO after hematopoietic stem cell transplantation are few, some cases have survived to hospital discharge; however, long term cancer survival was not reported.
Complications occurring after institution of ECMO that reflect either inadequate cardiorespiratory support or end organ failure arising from pre-ECMO injury have consistently been associated with increased risk of mortality[48,87]. Thus care to avoid complications during ECMO may increase survival. Although most pediatric patients with respiratory failure are able to be liberated from ECMO within 3 wk, survival decreases with prolonged duration; however, no studies have enumerated factors that clearly predict death and typically support is continued till either complications ensue causing multiple organ dysfunction or pulmonary status improves and the patient can be supported without ECMO. Survival among patients treated with ECMO for longer than 3 wk decreases to 38%.
CARE OF PEDIATRIC RESPIRATORY FAILURE ECMO PATIENTS
Many management aspects of respiratory failure for patients treated with ECMO are not based on medical evidence specific to ECMO care, but mirrors care recommended for similar patients treated without ECMO. However, ECMO does affect management. For instance some medications bind to or interact with the ECMO circuit (e.g., fentanyl) or clearance may be altered by addition of dialysis (e.g., barbiturates) so critical care teams should include expertise from a pharmacologist. Certainly patients must be able to cooperate with care without risk of unplanned medical appliance dislodgement. Some pediatric age patients require high levels of sedation; however, when possible neuromuscular blocking agents (NMBA) should be avoided and sedation minimized to allow spontaneous coughing, optimize respiratory secretion clearance and decrease respiratory and peripheral muscle de-conditioning. In addition, reduced use of deep sedation and NMBA will allow better assessment of patient neurological status[62,89]. As with survivors of any critical illness, ECMO survivors may be left with significant psychological and functional disabilities[90]. Recently more attention is paid to early recognition of possible neurological injury which should lead to early implementation of rehabilitation. Although implementation of physical therapy (PT) for patients while still on ECMO is very challenging due to increased risk of acute decompensation, medical device dislodgment , some authors suggest use of simple PT interventions to avoid motor and cardio-respiratory deconditioning[91]. Reports from multiple centers show that cooperative patients can assist with mobility including pulmonary rehabilitation while on ECMO without high risk of appliance movement and that prone positioning is routinely possible in pediatric patients[91-94]. However, it is unclear which components of rehabilitation are essential to maintain and improve functional outcomes (e.g., passive limb range of motion, splinting, active limb exercises/strengthening, ambulation) are not known. If tolerated by the patient, rehabilitation should be continued through post-ECMO hospitalization period and following hospital discharge[94].
No studies have evaluated optimal nutrition goals, but many critical care providers consider enteral feeds preferable to intravenous nutrition. Our center routinely uses trans pyloric feeding tubes rather than nasogastric to decrease risk for aspiration of formula. The optimal transfusion threshold during ECMO also remains unclear. Especially for patients treated with VV ECMO, providers must weigh potential benefits from increased oxygen deliver and potential harm from increased fluid overload. Recent trends in both adult and pediatric critical care medicine have found similar patient outcomes with lower red blood cell administration thresholds[95,96]. Many providers now accept lower transfusion thresholds (hgb > 10 g/dL) for ECMO pediatric patients with normal arterial saturations but maintain higher thresholds for desaturated patients. Trending mixed venous desaturation is an additional measure to aid transfusion decisions. If the mixed venous saturation increases after a transfusion then a higher hemoglobin concentration may provide physiologic benefit However, ECMO patients who tolerate fluid loss either using diuretics or hemoconcentration have higher rates of survival[88,97].
The mechanical ventilator strategy for respiratory ECMO should limit VILI[98-100]. Maintaining lung recruitment is ideal unless severe air-leak syndrome has developed, our practice is to reduce positive end expiratory pressure till the leak is minimized. Otherwise, PEEP is maintained and a low stretch ventilation strategy initiated as the circuit removal of carbon dioxide is efficient. No studies have compared conventional to high frequency oscillation ventilation in ECMO patients but patient care and avoidance of NMBA is easier to achieve during conventional ventilation.
Other care should be directed to decrease complications. Because they are intensively monitored ECMO patients have increased risk of catheter associated blood stream infections[101] and other nosocomial infections. Development of other organ failure substantially increases mortality. ECMO should be removed as soon as pulmonary compliance and gas exchange have improved and the patient can be maintained on non-toxic ventilator settings.
CONCLUSION
ECMO continues to be used as a rescue therapy for increasingly complex pediatric patients with respiratory failure. VV ECMO should be the used as the default mode due to lower complication rates and many aspects of ideal care remain unstudied. Linkage of the ELSO dataset with other databases or collaborative research networks will be required to address this knowledge deficit as most centers treat only a few pediatric respiratory failure patients each year.
Footnotes
P- Reviewers: Inaba H, Ntoumenopoulos G S- Editor: Gou SX L- Editor: A E- Editor: Lu YJ
References
- 1. Available from: http: //www.elsonet.org. Accessed 03-19-2013.
- 2. Available from: http: //www.elsonet.org/index.php/resources/guidelines.html. Accessed 03-19-2013.
- 3. Available from: http: //www.elsonet.org/index.php/registry/statistics/limited.html.
- 4.Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–768. doi: 10.1164/rccm.201109-1599OC. [DOI] [PubMed] [Google Scholar]
- 5.Diso D, Anile M, Patella M, Pecoraro Y, Rendina EA, Carillo C, Russo E, Onorati I, Angioletti D, Ruberto F, et al. Lung transplantation for cystic fibrosis: outcome of 101 single-center consecutive patients. Transplant Proc. 2013;45:346–348. doi: 10.1016/j.transproceed.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Magliocca JF, Magee JC, Rowe SA, Gravel MT, Chenault RH, Merion RM, Punch JD, Bartlett RH, Hemmila MR. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58:1095–101; discussion 1101-2. doi: 10.1097/01.ta.0000169949.82778.df. [DOI] [PubMed] [Google Scholar]
- 7.Fondevila C, Hessheimer AJ, Flores E, Ruiz A, Mestres N, Calatayud D, Paredes D, Rodríguez C, Fuster J, Navasa M, et al. Applicability and results of Maastricht type 2 donation after cardiac death liver transplantation. Am J Transplant. 2012;12:162–170. doi: 10.1111/j.1600-6143.2011.03834.x. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 9.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, Thomas AN, Proctor HJ, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 10.Hardart GE, Fackler JC. Predictors of intracranial hemorrhage during neonatal extracorporeal membrane oxygenation. J Pediatr. 1999;134:156–159. doi: 10.1016/s0022-3476(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 11.Hintz SR, Suttner DM, Sheehan AM, Rhine WD, Van Meurs KP. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics. 2000;106:1339–1343. doi: 10.1542/peds.106.6.1339. [DOI] [PubMed] [Google Scholar]
- 12.Roy BJ, Rycus P, Conrad SA, Clark RH. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics. 2000;106:1334–1338. doi: 10.1542/peds.106.6.1334. [DOI] [PubMed] [Google Scholar]
- 13.deLemos R, Yoder B, McCurnin D, Kinsella J, Clark R, Null D. The use of high-frequency oscillatory ventilation (HFOV) and extracorporeal membrane oxygenation (ECMO) in the management of the term/near term infant with respiratory failure. Early Hum Dev. 1992;29:299–303. doi: 10.1016/0378-3782(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 14.Gerstmann DR, Minton SD, Stoddard RA, Meredith KS, Monaco F, Bertrand JM, Battisti O, Langhendries JP, Francois A, Clark RH. The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics. 1996;98:1044–1057. [PubMed] [Google Scholar]
- 15.Clark RH, Yoder BA, Sell MS. Prospective, randomized comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. J Pediatr. 1994;124:447–454. doi: 10.1016/s0022-3476(94)70374-4. [DOI] [PubMed] [Google Scholar]
- 16.Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, deLemos RA, Sardesai S, McCurnin DC, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- 17.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 18.Christou H, Van Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, Stark AR, Kourembanas S. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med. 2000;28:3722–3727. doi: 10.1097/00003246-200011000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Dobyns EL, Anas NG, Fortenberry JD, Deshpande J, Cornfield DN, Tasker RC, Liu P, Eells PL, Griebel J, Kinsella JP, et al. Interactive effects of high-frequency oscillatory ventilation and inhaled nitric oxide in acute hypoxemic respiratory failure in pediatrics. Crit Care Med. 2002;30:2425–2429. doi: 10.1097/00003246-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative European Multicenter Study Group. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics. 1988;82:683–691. [PubMed] [Google Scholar]
- 22.Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A, Denson SE, Sehgal SS, Gross I, Stevens D. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics. 1991;88:19–28. [PubMed] [Google Scholar]
- 23.Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271–1279. doi: 10.1016/s0140-6736(95)91868-x. [DOI] [PubMed] [Google Scholar]
- 24.Mercer BM, Miodovnik M, Thurnau GR, Goldenberg RL, Das AF, Ramsey RD, Rabello YA, Meis PJ, Moawad AH, Iams JD, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989–995. [PubMed] [Google Scholar]
- 25.Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2009;44:1315–1321. doi: 10.1016/j.jpedsurg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, Stolar CJ, Hahn E, Pietsch J, Chung D, Moore D, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. 2013;163:114–119. e1. doi: 10.1016/j.jpeds.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens TP, Chess PR, McConnochie KM, Sinkin RA, Guillet R, Maniscalco WM, Fisher SG. Survival in early- and late-term infants with congenital diaphragmatic hernia treated with extracorporeal membrane oxygenation. Pediatrics. 2002;110:590–596. doi: 10.1542/peds.110.3.590. [DOI] [PubMed] [Google Scholar]
- 28.Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 30.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, et al. Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–e1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zangrillo A, Biondi-Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, Pappalardo F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green TP, Timmons OD, Fackler JC, Moler FW, Thompson AE, Sweeney MF. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Crit Care Med. 1996;24:323–329. doi: 10.1097/00003246-199602000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 34.Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J, Pandya H, Firmin R, Liddell M, Davis C, et al. Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK. Arch Dis Child Fetal Neonatal Ed. 2009;94:F129–F132. doi: 10.1136/adc.2008.141051. [DOI] [PubMed] [Google Scholar]
- 35.Petrou S, Bischof M, Bennett C, Elbourne D, Field D, McNally H. Cost-effectiveness of neonatal extracorporeal membrane oxygenation based on 7-year results from the United Kingdom Collaborative ECMO Trial. Pediatrics. 2006;117:1640–1649. doi: 10.1542/peds.2005-1150. [DOI] [PubMed] [Google Scholar]
- 36.Sananes R, Manlhiot C, Kelly E, Hornberger LK, Williams WG, MacGregor D, Buncic R, McCrindle BW. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93:1577–1583. doi: 10.1016/j.athoracsur.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld RE, Clark BG, Robertson CM, Moddemann DM, Dinu IA, Joffe AR, Sauve RS, Creighton DE, Zwaigenbaum L, Ross DB, et al. Five-year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2008;136:1413–1421, 1413-1421. doi: 10.1016/j.jtcvs.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, Lin M, Bellinger DC. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 39.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 40.Costello JM, O’Brien M, Wypij D, Shubert J, Salvin JW, Newburger JW, Laussen PC, Arnold JH, Fynn-Thompson F, Thiagarajan RR. Quality of life of pediatric cardiac patients who previously required extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012;13:428–434. doi: 10.1097/PCC.0b013e318238ba21. [DOI] [PubMed] [Google Scholar]
- 41.The collaborative UK ECMO (Extracorporeal Membrane Oxygenation) trial: follow-up to 1 year of age. Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.4.e1. [DOI] [PubMed] [Google Scholar]
- 42.Friedman S, Chen C, Chapman JS, Jeruss S, Terrin N, Tighiouart H, Parsons SK, Wilson JM. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg. 2008;43:1035–1043. doi: 10.1016/j.jpedsurg.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Rollins MD, Yoder BA, Moore KR, Barnhart DC, Jones C, Null DM, DiGeronimo RJ. Utility of neuroradiographic imaging in predicting outcomes after neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2012;47:76–80. doi: 10.1016/j.jpedsurg.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Hervey-Jumper SL, Annich GM, Yancon AR, Garton HJ, Muraszko KM, Maher CO. Neurological complications of extracorporeal membrane oxygenation in children. J Neurosurg Pediatr. 2011;7:338–344. doi: 10.3171/2011.1.PEDS10443. [DOI] [PubMed] [Google Scholar]
- 45.Hamrick SE, Gremmels DB, Keet CA, Leonard CH, Connell JK, Hawgood S, Piecuch RE. Neurodevelopmental outcome of infants supported with extracorporeal membrane oxygenation after cardiac surgery. Pediatrics. 2003;111:e671–e675. doi: 10.1542/peds.111.6.e671. [DOI] [PubMed] [Google Scholar]
- 46.Danzer E, Gerdes M, D’Agostino JA, Partridge EA, Hoffman-Craven CH, Bernbaum J, Rintoul NE, Flake AW, Adzick NS, Hedrick HL. Preschool neurological assessment in congenital diaphragmatic hernia survivors: outcome and perinatal factors associated with neurodevelopmental impairment. Early Hum Dev. 2013;89:393–400. doi: 10.1016/j.earlhumdev.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Waitzer E, Riley SP, Perreault T, Shevell MI. Neurologic outcome at school entry for newborns treated with extracorporeal membrane oxygenation for noncardiac indications. J Child Neurol. 2009;24:801–806. doi: 10.1177/0883073808330765. [DOI] [PubMed] [Google Scholar]
- 48.Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011;39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 49.Javidfar J, Wang D, Zwischenberger JB, Costa J, Mongero L, Sonett J, Bacchetta M. Insertion of bicaval dual lumen extracorporeal membrane oxygenation catheter with image guidance. ASAIO J. 2011;57:203–205. doi: 10.1097/MAT.0b013e3182155fee. [DOI] [PubMed] [Google Scholar]
- 50.de Bucourt M, Teichgräber UK. Image guided placement of extracorporeal life support through bi-caval dual lumen venovenous membrane oxygenation in an interventional radiology setting--initial experience. J Vasc Access. 2012;13:221–225. doi: 10.5301/jva.5000033. [DOI] [PubMed] [Google Scholar]
- 51.Camboni D, Philipp A, Lubnow M, Bein T, Zausig Y, Hilker M, Flörchinger B, Rupprecht L, Keyser A, Kobuch R, et al. Extracorporeal membrane oxygenation by single-vessel access in adults: advantages and limitations. ASAIO J. 2012;58:616–621. doi: 10.1097/MAT.0b013e31826a8a32. [DOI] [PubMed] [Google Scholar]
- 52.Reeb J, Falcoz PE, Santelmo N, Massard G. Double lumen bi-cava cannula for veno-venous extracorporeal membrane oxygenation as bridge to lung transplantation in non-intubated patient. Interact Cardiovasc Thorac Surg. 2012;14:125–127. doi: 10.1093/icvts/ivr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazar DA, Cass DL, Olutoye OO, Kim ES, Welty SE, Fernandes CJ, Lee TC. Venovenous cannulation for extracorporeal membrane oxygenation using a bicaval dual-lumen catheter in neonates. J Pediatr Surg. 2012;47:430–434. doi: 10.1016/j.jpedsurg.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 54.Fallon SC, Shekerdemian LS, Olutoye OO, Cass DL, Zamora IJ, Nguyen T, Kim ES, Larimer EL, Lee TC. Initial experience with single-vessel cannulation for venovenous extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Crit Care Med. 2013;14:366–373. doi: 10.1097/PCC.0b013e31828a70dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirose H, Yamane K, Marhefka G, Cavarocchi N. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg. 2012;7:36. doi: 10.1186/1749-8090-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barrett CS, Jaggers JJ, Cook EF, Graham DA, Rajagopal SK, Almond CS, Seeger JD, Rycus PT, Thiagarajan RR. Outcomes of neonates undergoing extracorporeal membrane oxygenation support using centrifugal versus roller blood pumps. Ann Thorac Surg. 2012;94:1635–1641. doi: 10.1016/j.athoracsur.2012.06.061. [DOI] [PubMed] [Google Scholar]
- 57.Barrett CS, Jaggers JJ, Cook EF, Graham DA, Yarlagadda VV, Teele SA, Almond CS, Bratton SL, Seeger JD, Dalton HJ, et al. Pediatric ECMO outcomes: comparison of centrifugal versus roller blood pumps using propensity score matching. ASAIO J. 2013;59:145–151. doi: 10.1097/MAT.0b013e31828387cd. [DOI] [PubMed] [Google Scholar]
- 58.Meyer AD, Wiles AA, Rivera O, Wong EC, Freishtat RJ, Rais-Bahrami K, Dalton HJ. Hemolytic and thrombocytopathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. 2012;13:e255–e261. doi: 10.1097/PCC.0b013e31823c98ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan S, Vasavada R, Qiu F, Kunselman A, Undar A. Extracorporeal life support systems: alternative vs. conventional circuits. Perfusion. 2011;26:191–198. doi: 10.1177/0267659110395060. [DOI] [PubMed] [Google Scholar]
- 60.Iwahashi H, Yuri K, Nosé Y. Development of the oxygenator: past, present, and future. J Artif Organs. 2004;7:111–120. doi: 10.1007/s10047-004-0268-6. [DOI] [PubMed] [Google Scholar]
- 61.Lim MW. The history of extracorporeal oxygenators. Anaesthesia. 2006;61:984–995. doi: 10.1111/j.1365-2044.2006.04781.x. [DOI] [PubMed] [Google Scholar]
- 62.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14:e77–e84. doi: 10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buck ML. Control of Coagulation during Extracorporeal Membrane Oxygenation. J Pediatr Pharmacol Ther. 2005;10:26–35. doi: 10.5863/1551-6776-10.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Görlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best Pract Res Clin Anaesthesiol. 2012;26:179–198. doi: 10.1016/j.bpa.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Rollins MD, Hubbard A, Zabrocki L, Barnhart DC, Bratton SL. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg. 2012;47:68–75. doi: 10.1016/j.jpedsurg.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68:1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 68.Barrett CS, Bratton SL, Salvin JW, Laussen PC, Rycus PT, Thiagarajan RR. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 69.Raymond TT, Cunnyngham CB, Thompson MT, Thomas JA, Dalton HJ, Nadkarni VM. Outcomes among neonates, infants, and children after extracorporeal cardiopulmonary resuscitation for refractory inhospital pediatric cardiac arrest: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatr Crit Care Med. 2010;11:362–371. doi: 10.1097/PCC.0b013e3181c0141b. [DOI] [PubMed] [Google Scholar]
- 70.Lidegran MK, Mosskin M, Ringertz HG, Frenckner BP, Lindén VB. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: Clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14:62–71. doi: 10.1016/j.acra.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46:630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 72.Meyer RJ, Brophy PD, Bunchman TE, Annich GM, Maxvold NJ, Mottes TA, Custer JR. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med. 2001;2:238–242. doi: 10.1097/00130478-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Paden ML, Warshaw BL, Heard ML, Fortenberry JD. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:153–158. doi: 10.1097/PCC.0b013e3181e2a596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Askenazi DJ, Selewski DT, Paden ML, Cooper DS, Bridges BC, Zappitelli M, Fleming GM. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7:1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, Gaies MG, Annich GM, Kershaw DB, Shanley TP, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40:2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolf MJ, Chanani NK, Heard ML, Kanter KR, Mahle WT. Early renal replacement therapy during pediatric cardiac extracorporeal support increases mortality. Ann Thorac Surg. 2013;96:917–922. doi: 10.1016/j.athoracsur.2013.05.056. [DOI] [PubMed] [Google Scholar]
- 77.Meyer DM, Jessen ME, Eberhart RC. Neonatal extracorporeal membrane oxygenation complicated by sepsis. Extracorporeal Life Support Organization. Ann Thorac Surg. 1995;59:975–980. doi: 10.1016/0003-4975(95)00044-l. [DOI] [PubMed] [Google Scholar]
- 78.Douglass BH, Keenan AL, Purohit DM. Bacterial and fungal infection in neonates undergoing venoarterial extracorporeal membrane oxygenation: an analysis of the registry data of the extracorporeal life support organization. Artif Organs. 1996;20:202–208. doi: 10.1111/j.1525-1594.1996.tb04428.x. [DOI] [PubMed] [Google Scholar]
- 79.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12:277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 80.Odetola F, Custer JR. Infections acquired while on extracorporeal membrane oxygenation: navigating the maze. Pediatr Crit Care Med. 2011;12:353–355. doi: 10.1097/PCC.0b013e3181e8b73c. [DOI] [PubMed] [Google Scholar]
- 81.Moler FW, Custer JR, Bartlett RH, Palmisano JM, Akingbola O, Taylor RP, Maxvold NJ. Extracorporeal life support for severe pediatric respiratory failure: an updated experience 1991-1993. J Pediatr. 1994;124:875–880. doi: 10.1016/s0022-3476(05)83174-9. [DOI] [PubMed] [Google Scholar]
- 82.Arnold JH, Anas NG, Luckett P, Cheifetz IM, Reyes G, Newth CJ, Kocis KC, Heidemann SM, Hanson JH, Brogan TV, et al. High-frequency oscillatory ventilation in pediatric respiratory failure: a multicenter experience. Crit Care Med. 2000;28:3913–3919. doi: 10.1097/00003246-200012000-00031. [DOI] [PubMed] [Google Scholar]
- 83.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124:87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 84.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 85.Brogan TV, Zabrocki L, Thiagarajan RR, Rycus PT, Bratton SL. Prolonged extracorporeal membrane oxygenation for children with respiratory failure. Pediatr Crit Care Med. 2012;13:e249–e254. doi: 10.1097/PCC.0b013e31824176f4. [DOI] [PubMed] [Google Scholar]
- 86.Smalley N, MacLaren G, Best D, Paul E, Butt W. Outcomes in children with refractory pneumonia supported with extracorporeal membrane oxygenation. Intensive Care Med. 2012;38:1001–1007. doi: 10.1007/s00134-012-2581-5. [DOI] [PubMed] [Google Scholar]
- 87.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–2114. doi: 10.1007/s00134-009-1661-7. [DOI] [PubMed] [Google Scholar]
- 88.Swaniker F, Kolla S, Moler F, Custer J, Grams R, Barlett R, Hirschl R. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35:197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 89.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30:134–142. doi: 10.1097/WNP.0b013e3182872af9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madderom MJ, Reuser JJ, Utens EM, van Rosmalen J, Raets M, Govaert P, Steiner K, Gischler SJ, Tibboel D, van Heijst AF, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: a nationwide multicenter study. Intensive Care Med. 2013;39:1584–1593. doi: 10.1007/s00134-013-2973-1. [DOI] [PubMed] [Google Scholar]
- 91.Thiagarajan RR, Teele SA, Teele KP, Beke DM. Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med. 2012;5:47–52. doi: 10.3233/PRM-2012-0195. [DOI] [PubMed] [Google Scholar]
- 92.Rahimi RA, Skrzat J, Reddy DR, Zanni JM, Fan E, Stephens RS, Needham DM. Physical rehabilitation of patients in the intensive care unit requiring extracorporeal membrane oxygenation: a small case series. Phys Ther. 2013;93:248–255. doi: 10.2522/ptj.20120336. [DOI] [PubMed] [Google Scholar]
- 93.Haefner SM, Bratton SL, Annich GM, Bartlett RH, Custer JR. Complications of intermittent prone positioning in pediatric patients receiving extracorporeal membrane oxygenation for respiratory failure. Chest. 2003;123:1589–1594. doi: 10.1378/chest.123.5.1589. [DOI] [PubMed] [Google Scholar]
- 94.Golej J, Trittenwein G. Early detection of neurologic injury and issues of rehabilitation after pediatric cardiac extracorporeal membrane oxygenation. Artif Organs. 1999;23:1020–1025. doi: 10.1046/j.1525-1594.1999.06446.x. [DOI] [PubMed] [Google Scholar]
- 95.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 96.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 97.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, Brett M, Convery M, Christie LM, Karamessinis L, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan E, Villar J, Slutsky AS. Novel approaches to minimize ventilator-induced lung injury. BMC Med. 2013;11:85. doi: 10.1186/1741-7015-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plataki M, Hubmayr RD. The physical basis of ventilator-induced lung injury. Expert Rev Respir Med. 2010;4:373–385. doi: 10.1586/ers.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;2:CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Odetola FO, Moler FW, Dechert RE, VanDerElzen K, Chenoweth C. Nosocomial catheter-related bloodstream infections in a pediatric intensive care unit: risk and rates associated with various intravascular technologies. Pediatr Crit Care Med. 2003;4:432–436. doi: 10.1097/01.PCC.0000090286.24613.40. [DOI] [PubMed] [Google Scholar]


