Abstract
Background: Data on the protein requirements of elderly adults are limited, because it is impractical to conduct repeated nitrogen balance protocols in these vulnerable humans.
Objective: This study was designed to determine the dietary protein requirement of elderly women by using the recently developed minimally invasive indicator amino acid oxidation (IAAO) technique.
Design: Six white women aged 80–87 y [mean ± SEM: 82 ± 1 y and body mass index (in kg/m2) 26 ± 2] completed a 3-d protocol 7 times. Each woman consumed an adaptation diet for 2 d and on day 3 consumed a complete test diet with a crystalline amino acid mixture containing 1 of 7 protein intakes (0.1, 0.3, 0.6, 0.9, 1.2, 1.5, or 1.8 g · kg−1 · d−1) tested randomly. A group-based protein requirement was assessed by using a nonlinear mixed model of protein intake and l-[1-13C]phenylalanine oxidation. The breakpoint, at which there was no further decline in the rate of appearance of 13C in the breath, was used as an index of the mean protein requirement.
Results: The mean protein requirement (95% CI) was 0.85 (0.60, 1.09) g · kg−1 · d−1. This requirement is 29% higher than the current Estimated Average Requirement (EAR) for adults of 0.66 g · kg−1 · d−1 based on the nitrogen balance technique, although the 95% CI includes the current EAR. The corresponding adequate protein allowance of 1.15 (0.77, 1.54) g · kg−1 · d−1 is 44% higher, although the 95% CI includes the Recommended Dietary Allowance (RDA) of 0.80 g · kg−1 · d−1.
Conclusions: Notwithstanding uncertainty about the validity of the use of the IAAO technique to assess protein requirements, the results of this study with octogenarian women suggest that the current EAR and RDA for elderly women may be underestimated. The limitations of this short-term, noninvasive method underscore the need for new research that uses alternative experimental designs and measuring physiologic, morphologic, and health-related outcomes. This trial was registered at clinicaltrials.gov as NCT01193946.
See corresponding article on page 761.
INTRODUCTION
The dietary protein needs of humans are described by using the terms Estimated Average Requirement (EAR)4 and Recommended Dietary Allowance (RDA) (1). In healthy adults, the EAR of protein is 0.66 g · kg−1 · d−1 and presumably meets the protein needs of 50% of the adult population. The RDA is 0.80 g · kg−1 · d−1—an intake that theoretically meets the protein needs of 97.5% of the adult population. These values are derived from a meta-analysis of 19 nitrogen balance studies (2), among which only one studied elderly adults (3). Although the meta-analysis found a 26% greater protein requirement in elderly than in young adults, this difference was not statistically significant; thus, the current EAR and RDA for protein are the same for all apparently healthy adults. Metabolic and physiologic changes occur with aging and may lead to protein requirement changes in elderly adults (4). Indeed, a weighted mean protein requirement was calculated at 0.91 g · kg−1 · d−1 (38% higher than the EAR) (7) when data from 4 short-term nitrogen balance studies in elderly subjects were integrated (3, 5–7). However, after the current Dietary Reference Intakes (DRIs) for protein was released, Campbell et al (4) measured nitrogen balance in young and elderly adults; the results suggest that protein requirements are not different between age groups and are not different from the EAR. Currently, data obtained from elderly adults are very limited, and debate continues about whether the RDA for protein is adequate for elderly adults (8–14).
Nitrogen balance is the foundation of the current EAR and RDA for protein. It has served as the standard technique to measure protein requirements and remains the only method with sufficient data to make recommendations. The primary reason that data in elderly adults are limited is that nitrogen balance requires strict dietary control (ie, consumption of prescribed amounts of protein for several weeks) and multiple 24-h collections of urine and stool, which are logistically very demanding and not practical for elderly adults. Alternative methods that are short term and less invasive are more suitable to use in such populations. The minimally invasive indicator amino acid oxidation (IAAO) technique may serve as a tool to address this important scientific need. It is based on the concept that dietary amino acids are partitioned between incorporation into protein or oxidation, because there is no significant storage of amino acids in the human body (15). When a range of test amino acids/test proteins are fed, monitoring the oxidation rate of the indicator amino acid (an indispensable amino acid), which reflects the utilization of other amino acids/proteins, can be used to determine the amino acid/protein requirement. The IAAO technique has been used extensively to measure amino acid requirements (16–18) and recently the protein requirements of children (19) and young men (20).
Because of limited data and the demanding procedures required to determine nitrogen balance, in 2005, the Institute of Medicine of the National Academies report on the DRIs for protein recommended research to assess the protein needs of elderly adults, especially those aged 80–100 y, with the use of new methods—other than nitrogen balance measures (1). Most of the elderly population is women and based on NHANES 2003–2004 data, elderly women consume the least amount of protein of the populations surveyed (21). Thus, the aim of this study was to assess the protein requirement of women aged ≥80 y by using the IAAO technique.
SUBJECTS AND METHODS
Subjects
Potential subjects were recruited via community postings in the greater Lafayette and West Lafayette, IN, area and Purdue University campus e-mail. An off-campus research laboratory was set up at a local retirement facility (University Place, West Lafayette, IN) and served as the clinical testing site. The Purdue University Biomedical Institutional Review Board approved the study protocol, and each subject signed an informed-consent form before enrollment. The inclusion criteria were as follows: 1) female aged 80 y or older, 2) BMI (in kg/m2) between 20 and 30, 3) stable weight (<4.5 kg weight gain or loss within the past 6 mo), 4) no smoking (within the past 6 mo), 5) ambulatory, 6) blood profiles within 10% of clinical normalcy, 7) no diseases or no use of medications known to influence protein or energy metabolism, 8) willingness and ability to consume the foods and beverages provided, 9) no allergies to phenylalanine, and 10) continence. Each subject received a monetary stipend for participating in the study. Eleven white women showed interest and completed the screening procedures (height, weight, fasting blood sample, and medical history questionnaire). Two were disqualified because of medical conditions, and one woman had a stroke and was no longer willing to participate. Eight women started and completed the study protocol. Data from 2 women who were diabetic were excluded from the final analysis because their patterns of response to the 7 protein intakes were different. Thus, data from 6 participants are reported.
Study design: 3-d protocol
This study was based on the minimally invasive IAAO protocol (22) used previously in young men (20). Each participant completed the same 3-consecutive-day protocol 7 times. On days 1 and 2, the subjects consumed an adaptation diet; on day 3, they participated in an 8-h testing period. Each 3-d protocol was separated by ≥1 wk (washout period), and all 8 participants completed the study within 3 mo. At the start of the protocol, subjects were asked to stop consuming over-the-counter supplements and were provided with a multivitamin/mineral supplement to consume daily (Centrum Silver; Wyeth Consumer Health Care).
The adaptation diet for days 1 and 2 of each 3-d protocol was developed by using Pronutra (release 3.2; Viocare Technologies Inc) metabolic feeding study software. All foods were prepared and distributed at the Indiana Clinical Research Center bionutrition facility at Purdue University. The diet contained 30% of energy as fat, 1.0 g protein · kg−1 · d−1 and variable amounts of carbohydrate. The energy need of each subject was calculated based on estimated energy requirements from the report of the Institute of Medicine DRIs for energy and macronutrients (1) with an activity level of 1.12 (low activity). A standard menu that was used for the adaptation diet is shown in Table 1. Food items were substituted if the subject was allergic to the item or could not tolerate certain foods. Each subject was provided all foods and beverages for 2 d before each testing day. Foods and beverages were packaged for consumption outside the bionutrition facility. The subjects were instructed to consume all of the foods and beverages provided and to not consume any other items (other than water, which was consumed ad libitum). The same diet was repeated on the 2 days before each of the 7 testing days.
TABLE 1.
Standard menu of the adaptation diet on days 1 and 2 of each 3-d protocol1
| Day 1 | Day 2 | |
| Breakfast | Cinnamon bread/toast with margarine | Cereals with milk |
| Yogurt with mixed berries | Strawberries | |
| Sausage patty | Sausage patty | |
| Decaffeinated coffee or tea (optional) | Decaffeinated coffee or tea (optional) | |
| Juice | Juice | |
| Lunch | Chicken salad plate | Ham and Swiss cheese on croissant |
| Chicken salad | Pasta salad | |
| Wheat crackers | Lemonade, fruit punch, or apple juice | |
| Mozzarella cheese | Shortbread cookies | |
| Cantaloupe and seedless grapes | ||
| Bottled water | ||
| Fudge striped cookies | ||
| Dinner | Pasta shells with meat sauce | Chicken rice casserole |
| Grated parmesan cheese | Mandarin oranges | |
| Cottage cheese with pineapple ring | Lemonade, fruit punch, or apple juice | |
| Lemonade, fruit punch, or apple juice | Cheesecake bites | |
| Petit four cakes |
The quantities of each menu item were customized for each subject based on their specified energy and macronutrient intakes.
Study design: testing day
On the morning of each of the 7 testing days, the subjects came to the research laboratory after having fasted for 10 h. After body weights and blood glucose concentrations were measured, the subjects consumed 8 isoenergetic testing-day drinks at hourly intervals. Each drink contained one-twelfth of the subject's total daily energy requirement, a protein- and amino acid–free diet powder (powders 1 and 2; Mead Johnson), and the crystalline amino acid mixture (Ajinomoto AminoScience LLC). They consumed 30% of energy from fat and 70% of energy from carbohydrate and protein. Seven dietary protein intakes were tested randomly on the 7 testing days: 0.10, 0.30, 0.60, 0.90, 1.2, 1.5, and 1.8 g protein · kg−1 · d−1. The amino acid composition was the same as egg protein, except for phenylalanine and tyrosine (which were held constant as described below), and the quantity of the mixture reflected various protein intakes on different testing days (Table 2).
TABLE 2.
Amino acid composition of reference protein and various test protein intakes as amino acid mixtures
| Reference protein1 | 0.1 g/kg protein | 0.3 g/kg protein | 0.6 g/kg protein | 0.9 g/kg protein | 1.2 g/kg protein | 1.5 g/kg protein | 1.8 g/kg protein | |
| mg/g | mg/0.1 g | mg/0.3 g | mg/0.6 g | mg/0.9 g | mg/1.2 g | mg/1.5 g | mg/1.8 g | |
| l-Alanine | 61.4 | 6.1 | 18.4 | 36.8 | 55.3 | 73.7 | 92.1 | 110.5 |
| l-Arginine · HCl2 | 90.5 | 7.5 | 22.5 | 45.1 | 67.6 | 90.1 | 112.7 | 135.2 |
| l-Aspargine | 33.3 | 3.3 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 59.9 |
| l-Aspartic acid | 33.3 | 3.3 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 59.9 |
| l-Cysteine | 22.1 | 2.2 | 6.6 | 13.3 | 19.9 | 26.5 | 33.2 | 39.8 |
| l-Glutamine | 56.6 | 5.7 | 17.0 | 34.0 | 50.9 | 67.9 | 84.9 | 101.9 |
| l-Glutamic acid | 56.6 | 5.7 | 17.0 | 34.0 | 50.9 | 67.9 | 84.9 | 101.9 |
| l-Glycine | 33.3 | 3.3 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 59.9 |
| l-Histidine | 22.7 | 2.3 | 6.8 | 13.6 | 20.4 | 27.2 | 34.1 | 40.9 |
| l-Isoleucine | 62.8 | 6.3 | 18.8 | 37.7 | 56.5 | 75.4 | 94.2 | 113.0 |
| l-Leucine | 83.3 | 8.3 | 25.0 | 50.0 | 75.0 | 100.0 | 125.0 | 149.9 |
| l-Lysine · HCl2 | 94.6 | 7.6 | 22.7 | 45.4 | 68.1 | 90.8 | 113.6 | 136.3 |
| l-Methionine | 29.6 | 3.0 | 8.9 | 17.8 | 26.6 | 35.5 | 44.4 | 53.3 |
| l-Phenylalanine3 | 54.7 | 30.5 | 30.5 | 30.5 | 30.5 | 30.5 | 30.5 | 30.5 |
| l-Proline | 41.9 | 4.2 | 12.6 | 25.1 | 37.7 | 50.3 | 62.9 | 75.4 |
| l-Serine | 83.9 | 8.4 | 25.2 | 50.3 | 75.5 | 100.7 | 125.9 | 151.0 |
| l-Threonine | 47.1 | 4.7 | 14.1 | 28.3 | 42.4 | 56.5 | 70.7 | 84.8 |
| l-Tryptophan | 15.6 | 1.6 | 4.7 | 9.4 | 14.0 | 18.7 | 23.4 | 28.1 |
| l-Tyrosine4 | 40.7 | 40.7 | 40.7 | 40.7 | 40.7 | 40.7 | 40.7 | 40.7 |
| l-Valine | 70.3 | 7.0 | 21.1 | 42.2 | 63.3 | 84.4 | 105.5 | 126.5 |
Represents egg-protein composition.
Actual concentration of amino acids in hydrochloric acid form in the amino acid mixture: 62.1 mg arginine/g and 60.6 mg lysine/g.
l-Phenylalanine intake was kept constant at 30.5 mg · kg−1 · d−1 at all protein intakes.
l-Tyrosine intake was kept constant at 40.7 mg · kg−1 · d−1 at all protein intakes.
The tracer protocol started with drink 5, and l-[1-13C]phenylalanine (99 atom percentage access; Cambridge Isotope Laboratories) and NaH13CO3 (99 atom percentage access; Cambridge Isotope Laboratories) tracers were administered orally with the drinks. Oral priming doses of l-[1-13C]phenylalanine and NaH13CO3 were 0.176 and 0.66 mg/kg, respectively. The hourly dose of l-[1-13C] phenylalanine was 1.2 mg · kg−1 · d−1 and continued with drinks 6, 7, and 8. The total phenylalanine intake (labeled and unlabeled) of drinks 5 to 8 was 30.5 mg · kg−1 · d−1, and the tyrosine intake was maintained at 40 mg · kg−1 · d−1 to ensure an excess of tyrosine. The quantity of phenylalanine and tyrosine both exceed the requirements to make sure that their intakes were sufficient for protein synthesis at the various protein intakes. Breath and urine samples were collected periodically before and after the tracers were consumed and were later analyzed for 13CO2 and [1-13C]phenylalanine enrichments, respectively. A comparison of oral with intravenous administrations of the tracer was conducted to test the effect of splanchnic uptake of the tracer on the breakpoint estimate (23). Although oxidation was higher for the enteral tracer than for the intravenous tracer, the overall breakpoint estimation was not affected by the absolute oxidation level because it was higher at all protein intakes.
Sample collection and analysis
Three baseline breath samples were collected at 45, 30, and 15 min before and 2 baseline urine samples were collected 55 and 5 min before the tracer protocol began. Four plateau breath and urine samples were collected (one each at 30-min intervals) starting 2.5 h after the tracer protocol began. Breath samples were collected by having subjects breath into a plastic tube, and samples were stored in preevacuated glass tubes at room temperature. Urine samples were collected with the aid of a urine collection hat. The samples of urine collected into the container were quickly transferred into freezer tubes and stored at – 80°C until thawed and analyzed for [1-13C]phenylalanine enrichment. The rate of carbon dioxide production was measured on each testing day by using a ventilated-hood indirect calorimeter (Parvo TrueOne 2400) between testing drinks 5 and 6 to quantify 13CO2 excretion in breath. Also, each subject's resting energy expenditure (kcal/d) was recorded simultaneously by using the indirect calorimeter.
Enrichment of 13C in breath samples was analyzed by using continuous-flow isotope ratio mass spectrometry (Thermo Fisher GasBench II and Delta V mass spectrometer). The isotope enrichment of each sample was calculated as atom percentage excess. [1-13C]Phenylalanine enrichment in the urine samples was analyzed with gas chromatography–mass spectrometry and calculated as mole percentage excess (MPE). Amino acids in urine samples were derivatized to their heptafluorobutyryl isobutyl esters. The capillary gas chromatography–mass spectrometry analyses were carried out by using an Agilent 5975C (Agilent Laboratories) mass spectrometer system. Typical electron energy was 70 eV with the ion-source temperature maintained at 250°C. The individual components were separated by using a 30-m HP-5 capillary column (250 μm internal diameter × 0.25-μm film thickness). The flow rate was typically set at 1 mL/min. The m/z of the fragment ions at 148 and 149, which quantitatively represents unlabeled and labeled [1-13C]phenylalanine, was monitored on ion chromatograms.
Tracer kinetics
Tracer kinetics were calculated by using a single-pool model described previously (24). The isotope steady state of the tracer enrichment at baseline and plateau was represented by unchanging values of [1-13C]phenylalanine in urine and 13CO2 in breath samples. Uncertainties in the measurements of the urine [1-13C]phenylalanine plateau existed because, after each urine collection, the subject's bladder may not have been completely emptied. This could delay achievement of the plateau. Baseline enrichment was counted as background and subtracted from plateau values.
The 13CO2 rate of appearance (F13CO2, μmol · kg−1 · h−1) was calculated by using the following equation:
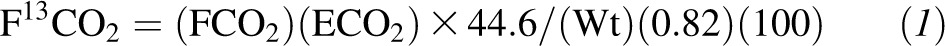 |
where FCO2 is the carbon dioxide production rate (mL/h), ECO2 is the 13C enrichment in breath, and Wt is body weight (kg). A factor of 0.82 was used to account for the fractional retention of 13CO2 in the bicarbonate pool. A factor of 100 was used to convert atom percentage excess to a fraction.
Phenylalanine flux (μmol · kg−1 · h−1), which equals the phenylalanine rate of appearance at steady state, was calculated from the dilution of orally administered [1-13C]phenylalanine into the plasma pool:
 |
where Ei is the enrichment of orally administered l-[1-13C]phenylalanine (MPE), Eu is the enrichment of [1-13C]phenylalanine in urine (MPE), and i is the infusion rate of l-[1-13C] phenylalanine (μmol · kg−1 · h−1).
Phenylalanine oxidation rate μmol · kg−1 · h−1) was calculated as follows:
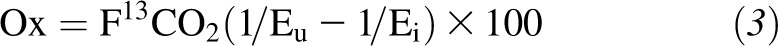 |
Plasma and urinary phenylalanine are not considered suitable pools for estimating phenylalanine hydroxylation/oxidation because they are not a true reflection of the pool where oxidation takes place. Enrichments measured from apolipoprotein B-100—a hepatic export protein—is a more appropriate estimator (25). However, the use of F13CO2 to estimate the breakpoint generates results similar to those with the use of apolipoprotein B-100 enrichment to estimate phenylalanine hydroxylation (26). Therefore, F13CO2 data were used to determine the protein requirements.
Clinical measurements
Fasting-state body weight was recorded on the morning of each testing day by using a platform scale. Percentage body fat was measured via bioelectric impedance (Tanita 2202/um-016) on the morning of the last testing day. Fat-free mass (FFM) was calculated as body weight (kg) × (1 − percentage body fat/100). The fasting blood glucose concentration was measured on every testing day with a finger-prick test (Accu-chek Aviva Blood Glucose Meter). Fasting-state blood samples were collected on testing days 1, 3, and 7, and data from a clinical chemistry panel, prealbumin, and complete blood count with differential were used to monitor each subject's health status throughout the study period.
Statistical analysis
Previously, Humayun et al (20) studied the protein requirements of 8 young men by using the IAAO technique. We proposed to recruit 10 subjects with ≥5 completing the trial; the potential dropout rate was 50% because of the age of the population to be studied. The Purdue Institutional Review Board approved our recruitment of up to 15 subjects.
The results are reported as means ± SEs. Statistical analyses were performed by using SAS (version 9.2.1; SAS Institute Inc). A PROC Mixed model was used to test the effect of protein intake on resting metabolic rate and phenylalanine flux and oxidation. Tukey-Kramer's multiple comparison was used for post hoc analysis. A P value <0.05 was considered significant.
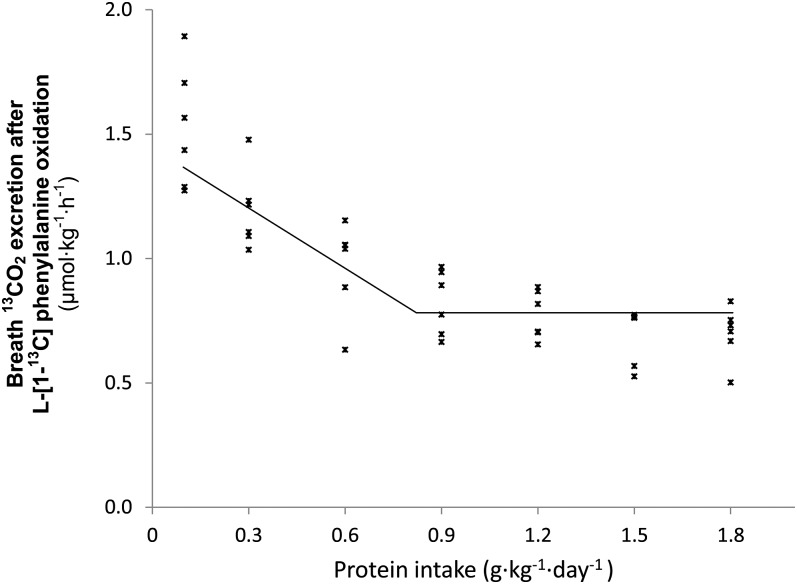
The data were analyzed by using a 4-parameter nonlinear mixed model. The model assumes that protein requirements are normally distributed. The mean and the variance of this distribution are 2 parameters of the model. A piecewise linear function is fit to the data. The breakpoint, or knot, is designated as the mean of the requirement. The data with the fit are shown in Figure 1. The additional 2 parameters of the model are the slope to the left of the breakpoint and the constant value of the function to the right of the breakpoint. The figure does not show the parameter that describes the variability of the protein requirement, the variance. We used the SAS Procedure NLMIXED for the analysis. Note that this mixed model is somewhat different from the mixed models fit by the SAS Procedures MIXED, GLM, and similar statistical software, where the random effect is an additive term in the model for the response variable.
FIGURE 1.
The relation between protein intake and breath 13CO2 production in 6 octogenarian women. The data points represent the 13CO2 excretion of individual subjects at each protein intake. The breakpoint represents the estimated mean protein requirement. The breakpoint was determined by using a nonlinear mixed model. The estimate for the mean protein requirement is 0.85 g · kg−1 · d−1, and the corresponding adequate protein allowance is 1.15 g · kg−1 · d−1.
The Institute of Medicine report on dietary protein needs (1) defines the EAR for protein as the median of the requirement distribution and the RDA as the 97.5th percentile of the requirement distribution. For a normally distributed data set, the median and mean are equal, so the EAR for protein is the mean of the requirement distribution. The RDA is the mean plus 1.96 (or 2) SD. The SAS Procedure NLMIXED used for our analysis not only provides estimates of the 4 parameters, but is also capable of providing estimates for arbitrary functions of the parameters with CIs. Using this approach, we obtained estimates with 95% CIs for the EAR and the RDA (the estimated mean of the requirement distribution plus 1.96 times the square root of the variance of this distribution).
In the section on protein requirements for adults, the Institute of Medicine report (1) suggests that the distribution of protein requirements is log normal, ie, the log of the requirement is normally distributed. Given the number of subjects in this study, the data do not provide sufficient information to guide a choice between the normal assumption and the log-normal assumption. We present the results for this alternative model and an approximation for the RDA (1.24 times the EAR) suggested in the Institution of Medicine report (1).
RESULTS
Subject characteristics
The age range of the 6 women who completed the study and were not diabetic was 80–87 y (82 ± 1 y). The average weight, height, BMI, and FFM of the subjects were 68 ± 4 kg, 160 ± 4 cm, 26 ± 2, and 40 ± 1 kg, respectively. The women maintained their body weights while completing the 7 testing periods, and protein intake did not affect the fed-state resting energy expenditure (1371 ± 66 kcal/d).
Phenylalanine flux and oxidation
Protein intake did not significantly affect phenylalanine flux (Table 3) (P = 0.46). The nonsignificant phenylalanine flux suggests that changes in protein synthesis are correspondingly reflected by changes in oxidation, although no changes in synthesis (flux minus oxidation) were observed. Phenylalanine oxidation was significantly affected by protein intake, and it declined overall with the increases in protein intake (Table 3). However, significant reductions were observed only at protein intakes of 1.5 g · kg−1 · d−1 compared with 0.1 and 0.3 g · kg−1 · d−1 (P < 0.05) but not at any other higher or lower intakes.
TABLE 3.
Phenylalanine flux and oxidation rate at different protein intakes1
| Protein intake | Phenylalanine flux2 | Phenylalanine oxidation3 |
| μmol · kg−1 · h−1 | μmol · kg−1 · h−1 | |
| 0.1 g · kg−1 · d−1 | 29.4 ± 4.0 | 6.2 ± 0.8a |
| 0.3 g · kg−1 · d−1 | 37.6 ± 4.1 | 6.4 ± 0.8a |
| 0.6 g · kg−1 · d−1 | 42.5 ± 9.1 | 5.9 ± 0.8a,b |
| 0.9 g · kg−1 · d−1 | 36.5 ± 9.3 | 4.4 ± 0.9a,b |
| 1.2 g · kg−1 · d−1 | 30.9 ± 3.7 | 3.4 ± 0.8a,b |
| 1.5 g · kg−1 · d−1 | 30.0 ± 3.5 | 2.4 ± 1.0b |
| 1.8 g · kg−1 · d−1 | 35.9 ± 9.4 | 3.6 ± 0.9a,b |
All values are means ± SEs; n = 6. Values in the same column with different superscript letters are significantly different, P < 0.05.
Repeated-measures ANOVA was used to assess the effects of different protein intakes on phenylalanine flux and oxidation. Tukey-Kramer's multiple comparison was used for post hoc analysis if the model was significant. Protein intake on different testing days had no significant effect on phenylalanine flux (P = 0.56).
The model of protein intake and phenylalanine oxidation was significant (P < 0.05). Post hoc analysis (Tukey-Kramer) was used to test which phenylalanine oxidation values were different from one another. Phenylalanine oxidation at an intake of 1.5 g protein · kg−1 · d−1 was significantly lower than other values, although it was not significantly different at any other higher or lower intakes. Protein synthesis as flux minus oxidation did not differ at any intake.
Protein requirement
The relation between F13CO2 and protein intake is shown in Figure 1. F13CO2 decreased as the protein intake increased, consistent with an increase in incorporation of l-[1-13C]phenylalanine into protein. This increase in label incorporation continued until protein intake reached the protein requirement and there was no further label incorporation into protein.
As described in the statistical analyses section of the methods, the protein requirement of these elderly women was estimated 3 ways. Assuming that the data were normally distributed, the estimate (95% CI) for the mean protein requirement is 0.85 (0.60, 1.09) g · kg−1 · d−1; for the adequate protein allowance, the corresponding values are 1.15 (0.77, 1.54) g · kg−1 · d−1. Based on the log normal assumption model, the protein requirement is 0.96 (0.71, 1.22) g · kg−1 · d−1, and the adequate protein allowance is 1.39 (0.84, 1.93) g · kg−1 · d−1. The approximation of the RDA using 1.24 times the EAR is 1.05 (0.75, 1.35). The log-normal assumption for the distribution of requirement gives a higher estimate than those based on the normal assumption, whereas the approximation of 1.24 times the mean protein requirement gives a lower estimate of the adequate protein allowance. The usual model-fit criteria (Akaike information criterion, Akaike information criterion for finite sample sizes, and Bayesian information criterion) did not provide an indication that one of the models (normal or log normal) was superior to the other. The estimates of the model parameters were as follows: slope, −0.987; breakpoint mean, 0.847; variance of breakpoint, 0.0234; constant value to the right of the breakpoint, 0.730; and residual variance, 0.0129.
DISCUSSION
In accordance with recommendations of the Institute of Medicine (1), this study assessed the protein needs of octogenarian women by using a technique other than nitrogen balance, namely the IAAO technique. This was the third study that measured protein requirements in humans with the use of the IAAO approach. We modified the IAAO method to make it minimally invasive so that it could be used in vulnerable groups, such as children and the elderly, in whom repeated nitrogen balance studies are an intolerable burden (19). The results of this study suggest that the protein requirement of octogenarian women may be higher than the current EAR, but multiple factors and issues must be considered before accepting these data and the interpretation that elderly women need to consume more protein to prevent inadequacy.
One important methodologic concern raised by Millward and Jackson (27) was that, because constant intakes of phenylalanine and tyrosine were used with increasing amounts of all other amino acids in the meals, the relative concentration of the indicator phenylalanine in the meals varied. Millward and Jackson argued that the curvilinear reduction in the relative phenylalanine concentration of the meal is similar to that of the reported oxidation of the tracer phenylalanine, as shown in Table 3. They also argued that the apparent breakpoint in tracer oxidation coincided with the amino acid intake, which was balanced in terms of the phenylalanine content of the egg-protein pattern used in the meals. In other words, Millward and Jackson suggested that phenylalanine given in the IAAO method became the limiting amino acid after it was balanced according to egg-protein amino acid composition. However, regardless of the protein intake, the total aromatic amino acid concentration is always 70 mg · kg−1 · d−1, which is higher than the aromatic amino acid requirement and thus could not be “balanced” by increasing protein intake. With sufficient tyrosine, the concept of the IAAO approach is to keep the phenylalanine content constant and sufficient at any protein (amino acid mixture) level to reflect the protein oxidation rate. The authors agree with Millward and Jackson that the relative concentration of phenylalanine is lower when the protein intake is higher. In their letter to the editor of The American Journal of Clinical Nutrition, Millward and Jackson (27), a theoretical figure was presented to show the relative concentration of the indicator amino acid phenylalanine with the increase of total protein intake. When the relative concentration of phenylalanine equals 1, protein intake calculates to 0.56 g · kg−1 · d−1, which is different from the observed breakpoint of the current study of 0.85 g · kg−1 · d−1. It is interesting to note that, in the hypothetical figure, the phenylalanine intake levels are included for the study of protein requirements from young men (20) and school-age children (19). For both studies, a similar relation in relative phenylalanine intake exists, but yields a breakpoint estimate of 0.93 g · kg−1 · d−1 for young men and of 1.3 g · kg−1 · d−1 for school-aged children. This suggests that the oxidation of the tracer is reflecting the overall pattern of the demand for net postprandial protein synthesis and not its own excess or limitation. Also, consistent with the results of the current study (Table 3), phenylalanine flux did not change with increasing intakes of protein (19, 20), and a plateau in tracer oxidation was observed beyond the breakpoint. All of these observations suggest that phenylalanine intakes were not limiting at any test protein intake.
After publication of the study of protein requirements in children (19), several concerns with the IAAO technique were raised and addressed (27). The key methodologic concern that Millward and Jackson (27) raised was that, because constant intakes of phenylalanine and tyrosine were used, the “relative concentration of phenylalanine indicator” fell in a curvilinear manner similar to that of the oxidation of the tracer phenylalanine. They suggested that this cast into question the application of the IAAO approach to determine the protein requirement. However, this concern presupposes that the rate of phenylalanine oxidation is determined by the “relative concentration of phenylalanine indicator” rather than by (as we argue and designed our study) the total amino acids available to optimize whole-body protein synthesis—indicator oxidation being the reciprocal of whole-body protein synthesis. A fundamental point is that the pattern of indicator oxidation is what is critical and not the absolute rate of oxidation. A clear example of this point is an earlier study in which the lysine requirement was determined by using either an intravenous tracer or an oral tracer (23). Oxidation of the oral tracer was greater than the intravenous tracer, but the requirement breakpoint was the same.
The mathematical approach used to analyze data is another important issue that limits confidence in accepting the current results. The IAAO-derived EAR of protein is calculated by using a nonlinear (breakpoint) model. For the current protein DRIs (1), the nitrogen balance–derived EAR was obtained by fitting a straight line between protein intakes and nitrogen balance, with the protein intake at which the nitrogen balance is interpolated to zero considered the protein requirement. Using this linear mathematical approach, we determined that age does not influence the protein requirement for adults, and the current EAR is apparently adequate for young and elderly men and women (4). We studied young (21–46 y) and elderly adults (63–81 y) consuming 0.50, 0.75, and 1.00 g · kg−1 · d−1 protein during three 18-d controlled feeding trials and determined that the protein allowance was 0.85 g · kg−1 · d−1 [not statistically different from the RDA (4)].
The linear model was questioned because nitrogen utilization changes with protein intakes (20): when the intake of protein increases, the efficiency of protein utilization decreases. A biphase linear regression was proposed as an alternative approach and better fit the data. However, the paucity of nitrogen balance data with protein intakes >1 g · kg−1 · d−1 limits the utility of applying the biphase model. Rand et al (2) commented that, “whereas a biologically more realistic equation is certainly the best approach… (when) data exist covering a wide range of intakes… in this relatively narrow range of intake data in the adult, the response curve is best approximated by a (simple) linear regression.” Thus, although it is desirable to use a biphase linear model, the currently available nitrogen balance data are not ideal for the model. Nonetheless, in addition to using a linear model, Rand et al (2) applied a biphase model to the data set used for the meta-analysis on which the current EAR and RDA are based. The resulting EAR was 18% higher than the EAR obtained with the linear model. Later, Humayun et al (20) included 28 nitrogen balance studies to reanalyze the data by using a biphase linear regression, and the estimated protein requirement was 40% higher. Use of nonlinear regression to reanalyze the existing nitrogen balance data gave a mean breakpoint estimate of 0.91 g · kg−1 · d−1, which was virtually the same as the IAAO estimate for men of 0.93 g · kg−1 · d−1 (20).
These varied mathematical approaches to analyzing nitrogen balance data have important implications for how the IAAO-derived protein EAR is viewed. The IAAO-derived EAR for protein is quantitatively more consistent with the corresponding nitrogen balance–based value estimated by using nonlinear rather than linear regression. However, the EAR from nonlinear regression is not the accepted value for the DRIs, and the decision regarding which nitrogen balance–based EAR value to accept is unlikely to be experimentally resolved because large-scale controlled feeding studies with concurrent measurements of nitrogen balance and IAAO are unlikely to be done. In light of the uncertainties and limitations of nitrogen balance and IAAO, both of which are short-term estimates of protein needs, the suggestion from the current study that the protein requirement of elderly women is higher than currently thought must be confirmed by longer-term studies of body composition, physical and cognitive function, and health outcomes (28). We recognize that these types of studies are very difficult and costly to conduct in elderly adults, and it is not ethical to test a very-low protein/nitrogen diet for a prolonged time because of profound adverse physiologic and functional effects (29, 30). Nonetheless, the accurate assessment of dietary protein needs of elderly adults will be greatly aided by systematically measuring longer-term physiologic outcomes.
Another limitation of the current study was that the estimation of RDA had a large CI because of the relatively large between-subject variance. We acknowledge that the sample size of the current study was relatively small; thus, the CI of the estimated protein requirements (mean and lower CI level) overlapped with the current EAR and RDA. Although the estimated means of the current study are higher than the EAR and RDA, they may not be significantly different based on the sample size. Also, although adaptation length (1, 3, or 7 d) did not affect amino acid oxidation (31), whether longer adaptation would affect the results is still unknown. Future studies should consider measuring longer-term functional and physiologic outcomes that could be affected by a low protein intake.
Findings from some nitrogen balance studies support that the current RDA for protein is adequate for elderly adults (4, 5), whereas other findings do not (7, 30, 32). Healthy elderly adults retain the capacity to adjust protein metabolism when different protein intakes that span the range of adequacy are consumed (7–9, 30, 33, 34). When elderly adults consume the RDA, these adjustments are necessary to help them adapt, as opposed to accommodate (ie, compromise physiologic functions) (7–9). On the basis of findings from prospective, short-term controlled feeding studies (8, 9, 30), the current RDA provides adequate protein to blunt negative accommodations to skeletal muscle and immune function (30) but may not be sufficient to completely prevent more subtle accommodative responses to body composition. In a controlled-feeding study, 10 men and women (aged 54–78 y) consumed an energy-balanced diet that provided the RDA of protein for 14 wk (8). Over time, muscle strength and whole-body muscle mass did not change, but FFM, midthigh muscle area, and total body water decreased, which suggests that the RDA for protein might be marginally inadequate for elderly people (8). Old adults (70–79 y) who consumed the highest quintile of protein intake (1.2 g · kg−1 · d−1) lost 40% less lean mass than did those who consumed the lowest quintile (0.7 g · kg−1 · d−1) over a 3-y period (10). This finding has been used to support that elderly adults need to consume protein intakes above the RDA (9). However, the protein intake of the lowest quintile group was below the RDA, and the changes in lean body mass over time were not different between the groups that consumed the RDA or 150% of the RDA. Also, the greater loss of lean body mass was only apparent when subjects consuming less than the RDA also lost weight (10). Thus, higher protein intakes may not help weight-stable elderly adults retain lean body composition, which could be interpreted as support for the adequacy of the RDA for protein. Collectively, these findings suggest that the current RDA for protein might be marginally inadequate for elderly adults, especially to promote long-term health (8), ie, it is a suggested minimum intake and not necessarily a preferred intake. The current IAAO results support this perspective.
Because FFM is the primary determinant of the protein requirement, it is important to consider protein needs per FFM as well. Elderly people typically have less FFM than young adults, which may lead to a higher protein requirement when expressed per FFM. In the current study, the mean protein requirement per FFM is 1.41 g · kg FFM−1 · d−1 and the corresponding RDA is 1.90 g · kg FFM−1 · d−1, which are higher than the values for young adults derived by using the IAAO method (20) (EAR: 1.14; RDA: 1.48 g · kg FFM−1 · d−1). These values are also higher than those estimated by using nitrogen balance (4).
Consumption of an inadequate amount of protein could accelerate muscle loss with aging (30) and lead to sarcopenia, which affects 30% of people older than 60 y and 50% of those older than 80 y (35). Muscle loss in elderly adults increases the likelihood of falls and the inability to perform routine daily activities (36). Increasing protein intake could increase muscle protein synthesis (37) because of increased amino acid availability (38). Finding the accurate protein requirement is important because the current RDA is widely used by health care professionals and government programs, such as Meals on Wheels, as the adequate protein intake for elderly people to consume (39). Research is also needed to assess the effects of age, sex, and sarcopenic status on protein intakes to maximize physical, cognitive, and metabolic functions in this fast growing population. Because the IAAO technique is short term, current research shows that it can be used in vulnerable groups, such as elderly people. However, it cannot be used to evaluate long-term health status. New approaches to determine protein needs based on indexes of health status must be established.
In conclusion, the results of this study, which assessed the protein requirement of elderly women by using the IAAO technique, indicate that this technique is feasible to use with this age group in a research setting and suggests that octogenarian women may have a higher protein requirement than currently indicated by the EAR. Caution is warranted before this result is used to question the accuracy of the current EAR because of the lack of direct comparisons between nitrogen balance and IAAO estimates of protein need, uncertainties and controversies surrounding the use of the method to determine protein requirements, and the need for confirmatory research based on physiologic and health outcomes.
Acknowledgments
We thank all of the subjects who participated in this study. We also thank the Purdue Center on Aging and the Life Course and the administrators at University Place (West Lafayette, IN) for their help in facilitating recruitment and clinical testings.
The authors’ responsibilities were as follows—MT and WWC: grant writing, study design, data collection, sample and data analysis, and manuscript writing; GPM: data analysis and manuscript writing; and RE, ROB, and PBP: expert consultation on study design and techniques and manuscript writing. The authors had no conflicts of interest.
Footnotes
Abbreviations used: DRI, Dietary Reference Intake; EAR, Estimated Average Requirement; FFM, fat-free mass; IAAO, indicator amino acid oxidation; MPE, mole percentage excess; RDA, Recommended Dietary Allowance.
REFERENCES
- 1.Institute of Medicine, Panel on Macronutrients. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes: Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press, 2005. [Google Scholar]
- 2.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr 2003;77:109–27. [DOI] [PubMed] [Google Scholar]
- 3.Uauy R, Scrimshaw NS, Young VR. Human protein requirements: nitrogen balance response to graded levels of egg protein in elderly men and women. Am J Clin Nutr 1978;31:779–85. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 2008;88:1322–9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AH, Gomez A, Bergan JG, Lee TC, Monckeberg F, Chichester CO. Comparative nitrogen balance study between young and aged adults using three levels of protein intake from a combination wheat-soy-milk mixture. Am J Clin Nutr 1978;31:12–22. [DOI] [PubMed] [Google Scholar]
- 6.Zanni E, Calloway DH, Zezulka AY. Protein requirements of elderly men. J Nutr 1979;109:513–24. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr 1994;60:501–9. [DOI] [PubMed] [Google Scholar]
- 8.Campbell WW, Trappe TA, Jozsi AC, Kruskall LJ, Wolfe RR, Evans WJ. Dietary protein adequacy and lower body versus whole body resistive training in older humans. J Physiol 2002;542:631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 2001;56:M373–80. [DOI] [PubMed] [Google Scholar]
- 10.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 11.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 2008;87:1562S–6S. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–82. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr 2008;27:675–84. [DOI] [PubMed] [Google Scholar]
- 15.Elango R, Ball RO, Pencharz PB. Indicator amino acid oxidation: concept and application. J Nutr 2008;138:243–6. [DOI] [PubMed] [Google Scholar]
- 16.Pillai RR, Elango R, Muthayya S, Ball RO, Kurpad AV, Pencharz PB. Lysine requirement of healthy, school-aged Indian children determined by the indicator amino acid oxidation technique. J Nutr 2010;140:54–9. [DOI] [PubMed] [Google Scholar]
- 17.Elango R, Humayun MA, Ball RO, Pencharz PB. Lysine requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr 2007;86:360–5. [DOI] [PubMed] [Google Scholar]
- 18.Riazi R, Wykes LJ, Ball RO, Pencharz PB. The total branched-chain amino acid requirement in young healthy adult men determined by indicator amino acid oxidation by use of L-[1-13C]phenylalanine. J Nutr 2003;133:1383–9. [DOI] [PubMed] [Google Scholar]
- 19.Elango R, Humayun MA, Ball RO, Pencharz PB. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am J Clin Nutr 2011;94:1545–52. [DOI] [PubMed] [Google Scholar]
- 20.Humayun MA, Elango R, Ball RO, Pencharz PB. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am J Clin Nutr 2007;86:995–1002. [DOI] [PubMed] [Google Scholar]
- 21.Fulgoni VL., III Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr 2008;87:1554S–7S. [DOI] [PubMed] [Google Scholar]
- 22.Bross R, Ball RO, Pencharz PB. Development of a minimally invasive protocol for the determination of phenylalanine and lysine kinetics in humans during the fed state. J Nutr 1998;128:1913–9. [DOI] [PubMed] [Google Scholar]
- 23.Kriengsinyos W, Wykes LJ, Ball RO, Pencharz PB. Oral and intravenous tracer protocols of the indicator amino acid oxidation method provide the same estimate of the lysine requirement in healthy men. J Nutr 2002;132:2251–7. [DOI] [PubMed] [Google Scholar]
- 24.Zello GA, Pencharz PB, Ball RO. Dietary lysine requirement of young adult males determined by oxidation of L-[1-13C]phenylalanine. Am J Physiol 1993;264:E677–85. [DOI] [PubMed] [Google Scholar]
- 25.Reeds PJ, Hachey DL, Patterson BW, Motil KJ, Klein PD. VLDL apolipoprotein B-100, a potential indicator of the isotopic labeling of the hepatic protein synthetic precursor pool in humans: studies with multiple stable isotopically labeled amino acids. J Nutr 1992;122:457–66. [DOI] [PubMed] [Google Scholar]
- 26.Rafii M, McKenzie JM, Roberts SA, Steiner G, Ball RO, Pencharz PB. In vivo regulation of phenylalanine hydroxylation to tyrosine, studied using enrichment in apoB-100. Am J Physiol Endocrinol Metab 2008;294:E475–9. [DOI] [PubMed] [Google Scholar]
- 27.Millward DJ, Jackson AA. Protein requirements and the indicator amino acid oxidation method. The American journal of clinical nutrition 2012, 95:1498–1501. [DOI] [PubMed] [Google Scholar]
- 28.International Dairy Federation. Does the nitrogen balance cover the various components of human protein needs? Available from: http://www.idfdairynutrition.org/Files/media/FactSheetsHP/Nitrogen-Balance-Choledoc-121-110209.pdf (cited 30 April 2012 ).
- 29.Castaneda C, Dolnikowski GG, Dallal GE, Evans WJ, Crim MC. Protein turnover and energy metabolism of elderly women fed a low-protein diet. Am J Clin Nutr 1995;62:40–8. [DOI] [PubMed] [Google Scholar]
- 30.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 1995;62:30–9. [DOI] [PubMed] [Google Scholar]
- 31.Elango R, Humayun MA, Ball RO, Pencharz PB. Indicator amino acid oxidation is not affected by period of adaptation to a wide range of lysine intake in healthy young men. J Nutr 2009;139:1082–7. [DOI] [PubMed] [Google Scholar]
- 32.Gersovitz M, Motil K, Munro HN, Scrimshaw NS, Young VR. Human protein requirements: assessment of the adequacy of the current Recommended Dietary Allowance for dietary protein in elderly men and women. Am J Clin Nutr 1982;35:6–14. [DOI] [PubMed] [Google Scholar]
- 33.Castaneda C, Gordon PL, Fielding RA, Evans WJ, Crim MC. Marginal protein intake results in reduced plasma IGF-I levels and skeletal muscle fiber atrophy in elderly women. J Nutr Health Aging 2000;4:85–90. [PubMed] [Google Scholar]
- 34.Thalacker-Mercer AE, Johnson CA, Yarasheski KE, Carnell NS, Campbell WW. Nutrient ingestion, protein intake, and sex, but not age, affect the albumin synthesis rate in humans. J Nutr 2007;137:1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 36.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr 1997;127:998S–1003S. [DOI] [PubMed] [Google Scholar]
- 37.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol 2006;41:215–9. [DOI] [PubMed] [Google Scholar]
- 38.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 1998;101:2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. JAMA 2008;299:2891–3. [DOI] [PubMed] [Google Scholar]