Abstract
Background: Docosahexaenoic acid (DHA) accumulates in the hippocampus and frontal lobes of the fetal brain during the last trimester of pregnancy. These areas of the brain contribute to attention and working memory and inhibitory control (WMIC).
Objective: We evaluated the effect of maternal omega-3 (n−3) long-chain polyunsaturated fatty acid supplementation in pregnancy on child attention and WMIC.
Design: A total of 185 term-born children of mothers who were randomly allocated to consume 800 mg DHA/d (treatment) or a placebo (control) from ∼20 wk of gestation until birth were assessed with multiple measures of attention and WMIC at a mean (±SD) of 27 ± 2 mo. Primary outcomes were the average time it took to be distracted when playing with a toy (distractibility) and the accuracy of remembering a new hiding location while inhibiting a learned response to search in the previous location (WMIC).
Results: Assessments were completed by 81 children in the treatment group (mean ± SD age: 835± 50.4 d) and 77 children in the control group (839 ± 65.6 d). There was no effect of supplementation on primary outcomes [distractibility mean difference: −0.2 s (95% CI: −0.7, 0.4 s); WMIC mean difference: 8.9 mm (95% CI: −10.6, 28.3 mm)]. There was no difference between DHA-supplemented and control groups except that treatment-group children looked away from the toys fewer times than controls when presented with multiple toys competing for attention but less accurately remembered a repeated hiding location. These secondary effects were not consistent with any other outcomes and may have been a result of chance. Cord plasma DHA was not consistently associated with attention and WMIC.
Conclusion: Maternal DHA supplementation during pregnancy does not enhance attention or WMIC in term-born preschoolers. The DHA for Maternal and Infant Outcomes trial was registered at www.anzctr.org.au as ACTRN1260500056906.
INTRODUCTION
The peak growth period of the brain is during the last trimester of pregnancy (1) when the accumulation of the omega-3 long-chain PUFA DHA in neural tissues is at the greatest velocity (2, 3). During this phase, the frontal lobes and hippocampus undergo an intense period of growth (1, 4). These areas of the brain are responsible for higher-order cognitive skills known as executive functions (EFs)4. Animal studies of omega-3 (n−3) fatty acid (FA) deprivation during pregnancy has shown a reduced concentration of offspring neural DHA (1, 4, 5) with deficits in abilities that reflect the functioning of the frontal lobes and hippocampus (6–8).
The amount of DHA required by the human fetus is thought to exceed the typical DHA intake of women of child-bearing age who consume a Westernized diet, which has led to the belief that supplementation during pregnancy will enhance the brain development of the child. Randomized controlled trials (RCTs) of omega-3 long-chain PUFA (LCPUFA) supplementation during pregnancy with neurodevelopment outcomes in the child have yielded inconsistent results (see reference 9 for a review). These inconsistencies may be attributable to the type of tests used to assess neurodevelopment; most RCTs have used standardized global tests of cognition (9). Although global tests capture abilities across major neurologic domains, they may lack the sensitivity to detect differences in specific neural functions such as EFs (10).
Researchers are increasingly recommending that nutrition intervention studies use outcome measures that involve neurologic pathways hypothesized to be influenced by dietary manipulation (10–13). In the DHA context, an appropriate measure would need to capture EFs. Attention and working memory and inhibitory control (WMIC) are EF skills that have a well-documented development in the psychology literature and have been shown to be predictive of later outcomes [attention (14); WMIC (15–19)] as well as reflective of frontal lobe and hippocampus functioning [attention (20, 21); WMIC (22, 23)]. Furthermore, performance in multiple measures of attention have been positively associated with maternal red blood cell DHA concentrations at delivery (24, 25).
Our aim was to test the effect of maternal supplementation of DHA during pregnancy on EFs of children. EFs were to be assessed by using age-appropriate tasks that incorporated functions attributed to the frontal lobes and hippocampus under the hypothesis that increased fetal DHA exposure will enhance the development of EFs.
SUBJECTS AND METHODS
Participants
This nested study involved a subset of infants born to mothers enrolled in a double-blind RCT called the DHA for Maternal and Infant Outcomes (DOMInO) trial (26) who underwent a visual acuity assessment at 4 mo of age (27). Women were eligible for the DOMInO trial if they had singleton pregnancies of 18–21-wk gestation with no fetal abnormalities. The EFs follow-up study excluded children who were born preterm (<37 wk of gestation), had low birth weight (<2500 g), or had clinician-diagnosed neurologic or visual pathologies. Parents were approached to enroll in the follow-up study between April 2009 and August 2010 with an information sheet and consent form posted via mail when the child turned 2 y old and a telephone call 2 wk later. Written informed consent was obtained from parents of all participating infants. All procedures were conducted in accordance with the trial protocol and with the approval of the Flinders Medical Centre Human Research Ethics Committee (Adelaide, Australia).
Random assignment and intervention
Methods for the group allocation and intervention have been described previously (26). Briefly, women were randomly assigned to an intervention group by a telephone randomization service that was stratified by site and parity (primiparous or multiparous). Women were given a unique identification number that was linked to their treatment allocation and known only to an independent statistician. Treatment and control capsules were identical in appearance and administered by hospital pharmacy departments. Trial investigators and study staff and participants were unaware of the group allocation. Treatment-group women were asked to consume three 0.5-g DHA-rich capsules/d, which provided 800 mg DHA/d and 100 mg EPA/d (Incromega 5000TG; Croda Chemicals) from enrollment until delivery. Control-group women were asked to consume three 0.5 g capsules that contained a blend of vegetable oils (Efamol Ltd). Treatment efficacy was shown by increased cord blood plasma phospholipid DHA concentrations (percentage of total FAs) (26) and breast-milk DHA (percentage of total FAs) (27) in the treatment compared with control groups.
Outcome assessments
All assessments were conducted at a 1.5-h clinic appointment by JFG at Flinders Medical Centre, South Australia, between June 2009 and August 2010 when children had mean (±SD) age of 27 ± 2 mo. At the appointment, children underwent measures of attention and WMIC. Parents were interviewed about the child's health, family situation, and dietary intake of DHA since birth and completed the Home Screening Questionnaire for children aged 0–3 y (28). Attention and WMIC assessments were conducted in accordance with previous research (24, 25, 29). All EF tasks took place in a plain, quiet room with children seated on the laps of their parents. Parents were asked to refrain from interacting with the child during tasks. JVC Enviro-S memory camcorders (GZ-MS120; JVC) recorded tasks so that data could be extracted after the assessment. All essential speech was preprepared as a standard script and JFG did not interact with the child during playtime.
Attention
Participants sat at a desk and were given a series of toys to explore and play with freely. A TEAC (LCDV2655HD) 26-in (66 cm) flat-screen liquid-crystal-display television was positioned 1 m away from the child on a 45° viewing angle while a 40 × 40-cm mirror behind the child reflected the television screen to the camcorder. There were 3 attention tasks in the assessment, each of which had one main outcome and 4 other outcomes. The first task was the single-object (SO) task, which measured the ability of the child to sustain attention on a toy in the absence of competition or distraction; the child was given a single, complex toy with multiple buttons and functions [Leapfrog Multifunction Play Centre (Leapfrog)] with which to play for 5 min. The second task was the multiple-object (MO) task, which measured the ability of the child to sustain attention on a toy in the presence of 4 other toys that competed for the child's attention; the child was given 5 toys [a Dora the Explorer figurine (Fisher Price), Bob the Builder quad bike (Learning Curve), rubber duck, plastic bowl with handles and lid, and a Mickey Mouse mobile flip telephone (Fisher Price)] to play with for 5 min. The third task was the distractibility task, which measured the ability of the child to maintain attention on a target object in the presence of a distracting stimulus; the child was given 4 toys [a Magnadoodle (Fisher Price), Little Mermaid building blocks (Mega Blocks), shape sorter, and wooden train set], one at a time, for 3 min each. The television played a DVD that consisted of 7-s distractor segments (segments of various children's programs) with pseudorandom 5–25-s intervals of a black, blank screen to distract the child's attention from the toy. There were ∼8 distractor segments/3 min toy play. Video recordings of each task were downloaded to a computer and viewed after the appointment by using Pinnacle Studio Plus (12th ed) Video Editing Software with a built in timer and a shuttle jog (Contour ShuttleExpress) for frame-by-frame viewing, which was necessary to record the exact timing of eye movements to and from the toys and television. An individual episode of attention [a look at the toy (s)] or inattention [not looking at the toy (s)] of any duration was included in the distractibility task but was only counted if it was ≥1-s duration in the SO task (24) or ≥0.5-s duration in the MO task (25). At the onset of each distractor in the distractibility task, the child's state of attention was coded as focused (looking at the toy and engaged in active learning), casual (looking at the toy but not engaged in active learning), or other (not looking at the toy) on the basis of child facial expression and behavior by using methods previously described (24). The primary outcome related to distractibility only when the child's attention had been focused. Any interruptions (such as parental interactions) that influenced the child's actions were coded as interference and not included as part of the assessment. Superior attention abilities were indicated through the greater duration of time spent looking at the toy (s) and longer latency to turn to the television.
WMIC
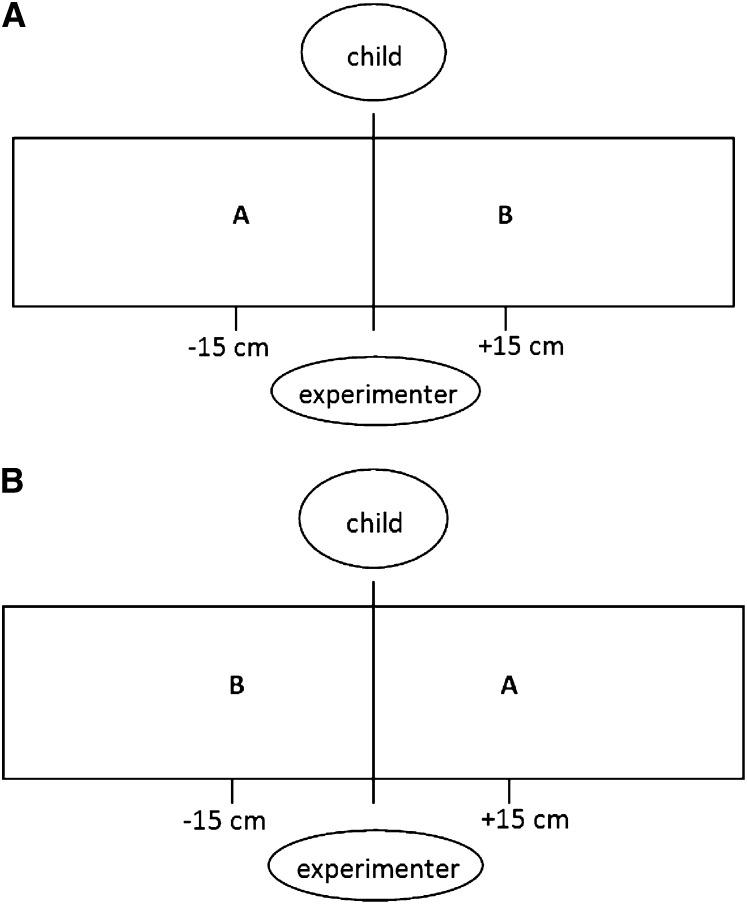
To assess WMIC, a lentil-box version of the A-not-B task was used (29). Children watched as a figurine [Bob the Builder or Wendy (Learning Curve); 7.6 × 2.5 cm] was hidden in a wooden box (150 × 40 × 20 cm) filled with dry lentils and were asked to retrieve the figurine after a delay. The WMIC task proceeded in 5 consecutive phases as follows: 1) learning trials (to familiarize children with the protocol and touching the lentils), 2) training trials set 1 (to develop the learned response to look for the hidden figurine in a specific location), 3) test trials set 1 (to test the ability to inhibit the learned response to look in the previous location while remembering the new hiding location), 4) training trials set 2 (repeat of set 1), and 5) test trials set 2 (repeat of set 1). the experimenter sat on one side of the lentil box; the child, who was seated on a parent's lap, sat on the opposite side, and a camcorder suspended from the ceiling directly above the center of the box recorded a bird's-eye view of the child's hand movements in the lentil box. The assessment commenced with 3 learning trials; the experimenter placed the figurine in location A (Figure 1A) and asked the child to retrieve it 3 times, with the figurine hidden from view more each time. This task was followed by set 1 of the training trials; the experimenter hid the figurine in location A and distracted the child for 3 s before allowing the child to retrieve the figurine. This task was administered 3 times to train the child to search for the hidden toy in location A. Next, we conducted set 1 of the test trials; the experimenter hid the toy in location B and distracted the child for 10 s before allowing the child to retrieve the toy. This task was administered twice to test the child's ability to inhibit the response learned in the training trials and remember the new hiding location. A failure to remember the new hiding location resulted in the child searching in location A and is known as the A-not-B error (29). Set 2 of the training and test trials followed but with locations A and B reversed (Figure 1B). The side of the box on which locations A and B first appeared alternated between assessments. The child accuracy of retrieving the toy from the lentils was measured by viewing the video recording frame by frame. Accuracy was defined as the distance (mm) between the middle of the hidden figurine and the tip of the child's index finger when it first touched the lentils. A shorter distance between the hidden toy and the location where the child searched indicated more-accurate searching and inferred better WMIC.
FIGURE 1.
Setup of the lentil box with locations A and B for the working memory and inhibitory control assessment for training and test trials set 1 (A) and training and test trials set 2 (B).
Twenty-five percent of children were coded by 2 people to verify the agreement of data-extraction procedures. Results were compared by using paired-samples t tests, 2-tailed Pearson's paired-samples correlations, and a κ calculation (to verify the agreement in coding of the child state of attention during the distractibility task). Correlations between extractors were high (0.96–0.99; P < 0.001), and t tests showed that extracted data did not differ between coders, and coders agreed on the state of attention 98% of the time (κ agreement = 0.91).
Cord plasma DHA
Cord blood was collected in the DOMInO trial, and plasma phospholipids were analyzed according to previously established methods (30).
Sample size and statistical analysis
The 185 children who participated in the visual acuity assessment were eligible for the current study (27). To our knowledge, at the commencement of this project, there were no published DHA-intervention trials from which to estimate effect size and power. Therefore, our study was powered to detect a plausible effect size by using the available sample. The effect size was within the range of WMIC and attention values collected from healthy children. With n = 185 children, we had 90% power to detect a difference (±SD) of 0.7 ± 1.5 s for the distractibility task and 18 ± 37 mm for the WMIC task with 95% significance.
Once all assessments were completed, the data analysis proceeded blinded to which children formed treatment and control groups. Statistical analyses were conducted with STATA version IC 11 software (StataCorp LP). No adjustment was made for multiple preplanned comparisons, and P = 0.05 was considered significant. Primary analyses were conducted and adjusted for sex [because girls and boys have different rates of development (31, 32)], smoking during pregnancy [because this has been linked to lower developmental outcomes (27)], and paternal completion of secondary education (because this differed by >10% between groups); however, analyses were also performed unadjusted for completeness and to potentially confirm results of adjusted analyses. Some variables were transformed to normalize their distribution; however, because the transformation made no difference to the results, untransformed data are reported for ease of interpretation. Groups were compared by using independent samples t tests and ANCOVA. A repeated-measures ANOVA was used to determine whether there was a learning effect in the distractibility task (4 trials, each with a different toy) that differed between groups. Associations between cord plasma DHA (as a percentage of total plasma phospholipids) and outcomes were analyzed by using linear regression with adjustment for covariables. The regression predictor was plasma DHA, outcomes were distractibility and WMIC outcomes, and covariables were sex, smoking during pregnancy, and paternal completion of secondary education. Analyses were conducted separately according to treatment compared with control groups because the trial intervened on FA status and, therefore, the combination of groups would have violated the regression assumption that the samples were drawn from the same population.
RESULTS
Participants
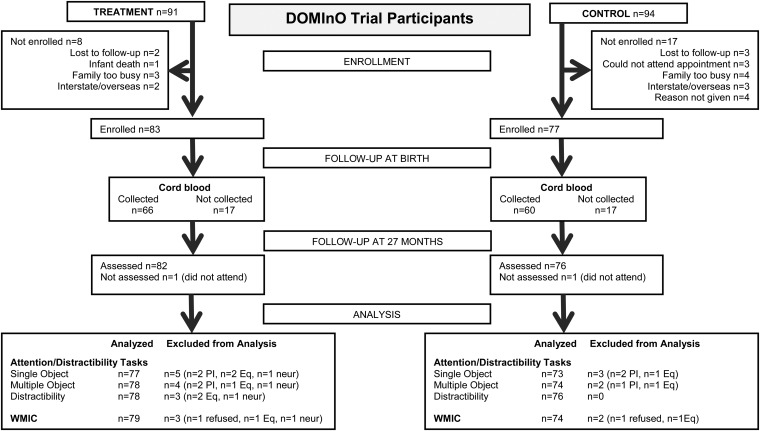
Of the 185 children from the visual study, 184 children were eligible to participate in the 27-mo follow-up because one child died before the age of 2 y. Rates of loss to follow-up rate were n = 8 (8.8%) in the treatment group and n = 17 (18.1%) in the control group. Baseline characteristics of consenters did not differ from those of nonconsenters, and reasons for losses are given in Figure 2. Some data were excluded from analyses because of excessive interference by parents, a subsequent neurologic diagnosis, equipment failure, and child noncompliance.
FIGURE 2.
Flow of participants in the DOMInO trial through an executive function follow-up study at 27 mo. DOMInO, DHA for Maternal and Infant Outcomes; Eq, equipment failure; neur, clinician-diagnosed neurologic disorder; PI, parental interference; WMIC, working memory and inhibitory control.
The follow-up sample was comparable to that in the overall DOMInO trial with the exception of the proportion of mothers in the follow-up who had completed tertiary education (77.8% compared with 68.4% of the DOMInO trial; P = 0.06), and compliance was slightly higher in the follow-up (47.5% compared with 35.6% in the DOMInO trial did not miss a capsule at 28 wk of gestation; P < 0.01). Baseline characteristics of mothers who participated in this nested follow-up study were similar across treatment and control groups as were characteristics of children at birth and the 27-mo follow-up (Table 1). More fathers in the treatment group had completed secondary education compared with in the control group (68.6% compared with 44.7%), and thus, analyses were adjusted for paternal completion of secondary education.
TABLE 1.
Characteristics of participants and their mothers at enrollment, birth, and follow-up at 27 mo of age
| Treatment group (n = 82) | Control group (n = 76) | |
| Parental data collected at enrollment1 | ||
| Maternal age at trial entry (y) | 29.7 ± 5.32 | 29.4 ± 5.0 |
| Mother completed secondary education [n (%)] | 53.0 (64.6) | 51.0 (67.1) |
| Mother smoked cigarettes at time of enrollment [n (%)] | 8.0 (9.8) | 9.0 (11.8) |
| Mother drank alcohol at time of enrollment [n (%)] | 8.0 (9.8) | 9.0 (11.8) |
| Father completed secondary education [n (%)] | 55.0 (68.6) | 34.0 (44.7) |
| Infant data collected at birth | ||
| Sex (M) [n (%)] | 41.0 (50.0) | 35.0 (46.1) |
| Cord plasma DHA (percentage of phospholipid fatty acids) | 8.4 ± 1.9 | 6.7 ± 1.6 |
| Weight (g) | 3585.0 ± 473 | 3625.0 ± 410 |
| Length (cm) | 50.4 ± 2.2 | 49.8 ± 3.3 |
| Head circumference (cm) | 35.2 ± 1.3 | 35.6 ± 3.3 |
| Child data collected at the 27-mo follow-up | ||
| Age at follow-up (d) | 835.0 ± 50.4 | 839.0 ± 65.6 |
| Fed breast milk or LCPUFA3-supplemented formula until ≥12 mo of age [n (%)] | 45.0 (54.9) | 34.0 (44.7) |
| Home screening score4 | 35.7 ± 2.8 | 35.5 ± 2.9 |
| Time watching television (h/d) | 1.4 ± 1.1 | 1.5 ± 1.0 |
| Fed ≥1 fish meal in the previous week [n (%)] | 48.0 (58.5) | 47.0 (61.8) |
| Fed ≥1 DHA-enriched serving of food in the previous week [n (%)] | 23.0 (28.1) | 21.0 (27.6) |
| Taking ≥3 DHA supplements/wk [n (%)] | 7.0 (8.5) | 8.0 (10.5) |
Enrollment in the DHA for Maternal and Infant Outcomes trial (a randomized controlled trial of DHA supplementation during pregnancy) at 18–21 wk of gestation.
Mean ± SD (all such values).
LCPUFA, long-chain PUFA.
Score <32 indicated a suspect score (28).
Attention
The primary outcome of distractibility did not differ between treatment and control groups (Table 2). Also, there was not a difference for main SO or MO outcomes as well as most other secondary outcomes, with one exception, ie, the number of times children looked away from the toys in the MO task was lower in the treatment group than control group, although the size of the difference was small [adjusted mean difference: −2.0 looks; 95% CI: −3.9 to −0.2 looks). To investigate this difference further, a post hoc analysis regarding the duration of time spent not looking at the toys during the MO task was conducted; however, the time spent not looking at the toys was the same in each group [treatment mean ± SD = 33.6 ± 23.4 s; control mean ± SD: 36.8 ± 19.4 s; adjusted mean difference: −1.5 s (95% CI: −8.7, 5.7 s; P = 0.69); unadjusted mean difference: −3.2 s (95% CI: −10.1, 3.7 s; P = 0.36), respectively]. The latency to being distracted significantly increased across distractibility trials (P < 0.001), which suggested the presence of a learning effect, although the effect did not differ between groups.
TABLE 2.
Outcomes of assessments of attention and working memory and inhibitory control at 27 mo of age by treatment group1
| Outcome | Treatment (n = 77) | Control (n = 73) | Unadjusted | P | Adjusted2 | P |
| Attention | ||||||
| Single-object task | ||||||
| Total duration of time spent looking at the toy (s)3 | 238.8 ± 51.14 | 246.6 ± 41.0 | −7.8 (−22.8, 7.2)5 | 0.31 | −12.0 (−27.7, 3.5) | 0.13 |
| Percentage of time spent looking at the toy (%) | 84.1 ± 11.8 | 86.0 ± 9.3 | −0.02 (−0.1, 0.02) | 0.28 | −0.02 (−0.1, 0.02) | 0.33 |
| Average length of look at the toy (s) | 27.1 ± 19.4 | 27.7 ± 21.9 | −0.5 (−7.2, 6.2) | 0.88 | −2.5 (−9.5, 4.4) | 0.47 |
| No. of looks at the toy | 11.4 ± 4.6 | 11.6 ± 4.6 | −0.2 (−1.7, 1.3) | 0.76 | 0.1 (−1.5, 1.7) | 0.90 |
| No. of times looked away from the toy | 10.0 ± 4.5 | 10.4 ± 4.3 | −0.3 (−1.7, 1.1) | 0.78 | −0.01 (−1.5, 1.5) | 0.99 |
| Multiple-object task | ||||||
| No. of times shifted looks between toys3 | 41.6 ± 11.8 | 42.0 ± 12.4 | −0.5 (−4.4, 3.4) | 0.45 | 2.7 (−4.2, 3.6) | 0.89 |
| Total duration of time looking at toys (s) | 250.6 ± 37.4 | 249.5 ± 30.8 | 1.1 (−9.9, 12.1) | 0.84 | −1.6 (−13.0, 9.9) | 0.79 |
| Percentage of time spent looking at toys (%) | 87.8 ± 9.3 | 87.0 ± 7.2 | 0.01 (−0.02, 0.04) | 0.54 | 0.02 (−0.03, 0.03) | 0.91 |
| No. of times looked away from toys | 14.0 ± 5.8 | 15.7 ± 5.6 | −1.8 (−3.6, 0.02) | 0.05 | −2.0 (−3.9, −0.2) | 0.03 |
| Average length of a look at a toy (s) | 5.3 ± 1.4 | 5.2 ± 1.6 | 0.1 (−0.4, 0.6) | 0.78 | −0.1 (−0.6, 0.4) | 0.81 |
| Distractibility task | ||||||
| Average latency to turn to the distractor when attention was focused (s)367 | 3.5 ± 1.6 | 3.8 ± 1.9 | −0.3 (−0.9, 0.3) | 0.28 | −0.2 (−0.7, 0.4) | 0.58 |
| Percentage of times distracted when focused (%) | 66.2 ± 0.3 | 59.7 ± 0.3 | 0.1 (−0.02, 0.2) | 0.15 | 0.04 (−0.1, 0.1) | 0.34 |
| Average latency to look to the distractor when attention was casual (s)7 | 2.2 ± 1.7 | 2.6 ± 2.1 | −0.4 (−1.0, 0.2) | 0.23 | −0.3 (−0.9, 0.3) | 0.33 |
| Percentage of times distracted when casual (%) | 78.1 ± 0.3 | 74.4 ± 0.3 | 0.04 (−0.6, 0.1) | 0.43 | 0.04 (−0.1, 0.1) | 0.44 |
| Total duration of time spent looking at the distractor when the distractor was on (s) | 89.7 ± 49.7 | 79.9 ± 50.1 | 9.8 (−6.1, 25.7) | 0.23 | 3.9 (−11.7, 19.5) | 0.62 |
| Total duration of time spent looking at the distractor when the distractor was off (s) | 26.8 ± 23.4 | 24.6 ± 21.6 | 2.5 (−4.7, 9.7) | 0.49 | 0.8 (−6.3, 7.9) | 0.82 |
| Working memory and inhibitory control | ||||||
| Average accuracy of locating figurine during test trials (mm)6 | 132.6 ± 55.5 | 120.0 ± 63.4 | 12.6 (−6.4, 31.6) | 0.19 | 8.9 (−10.6, 28.3) | 0.37 |
| Average accuracy of locating figurine during training trials (mm) | 95.6 ± 47.4 | 83.7 ± 39.9 | 11.9 (−2.2, 25.9) | 0.10 | 14.4 (−0.2, 29.1) | 0.05 |
Groups were compared by using independent samples t tests and ANCOVA.
Adjusted for maternal smoking at baseline (∼18 wk of gestation), child sex, and father's secondary education.
Main outcome for the attention task.
Mean ± SD (all such values).
Mean difference; 95% CI in parentheses (all such values).
Primary outcome.
Maximum latency: 7 s.
WMIC
The primary outcome of the WMIC assessment (ie, the accuracy of locating the toy during test trials) did not differ between treatment and control groups (Table 2). However, the control group were more accurate at searching for the hidden toy during training trials than was the treatment group (14.4 mm; 95% CI: −0.2, 29.1 mm; P = 0.05).
Cord plasma FA associations
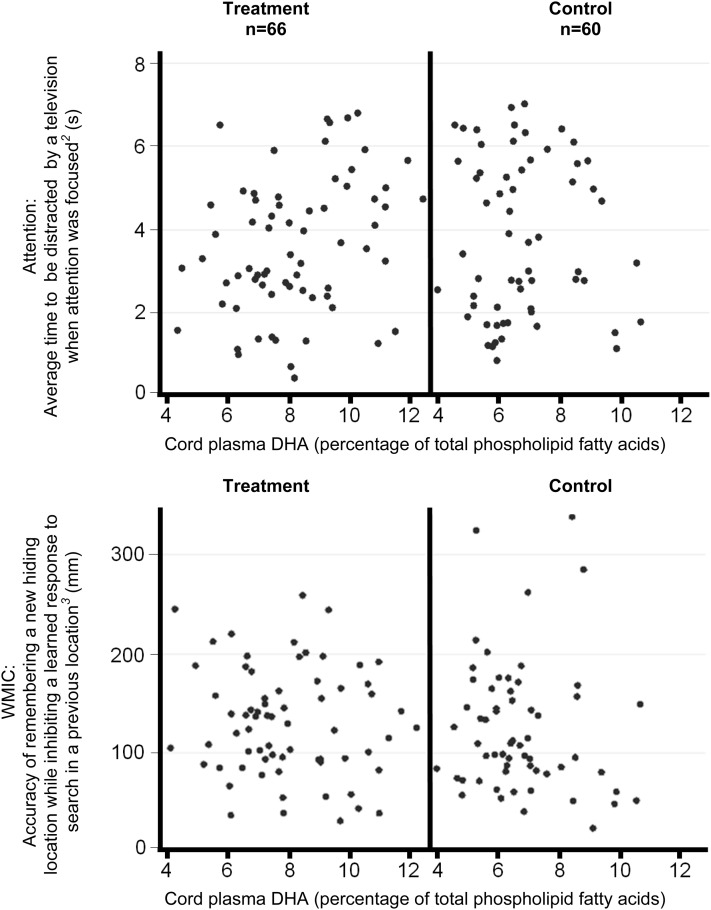
Cord blood samples were collected from 66 treatment-group participants (80%) and 60 control-group participants (79%). Scatterplots (Figure 3) as well as means (±SDs) (Table 1) showed an overlap of cord plasma DHA between groups. Regression analyses (Table 3) showed associations between the plasma DHA of treatment and control groups (groups analyzed separately) and some but not all assessment outcomes. For example, a 1% higher concentration of DHA in cord plasma was associated with an increase of 0.3 s in distractibility (95% CI: 0.1, 0.5 s) in treatment-group children (P = 0.01) but not in the control group (P = 0.38).
FIGURE 3.
Associations between cord blood plasma DHA (percentage of phospholipid fatty acids) and primary outcomes of the attention and WMIC for the treatment (attention-adjusted r2 = 0.07, P = 0.08; WMIC-adjusted r2 = –0.01, P = 0.47) and control groups (attention-adjusted r2 = 0.10, P = 0.05; WMIC-adjusted r2 = 0.01, P = 0.33). Associations were analyzed by using linear regression with adjustment for maternal smoking at baseline (∼18 wk of gestation), child sex, and father's secondary education. 2Average across all 4 toy trials; 3average across all 4 test trials. WMIC, working memory and inhibitory control.
TABLE 3.
Association between cord blood plasma DHA (percentage of phospholipid fatty acids) and the main outcome for each attention task in the attention and working memory and inhibitory control assessments at 27 mo of age1
| Treatment (n = 66) |
Control (n = 60) |
|||
| Outcome | β (95% CI) | P | β (95% CI) | P |
| SO: total amount of time spent looking at the toy (s) | 5.0 (−1.4, 11.2) | 0.12 | 10.1 (1.8, 18.5) | 0.02 |
| MO: no. of times the child looked between toys | 0.5 (−1.1, 2.1) | 0.53 | −0.6 (−2.6, 1.3) | 0.51 |
| D: latency to turn to the distractor when attention was focused (s) | 0.3 (0.1, 0.5) | 0.01 | −0.1 (−0.5, 0.2) | 0.38 |
| Accuracy of locating figurine during test trials (mm)2 | −3.8 (−11.1, 3.5) | 0.30 | −0.2 (−11.6, 11.2) | 0.97 |
Associations were analyzed by using linear regression with adjustment for maternal smoking at baseline (∼18 wk of gestation), child sex, and the father's secondary education. D, distractibility task of the attention assessment; MO, multiple-object task of the attention assessment; SO, single-object task of the attention assessment.
Average across all (4) test trials of the working memory and inhibitory control assessment.
DISCUSSION
We hypothesized that DHA supplementation during pregnancy would enhance children's visual attention and WMIC, and that this enhancement would be reflected across all outcomes. However, there was no effect of supplementation on primary outcomes of distractibility and WMIC. There was a minor difference between groups in the MO task, whereby treatment-group children looked away from the toys 2 times fewer than did controls, which implied that the treatment group was better at attending to the toys when there was competition for attention. Conversely, in the WMIC task, control children were able to more accurately remember the hiding location of the figurine when embedding the memory to search for the hidden figurine in location A (training trials) but not under test conditions. The lack of consistent effects across all outcomes coupled with the high number of comparisons indicated that these differences may have been a result of chance (33).
If fetal DHA exposure was linked to attention or WMIC outcomes, the association should have been present across both randomization groups and in the same direction, particularly given the degree of overlap of cord plasma DHA between groups. However, associations between plasma DHA with attention and WMIC outcomes were inconsistent, which suggested that any significant findings may also have been a result of chance (33).
The null findings of specialized measures of frontal lobe and hippocampus functioning used in this study supported the null findings of the globalized psychometric tests in the DOMInO trial at 18 mo of age (26) as well as the majority of globalized and other neurodevelopment tests in other RCTs of DHA supplementation during pregnancy (9).
The attention methods that we used were based on studies by Colombo et al (24) and Kannass et al (25) after an intervention during pregnancy in which women were provided with eggs that contained DHA (treatment: 135 mg DHA/egg; control: 35 mg DHA/egg). In contrast to our findings, the authors reported that children born to mothers with high erythrocyte membrane DHA at delivery had an accelerated development of attention from 4 to 18 mo of age compared with that of children born to mothers who had low DHA (24, 25). The sample of 50 children was dichotomized into high compared with low DHA exposure on the basis of the median maternal DHA status at delivery. In comparison, our study involved a larger sample (n = 158) in which treatment and control groups had a substantial overlap in cord blood DHA concentrations despite the higher DHA dose (800 mg DHA/d). Colombo et al (24) also used a heart rate monitor as an objective measure of the attention states in infants 4–8 mo of age; however, such a measure is problematic in older children who find the monitor intrusive. There are important differences in analyses between our studies; Colombo et al (24) conducted a longitudinal, repeated-measures analysis that captured the change in attention over time, whereas our analysis involved a per-group analysis of attention measured once. Even if attention develops more rapidly in 4–18-mo-old infants whose mothers consume increased DHA during pregnancy, our findings indicated that the difference in attention may no longer be present by 2 y of age.
It may be that the DHA supplementation during pregnancy had no effect on term-born children's neurodevelopment because the growth of the brain is protected during in utero development. Maternal stores of DHA, the upregulation of DHA synthesis (34), and the preferential transfer of DHA across the placenta (see reference 35 for a review) during pregnancy may protect fetal neurologic structures from suboptimal development. The term-born children in the 27-mo follow-up had the benefit of receiving an intrauterine supply of DHA during the peak period of fetal DHA accrual (2, 3). This benefit could explain the overlap in cord plasma DHA between intervention groups despite the fact that the high dose of DHA (∼800 mg/d) used to supplement women in this trial greatly exceeded the average DHA intake of the population from which the sample was derived. The majority of other DHA RCTs have likewise assessed infants who received a full in utero DHA supply (9). Preterm infants are denied normal DHA provisions when the velocity of brain growth is at its greatest but have been underrepresented in trials of DHA supplementation during pregnancy. Future research is needed to determine whether supplementation of specific populations, such as those born preterm, is of benefit to their cognitive development.
To our knowledge, this is the first study to compare early childhood EFs after maternal supplementation with DHA or a placebo during pregnancy. Attention outcomes were chosen over other EF assessments because maternal DHA at delivery had previously been associated with attention and distractibility (24, 25), and these tasks have been widely used in developmental psychology although not used clinically, and normal performance variables in these assessments have not been established. Thus, there are no known clinically relevant effect sizes or SDs for these tests that can be referred to when interpreting our findings. Our results can be used to inform future research that use these tests.
The WMIC measure used in our study involved a homogenous space (lentils) to search for the hidden figurine; whereas previous studies have used wells to hide toys. The advantage of lentils is that there are no visible cues to assist in remembering the location of the hidden figurine, and the exact magnitude of a search error can be measured. The attention and WMIC protocols matched previously published methods, except that children in our study were slightly older [27 compared with 24 mo (29); 12 or 18 mo of age (24, 25)]. It is possible that the tasks were not sufficiently challenging for children aged 27 mo.
Another potential limitation of our study was that we did not adjust significance tests for the number of comparisons (36). In addition, the power to detect a difference in the WMIC test was less than originally planned because the SD in the current study (treatment SD: 55.5 mm; control SD: 63.4 mm) was larger than anticipated (SD: 37 mm). We could not rule out whether a larger sample may have resulted in a different outcome in the WMIC measure; however, means and SDs in attention outcomes, together with the lack of enhanced performance across all tasks, made it unlikely that a larger sample would have resulted in consistent differences in attention tasks between treatment and control groups.
More treatment-group than control-group mothers correctly guessed their group allocation; however, selection and response biases were unlikely because all assessments and data analyses were conducted blinded to group allocation. Other subgroup characteristics were comparable to those of the 4-mo sample and DOMInO trial, which suggested that the integrity of the 27-mo study was retained as representative of the overall cohort.
In conclusion, the findings of null effects of prenatal DHA supplementation on specialized measures of EFs attention and WMIC are consistent with other findings in the literature by using globalized assessments (9). To this point, DHA supplementation during pregnancy has not yielded any obvious benefits for early childhood cognitive development in well-nourished healthy term-born children.
Acknowledgments
We thank the families and their children for their participation in the study, Susan Carlson for training LGS in the administration of assessment methods in their psychometric laboratory in Kansas, Robert A Gibson for his input in overviewing and reviewing the study, and the DOMInO Trial Steering Committee (Maria Makrides, Robert A Gibson, Andrew J McPhee, Lisa Yelland, Julie Quinlivan, and Phillip Ryan) for their role in the management of the DOMInO trial.
The authors’ responsibilities were as follows—LGS and MM: conceived the idea for the study and obtained funding; JC: developed the attention- and WMIC-assessment methods and provided support in the administration of these methods for the duration of the study; LGS: trained and supervised JFG in the administration and data extraction of assessments and performed the data-extraction reliability check; JFG: managed the follow-up study, conducted assessments, collected and processed data, performed analyses, and wrote the manuscript under the supervision of LGS and MM; and all authors: contributed to the scientific interpretation of the analysis, commented on drafts of the manuscript, read and approved the final version of the manuscript, and accepted final responsibility for the manuscript. MM serves on the advisory boards for Nestle, Fonterra, and Nutricia. JC serves on the advisory board for Fonterra and Mead Johnson Nutrition. JFG and LGS had no conflicts of interest.
Footnotes
Abbreviations used: DOMInO, DHA for Maternal and Infant Outcomes; EF, executive function; FA, fatty acid; MO, multiple object; RCT, randomized controlled trial; SO, single object; WMIC, working memory and inhibitory control.
REFERENCES
- 1.Volpe JJ. Neuronal proliferation, migration, organization, and myelination. 2nd ed. In: Volpe JJ, ed. Neurology of the newborn. Philadelphia, PA: WB Saunders Co, . 1987:33–68.
- 2.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirments. Early Hum Dev 1980;4:131–8. [DOI] [PubMed] [Google Scholar]
- 3.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 1992;120:S129–38. [DOI] [PubMed] [Google Scholar]
- 4.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child 1973;48:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawlosky RJ, Denkins Y, Ward G, Salem N., Jr Retinal and brain accretion of long-chain polyunsaturated fatty acids in developing felines: the effects of corn oil-based maternal diets. Am J Clin Nutr 1997;65:465–72. [DOI] [PubMed] [Google Scholar]
- 6.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem 2000;75:2563–73. [DOI] [PubMed] [Google Scholar]
- 7.Catalan J, Moriguchi T, Slotnick B, Murthy M, Greiner RS, Salem N., Jr Cognitive deficits in docosahexaenoic acid-deficient rats. Behav Neurosci 2002;116:1022–31. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A, Murthy M, Greiner RS, Moriguchi T, Salem N., Jr A decrease in cell size accompanies a loss of docosahexaenoate in the rat hippocampus. Nutr Neurosci 2002;5:103–13. [DOI] [PubMed] [Google Scholar]
- 9.Gould JF, Smithers LG, Makrides M. The effect of maternal omega-3 (n−3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2013;97:531–44. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am J Clin Nutr 2006;84:961–70. [DOI] [PubMed] [Google Scholar]
- 11.Hughes D, Bryan J. The assessment of cognitive performance in children: considerations for detecting nutritional influences. Nutr Rev 2003;61:413–22. [DOI] [PubMed] [Google Scholar]
- 12.Cheatham CL, Colombo J, Carlson SE. n−3 Fatty acids and cognitive and visual acuity development: methodologic and conceptual considerations. Am J Clin Nutr 2006;83(supplement):1458S–66S. [DOI] [PubMed] [Google Scholar]
- 13.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr 2007;85(suppl):614S–20S. [DOI] [PubMed] [Google Scholar]
- 14.Anderson V, Fenwick T, Manly T, Robertson I. Attentional skills following traumatic brain injury in childhood: a componential analysis. Brain Inj 1998;12:937–49. [DOI] [PubMed] [Google Scholar]
- 15.McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Dev Psychol 2007;43:947–59. [DOI] [PubMed] [Google Scholar]
- 16.Kochanska G, Murray K. Inhibitory control in young children and its role in emerging internalization. Child Dev 1996;67:490–507. [PubMed] [Google Scholar]
- 17.Espy KA, McDiarmid MM, Cwik MF, Stalets MM, Hamby A, Senn TE. The contribution of executive functions to emergent mathematic skills in preschool children. Dev Neuropsychol 2004;26:465–86. [DOI] [PubMed] [Google Scholar]
- 18.Riggs NR, Blair CB, Greenberg MT. Concurrent and 2-year longitudinal relations between executive function and the behavior of 1st and 2nd grade children. Child Neuropsychol 2003;9:267–76. [DOI] [PubMed] [Google Scholar]
- 19.van de Weijer-Bergsma E, Wijnroks L, Boom J, de Vries LS, van Haastert IC, Jongmans MJ. Individual differences in developmental trajectories of A-not-B performance in infants born preterm. Dev Neuropsychol 2010;35:605–21. [DOI] [PubMed] [Google Scholar]
- 20.Bell MA. Frontal lobe function during infancy: implications for the development of cognition and attention. In: Richards JE, ed. Cognitive neuroscience of attention; a developmental perspective. Mahwah, NJ: Lawrence Erlbaum Associates, 1998:28–316.
- 21.Ruff HA. Summary and commentary. Selective attention: its measurement in a developmental framework. In: Richards JE, ed. Cognitive neuroscience of attention; a developmental perspective. Mahwah, NJ: Lawrence Erlbaum Associates, 1998:419–26.
- 22.Diamond A, Zola-Morgan S, Squire LR. Successful performance by monkeys with lesions of the hippocampal formation on AB and object retrieval, two tasks that mark developmental changes in human infants. Behav Neurosci 1989;103:526–37. [DOI] [PubMed] [Google Scholar]
- 23.Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Dev 1992;63:1142–63. [PubMed] [Google Scholar]
- 24.Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 2004;75:1254–67. [DOI] [PubMed] [Google Scholar]
- 25.Kannass KN, Colombo J, Carlson SE. Maternal DHA levels and toddler free-play attention. Dev Neuropsychol 2009;34:159–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA 2010;304:1675–83. [DOI] [PubMed] [Google Scholar]
- 27.Smithers LG, Gibson RA, Makrides M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: a randomized controlled trial. Am J Clin Nutr 2011;93:1293–9. [DOI] [PubMed] [Google Scholar]
- 28.Frankenburg WK, Coons CE. Home Screening Questionnaire: its validity in assessing home environment. J Pediatr 1986;108:624–6. [DOI] [PubMed] [Google Scholar]
- 29.Spencer JP, Smith LB, Thelen E. Tests of a dynamic systems account of the A-not-B error: the influence of prior experience on the spatial memory abilities of two-year-olds. Child Dev 2001;72:1327–46. [DOI] [PubMed] [Google Scholar]
- 30.Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: results from the DINO trial. Prostaglandins Leukot Essent Fatty Acids 2008;79:141–6. [DOI] [PubMed] [Google Scholar]
- 31.Bosacki S, Astington JW. Theory of mind in preadolescence: relations between social understanding and social competence. Soc Dev 1999;8:237–55. [Google Scholar]
- 32.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996;119:1763–74. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JAC, Smith GD. Sifting the evidence-what's wrong with significance tests? BMJ 2001;322:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 2005;45:581–97. [DOI] [PubMed] [Google Scholar]
- 35.Hanebutt FL, Demmelmair H, Schiessl B, Larque E, Koletzko B. Long-chain polyunsaturated fatty acid (LC-PUFA) transfer across the placenta. Clin Nutr 2008;27:685–93. [DOI] [PubMed] [Google Scholar]
- 36.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. [PubMed] [Google Scholar]