Abstract
Background: Magnesium is a necessary component of bone, but its relation to osteoporotic fractures is unclear.
Objective: We examined magnesium intake as a risk factor for osteoporotic fractures and altered bone mineral density (BMD).
Design: This prospective cohort study included 73,684 postmenopausal women enrolled in the Women's Health Initiative Observational Study. Total daily magnesium intake was estimated from baseline food-frequency questionnaires plus supplements. Hip fractures were confirmed by a medical record review; other fractures were identified by self-report. A baseline BMD analysis was performed in 4778 participants.
Results: Baseline hip BMD was 3% higher (P < 0.001), and whole-body BMD was 2% higher (P < 0.001), in women who consumed >422.5 compared with <206.5 mg Mg/d. However, the incidence and RR of hip and total fractures did not differ across quintiles of magnesium. In contrast, risk of lower-arm or wrist fractures increased with higher magnesium intake [multivariate-adjusted HRs of 1.15 (95% CI: 1.01, 1.32) and 1.23 (95% CI: 1.07, 1.42) for quintiles 4 and 5, respectively, compared with quintile 1; P-trend = 0.002]. In addition, women with the highest magnesium intakes were more physically active and at increased risk of falls [HR for quintile 4: 1.11 (95% CI: 1.06, 1.16); HR for quintile 5: 1.15 (95% CI: 1.10, 1.20); P-trend < 0.001].
Conclusions: Lower magnesium intake is associated with lower BMD of the hip and whole body, but this result does not translate into increased risk of fractures. A magnesium consumption slightly greater than the Recommended Dietary Allowance is associated with increased lower-arm and wrist fractures that are possibly related to more physical activity and falls. This trial was registered at clinicaltrials.gov as NCT00000611.
INTRODUCTION
Low magnesium intake has been implicated in neuromuscular disorders, hypertension, cardiac arrhythmias, mitral valve prolapse, atherogenesis, insulin resistance, eclampsia, and disordered bone metabolism (1, 2). Interest in the latter has arisen because magnesium intake may be a modifiable risk factor for fracture and osteoporosis.
Magnesium depletion has been associated with decreased osteoblastic and osteoclastic activity, osteopenia, bone fragility (3, 4), vitamin D resistance or reduction (4–7), and parathyroid hormone resistance (8) or reduction (6). In postmenopausal women, low magnesium intake has been correlated with more rapid bone loss or lower bone mineral density (BMD)5 (9–11). In the Framingham Heart Study, although no longitudinal association was shown between magnesium intake and BMD over 4 y, a 2% higher trochanteric BMD was noted for every 100 mg Mg consumed by women at baseline (9). Supplementation with magnesium has resulted in improvement in BMD or a reduction in bone-turnover markers in some human trials (12), but other trials have shown no benefit (9, 13–15). These data suggest that magnesium intake might favorably alter BMD, but the relation to fracture outcomes is unclear.
Although magnesium deficiency has been shown to be deleterious to skeletal health, intake of amounts greater than the Recommended Dietary Allowance (RDA) may potentially pose risks. The RDA of magnesium for women was raised from 280 to 320 mg/d by the National Academy of Science in 1997 (16). Magnesium excess (5–10 times nutrient requirements) in rats had no effect on BMD in shorter-term studies (17) but lowered BMD in longer-term studies (18). There have been reports from human studies of bone lesions and lower BMD in cases of acute exposure to high-dose magnesium (19–22), but to our knowledge, there are no data on chronic exposure to excess magnesium intake in relation to BMD and fracture risk. This prospective cohort study examined the role of magnesium intake as an independent risk factor for altered BMD and fracture of the hip, forearm and wrist, and total fractures after accounting for important covariates in postmenopausal women enrolled in the Women's Health Initiative (WHI) Observational Study.
SUBJECTS AND METHODS
Study group
The WHI Observational Study is a prospective cohort study that is based on 40 clinical centers throughout the United States. A total of 93,676 postmenopausal women aged 50–79 y (mean: 64 y) were enrolled between 1994 and 1998. These women completed screening and enrollment questionnaires by a self-report, interview, physical examination, and blood specimen collection as previously described (23). Procedures followed were in accordance with ethical standards of institutional review boards of all participating institutions. From this sample, a sample of 73,684 participants with no missing data on magnesium or other model covariates was selected for all analyses.
Outcome ascertainment
Baseline BMD at the hip, posterior-anterior spine, and total body was measured in a subgroup of 6108 women who participated in the WHI Observational Study BMD Cohort. Three clinical centers participated (Pittsburgh, PA; Birmingham, AL; and Phoenix and Tucson, AZ). These clinics were chosen to provide maximum racial and ethnic diversity and, hence, were not representative of the WHI as a whole. BMD was obtained by using dual-energy X-ray absorptiometry with a Hologic QDR densitometer (Hologic Inc). Technicians were trained and certified by the University of California, San Francisco, Bone Density Coordinating Center. Standard protocols for positioning and analysis, routine spine and hip phantoms, and a random-sample review were used. Hardware and software changes were centralized, and calibration phantoms, which were scanned across instruments and clinical sites, were in close agreement (interscanner variability <1.5% for the spine, <4.8% for the hip, and <1.7% for linearity) (24, 25).
Questionnaires were sent to subjects annually to report hospitalizations and other clinical outcomes, including incident falls, hip, lower-arm and wrist, and other fractures. Proxy interviews regarding health outcomes were conducted for women who were unable to attend clinic visits or deceased (26). A fall history was obtained by asking about the number of times the participant fell or landed on the ground (excluding sporting activities) in the past year. The falls outcome variable for this analysis was based on a participant reporting ≥2 falls in the past year. All hip fractures were verified by review of an X-ray, MRI, or operative reports by centrally trained and blinded physician adjudicators at each clinical center. Lower-arm and wrist fractures were self-reported. Total fractures were defined as all reported clinical fractures other than those of the ribs, sternum, skull or face, fingers, toes, and cervical vertebrae. WHI Clinical Trial data showed the validity of self-reports because 71% of self-reported single-site fractures were subsequently confirmed by a physician review (27).
Exposure ascertainment
Total daily magnesium intake included dietary and supplemental sources (ie, magnesium from dietary supplements or medications). Baseline dietary magnesium was ascertained via a modified food-frequency questionnaire (FFQ) as previously described (28). Current supplements and medications were directly observed by an interviewer at baseline clinic visits and coded into Medispan database (First DataBank Inc). A standardized interviewer-administered form was used to collect information on supplement ingredients, frequency (pills/wk) and duration (mo and y) of use for each supplement. Only supplements used ≥1 time/wk were recorded (29). Nutrient intakes were estimated from the FFQ by using a database derived from the University of Minnesota's Nutrition Coordinating Center (Minnesota Nutrition Data System for Research, version 30) (30). Magnesium-intake estimates from the FFQ and means from 8-d food-intake records correlated well, with Pearson's correlation coefficients of 0.68 for energy-adjusted intake and 0.61 for unadjusted intake (28).
Other covariates
Covariates included age (50–59, 60–69, and 70–79 y), race-ethnicity, parental history of fracture, personal fracture at ≥55 y of age, BMI, history of coronary artery disease (CAD; defined as a history of angina or myocardial infarction), treated diabetes, self-reported health, hormone therapy (HT) use, alcohol intake, total calcium intake, current and past smoking, and physical activity in metabolic equivalent tasks (METs) per week. Physical activity was assessed by using a detailed questionnaire that asked about the number of minutes per week spent walking outside the home and the frequency and duration of recreational activities, which were classified by using standardized codes of the energy expenditure associated with activities in METs (31). Calcium intake and alcohol consumption were estimated from the FFQ. Weight measured to the nearest 0.1 kg and height measured to the nearest 0.1 cm per protocol were used to generate BMI (in kg/m2), which was stratified into <25 and ≥25.
Statistical analysis
Data on magnesium intake and all included covariates were available for 73,684 women. In the primary analysis, the cohort was stratified by quintiles of daily magnesium intake. A series of linear models were run to look at baseline BMD by modeling BMD at each site as a function of quintiles of magnesium intake 1) in an unadjusted model and 2) in a model adjusted for all of the previously mentioned covariates. Least-squares means and 95% CIs are presented. In addition, a second set of models that evaluated baseline BMD at each site as a function of a linear trend of magnesium were run with P-linear trend values presented.
The RR of fracture was estimated for each quintile and compared with magnesium intake in the lowest quintile. Proportional hazards models were fit for each outcome by modeling the outcome of interest as a function of quintiles of magnesium intake. Models were initially adjusted for age and then further adjusted for all covariates. To examine the effect of missing data, we reran our age-adjusted model on a full set of 89,547 participants who had both outcome and magnesium data. Because results were nearly identical, results are presented for the final sample that contained no missing data with event totals, annualized percentages, and HRs with 95% CIs. As with BMD models, a second model to evaluate the linear trend on each fracture site was run with corresponding P-trend values presented. P = 0.05 was set a priori to determine significant differences between groups.
In addition to main-effect models, we examined whether the effect of magnesium on hip, lower-arm and wrist, and total fractures was modified by baseline characteristics, including age, HT, history of CAD, history of fracture at ≥55 of age, BMI, physical activity, total calcium intake, total potassium intake, and total vitamin D intake. For each subgroup analysis, HRs for fracture or fall were computed for each category of the subgroup, as well as corresponding interaction model to evaluate the interaction between the subgroup and linear trend over magnesium groups. We also used this method to test for interaction with use of magnesium supplements or magnesium-containing medications. SAS software (version 8.2; SAS Institute Inc) was used in all analyses.
RESULTS
Descriptive data
The 73,684 women included in this analysis accrued 563,231 person-years of follow-up. The mean age of enrollees was 63 y. Approximately 85% of the cohort was white. Approximately one-half of enrollees had never smoked, and 40% of enrollees had never used HT. Approximately 58% of women had BMI ≥25, and 40% of women denied walking for exercise.
Characteristics of women enrolled in the WHI Observational Study and their stratification by quintiles of magnesium intake are summarized in Table 1. Women in the highest quintile of magnesium intake tended to be thinner, reported better health and less heart disease or diabetes, consumed more alcohol and calcium, did not currently smoke, had a higher family income, had a history of parental fracture and personal fracture at ≥55 y of age, and were more active as measured by both METs and minutes walking outside the home. The percentage composition of blacks decreased with increasing magnesium-intake quintiles, with blacks comprising 13.0% of the lowest quintile compared with 4.0% of the highest quintile.
TABLE 1.
Baseline characteristics of the WHI Observational Study participants by quintile of total magnesium intake1
| <206.5 mg | 206.5–270.2 mg | 270.3–333.7 mg | 333.8–422.4 mg | ≥422.5 mg | P | |
| Age [n (Ann %)] | <0.001 | |||||
| 50–59 y | 4923 (33.9) | 4825 (32.7) | 4848 (32.6) | 4737 (31.6) | 4987 (33.5) | |
| 60–69 y | 6191 (42.6) | 6400 (43.3) | 6441 (43.4) | 6656 (44.4) | 6592 (44.3) | |
| 70–79 y | 3426 (23.6) | 3544 (24.0) | 3560 (24.0) | 3607 (24.0) | 3294 (22.1) | |
| Race-ethnicity [n (Ann %)] | <0.001 | |||||
| White | 10,877 (74.8) | 12,419 (84.1) | 12,999 (87.5) | 13,464 (89.8) | 13,326 (89.6) | |
| Black | 1888 (13.0) | 1074 (7.3) | 802 (5.4) | 668 (4.5) | 601 (4.0) | |
| Hispanic | 821 (5.6) | 577 (3.9) | 425 (2.9) | 360 (2.4) | 344 (2.3) | |
| American Indian | 82 (0.6) | 61 (0.4) | 47 (0.3) | 34 (0.2) | 49 (0.3) | |
| Asian/Pacific Islander | 630 (4.3) | 447 (3.0) | 410 (2.8) | 319 (2.1) | 356 (2.4) | |
| Unknown | 242 (1.7) | 191 (1.3) | 166 (1.1) | 155 (1.0) | 197 (1.3) | |
| Parental history of fracture [n (Ann %)] | 5353 (36.8) | 5772 (39.1) | 5890 (39.7) | 6186 (41.2) | 6184 (41.6) | <0.001 |
| Fracture at ≥55 y of age [n (Ann %)] | 1919 (13.2) | 2170 (14.7) | 2156 (14.5) | 2223 (14.8) | 2299 (15.5) | <0.001 |
| BMI [n (Ann %)] | <0.001 | |||||
| <25 kg/m2 | 5545 (38.1) | 6102 (41.3) | 6268 (42.2) | 6359 (42.4) | 6483 (43.6) | |
| ≥25 kg/m2 | 8995 (61.9) | 8667 (58.7) | 8581 (57.8) | 8641 (57.6) | 8390 (56.4) | |
| History of MI or angina [n (Ann %)] | 1076 (7.4) | 1018 (6.9) | 888 (6.0) | 943 (6.3) | 928 (6.2) | <0.001 |
| Treated diabetes [n (Ann %)] | 699 (4.8) | 599 (4.1) | 528 (3.6) | 542 (3.6) | 486 (3.3) | <0.001 |
| Self-reported health [n (Ann %)] | <0.001 | |||||
| Excellent | 2260 (15.5) | 2719 (18.4) | 2875 (19.4) | 2906 (19.4) | 3017 (20.3) | |
| Very good | 5537 (38.1) | 5975 (40.5) | 6294 (42.4) | 6482 (43.2) | 6385 (42.9) | |
| Good | 4993 (34.3) | 4765 (32.3) | 4504 (30.3) | 4475 (29.8) | 4370 (29.4) | |
| Fair/poor | 1750 (12.0) | 1310 (8.9) | 1176 (7.9) | 1137 (7.6) | 1101 (7.4) | |
| HT use [n (Ann %)] | <0.001 | |||||
| Never/past user | 9704 (66.7) | 9494 (64.3) | 9474 (63.8) | 9522 (63.5) | 9403 (63.2) | |
| <5 y | 1748 (12.0) | 1925 (13.0) | 1945 (13.1) | 1897 (12.6) | 1974 (13.3) | |
| 5 to <10 y | 1262 (8.7) | 1359 (9.2) | 1471 (9.9) | 1533 (10.2) | 1516 (10.2) | |
| 10 to <15 y | 776 (5.3) | 885 (6.0) | 880 (5.9) | 967 (6.4) | 929 (6.2) | |
| ≥15 y | 1050 (7.2) | 1106 (7.5) | 1079 (7.3) | 1081 (7.2) | 1051 (7.1) | |
| Thiazide use [n (Ann %)] | <0.001 | |||||
| Nonuser | 13,689 (94.1) | 13,983 (94.7) | 14,059 (94.7) | 14,249 (95.0) | 14,185 (95.4) | |
| <10 y | 616 (4.2) | 566 (3.8) | 532 (3.6) | 514 (3.4) | 480 (3.2) | |
| ≥10 y | 235 (1.6) | 220 (1.5) | 258 (1.7) | 236 (1.6) | 208 (1.4) | |
| Current corticosteroid use [n (Ann %)] | 208 (1.4) | 207 (1.4) | 184 (1.2) | 187 (1.2) | 181 (1.2) | 0.380 |
| Alcohol intake [n (Ann %)] | <0.001 | |||||
| Nondrinker | 2088 (14.4) | 1649 (11.2) | 1556 (10.5) | 1406 (9.4) | 1350 (9.1) | |
| Past drinker | 3026 (20.8) | 2682 (18.2) | 2503 (16.9) | 2481 (16.5) | 2722 (18.3) | |
| <1 drink/mo | 1879 (12.9) | 1825 (12.4) | 1667 (11.2) | 1566 (10.4) | 1626 (10.9) | |
| <1 drink/wk | 2922 (20.1) | 2920 (19.8) | 3034 (20.4) | 3023 (20.2) | 2999 (20.2) | |
| 1 to <7 drinks/wk | 3231 (22.2) | 3872 (26.2) | 4040 (27.2) | 4269 (28.5) | 4128 (27.8) | |
| ≥7 drinks/wk | 1394 (9.6) | 1821 (12.3) | 2049 (13.8) | 2255 (15.0) | 2048 (13.8) | |
| Smoking status [n (Ann %)] | <0.001 | |||||
| Never | 7449 (51.2) | 7571 (51.3) | 7570 (51.0) | 7649 (51.0) | 7617 (51.2) | |
| Past | 5758 (39.6) | 6211 (42.1) | 6477 (43.6) | 6631 (44.2) | 6719 (45.2) | |
| Current | 1333 (9.2) | 987 (6.7) | 802 (5.4) | 720 (4.8) | 537 (3.6) | |
| Total daily calcium intake [n (Ann %)]2 | <0.001 | |||||
| <600 mg | 8233 (56.6) | 3331 (22.6) | 1168 (7.9) | 323 (2.2) | 125 (0.8) | |
| 600–1500 mg | 5283 (36.3) | 9325 (63.1) | 10,029 (67.5) | 8814 (58.8) | 4879 (32.8) | |
| >1500 mg | 1024 (7.0) | 2113 (14.3) | 3652 (24.6) | 5863 (39.1) | 9869 (66.4) | |
| Total daily vitamin D intake [n (Ann %)]2 | <0.001 | |||||
| <200 IU | 11,303 (77.7) | 7205 (48.8) | 3898 (26.3) | 1974 (13.2) | 1460 (9.8) | |
| 200–500 IU | 2516 (17.3) | 5253 (35.6) | 5804 (39.1) | 4778 (31.9) | 3435 (23.1) | |
| >500 IU | 721 (5.0) | 2311 (15.6) | 5147 (34.7) | 8248 (55.0) | 9978 (67.1) | |
| Physical activity [n (Ann %)] | <0.001 | |||||
| Inactive | 2863 (19.7) | 2146 (14.5) | 1795 (12.1) | 1558 (10.4) | 1385 (9.3) | |
| <5 METs/wk | 3460 (23.8) | 2956 (20.0) | 2759 (18.6) | 2573 (17.2) | 2191 (14.7) | |
| 5–12 METs/wk | 3349 (23.0) | 3667 (24.8) | 3592 (24.2) | 3609 (24.1) | 3339 (22.5) | |
| >12 METs/wk | 4792 (33.0) | 5918 (40.1) | 6602 (44.5) | 7155 (47.7) | 7860 (52.8) | |
| Time spent walking [n (Ann %)] | <0.001 | |||||
| 0 min/wk | 7250 (49.9) | 6289 (42.6) | 5628 (37.9) | 5385 (35.9) | 4951 (33.3) | |
| >0–150 min/wk | 5423 (37.3) | 6237 (42.2) | 6702 (45.1) | 6989 (46.6) | 6905 (46.4) | |
| >150 min/wk | 1867 (12.8) | 2243 (15.2) | 2519 (17.0) | 2626 (17.5) | 3017 (20.3) | |
| Family income [n (Ann %)] | <0.001 | |||||
| <$10,000 | 818 (5.6) | 538 (3.6) | 421 (2.8) | 382 (2.5) | 422 (2.8) | |
| $10,000–$19,999 | 1866 (12.8) | 1515 (10.3) | 1356 (9.1) | 1357 (9.0) | 1312 (8.8) | |
| $20,000–$34,999 | 3316 (22.8) | 3234 (21.9) | 3128 (21.1) | 3155 (21.0) | 3045 (20.5) | |
| $35,000–$49,999 | 2606 (17.9) | 2762 (18.7) | 2857 (19.2) | 2900 (19.3) | 2915 (19.6) | |
| $50,000–$74,999 | 2435 (16.7) | 2760 (18.7) | 2970 (20.0) | 2946 (19.6) | 3098 (20.8) | |
| ≥$75,000 | 2354 (16.2) | 2906 (19.7) | 3112 (21.0) | 3166 (21.1) | 3142 (21.1) | |
| US region [n (Ann %)] | <0.001 | |||||
| Northeast | 3381 (23.3) | 3573 (24.2) | 3597 (24.2) | 3574 (23.8) | 3290 (22.1) | |
| South | 4298 (29.6) | 3816 (25.8) | 3700 (24.9) | 3559 (23.7) | 3257 (21.9) | |
| Midwest | 2877 (19.8) | 3269 (22.1) | 3444 (23.2) | 3776 (25.2) | 3376 (22.7) | |
| West | 3984 (27.4) | 4111 (27.8) | 4108 (27.7) | 4091 (27.3) | 4950 (33.3) |
All variables were assessed at the baseline screening visit. Magnesium values include the combined intake from food and supplements (range: 0.38–9274 mg/d; 99th percentile: 958 mg/d). P values were based on significant differences between all quintiles. Ann, annual; HT, hormone therapy; MET, metabolic equivalent task; MI, myocardial infarction; WHI, Women's Health Initiative.
Calcium and vitamin D values include the combined intake from food and supplements.
Magnesium intake in this WHI Observational Study cohort study ranged from 0.38 to 9274 mg/d, with the 99th percentile at 958 mg/d. The average daily magnesium intake for the cohort was 335 mg/d, with 85% of this value obtained from the diet and the remainder from supplements.
Magnesium and BMD
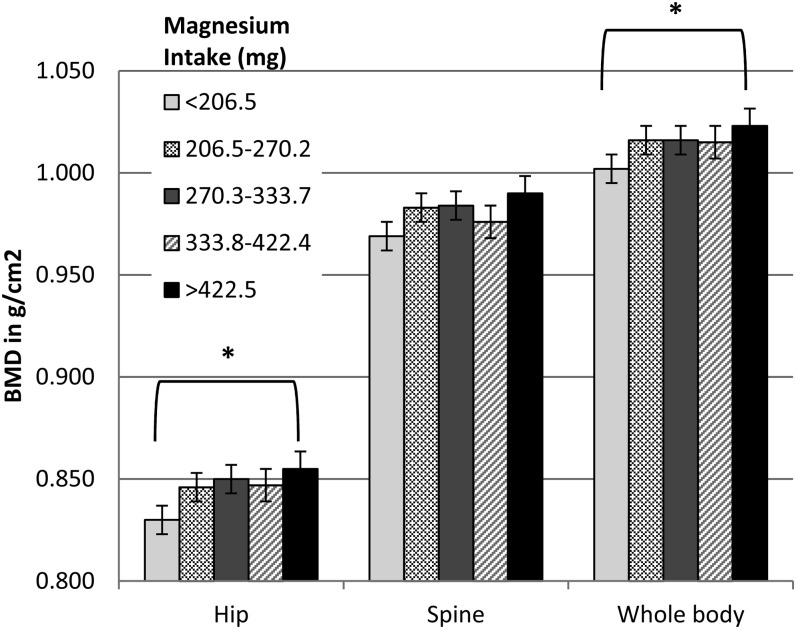
A total of 4778 women had BMD data in our sample. The relation of magnesium intake to baseline BMD after multivariate adjustment is shown in Figure 1. In women who consumed >422.5 compared with <206.5 mg Mg/d, there was a 3% higher total hip BMD (adjusted least-squares mean: 0.830 compared with 0.855 g/cm2, respectively; P < 0.001) and a 2% higher whole-body BMD (adjusted least-squares mean: 1.003 compared with 1.021 g/cm2, respectively; P < 0.001). BMD at 3 or 6 y of follow-up did not differ from baseline when stratified by quintiles of magnesium intake (data not shown).
FIGURE 1.
Least-squares means (±SDs) of BMD at specified sites on the basis of quintiles of magnesium intake. Values were adjusted for covariates (age, race-ethnicity, parental history of fracture, personal fracture at ≥55 y of age, BMI, history of coronary heart disease, treated diabetes, self-reported health, hormone therapy use, alcohol intake, total calcium intake, smoking, and physical activity). *Quintile 5 compared with quintile 1, P < 0.001 (tests for linear trend across quintiles). BMD, bone mineral density.
Magnesium intake, fracture, and falls
During an average of 7.6 y of follow-up, a total of 11,510 participants reported fractures including 844 hip and 2590 forearm and wrist fractures (Table 2). Incidences and RRs of hip and total fractures were not significantly different across quintiles of magnesium intake. In contrast, the RR of lower-arm and wrist fractures increased with increasing magnesium intake with a multivariate adjusted HR of 1.15 (95% CI: 1.01, 1.32) in women who consumed 333.8–422.4 mg Mg/d and an HR of 1.23 (95% CI: 1.07, 1.42) in women in the highest quintile of magnesium intake (≥422.5 mg/d) (P-trend = 0.002). The relation between quintiles of magnesium and fracture risk was not modified by HT use, magnesium supplement or medication use, history of CAD, history of fracture at ≥55 y of age, BMI, or physical activity (data not shown). However, there was a significant interaction between age at screening and lower-arm and wrist fractures. Although both younger (50–64 y of age) and older (≥65 of age) women had increased risk in higher quintiles, the increase in younger women was considerably greater (quintiles 5 compared with 1: HR of 1.41 (95% CI: 1.16, 1.71) in younger women compared with 1.10 (95% CI: 0.91, 1.32) in older women; P-interaction between age and magnesium linear quintiles = 0.03).
TABLE 2.
HRs for fracture and falls on the basis of total magnesium intake1
| Outcome and magnesium intake | Age adjusted |
Fully adjusted |
|||
| n (Ann %) | HR (95% CI) | P | HR (95% CI) | P | |
| Hip fracture | 0.162 | 0.563 | |||
| <206.5 mg | 169 (0.15) | 1.00 | 1.00 | ||
| 206.5–270.2 mg | 198 (0.18) | 1.12 (0.91, 1.37) | 1.11 (0.90, 1.36) | ||
| 270.3–333.7 mg | 156 (0.14) | 0.88 (0.71, 1.09) | 0.90 (0.71, 1.12) | ||
| 333.8–422.4 mg | 157 (0.14) | 0.87 (0.70, 1.09) | 0.90 (0.71, 1.14) | ||
| ≥422.5 mg | 164 (0.15) | 0.96 (0.78, 1.19) | 1.04 (0.81, 1.34) | ||
| Lower-arm and wrist fractures | <0.001 | 0.002 | |||
| <206.5 mg | 436 (0.40) | 1.00 | 1.00 | ||
| 206.5–270.2 mg | 494 (0.45) | 1.10 (0.97, 1.25) | 1.06 (0.93, 1.20) | ||
| 270.3–333.7 mg | 517 (0.46) | 1.14 (1.01, 1.30) | 1.09 (0.95, 1.24) | ||
| 333.8–422.4 mg | 555 (0.49) | 1.22 (1.07, 1.38) | 1.15 (1.01, 1.32) | ||
| ≥422.5 mg | 588 (0.53) | 1.32 (1.17, 1.49) | 1.23 (1.07, 1.42) | ||
| Total fractures | 0.027 | >0.999 | |||
| <206.5 mg | 2145 (4.57) | 1.00 | 1.00 | ||
| 206.5–270.2 mg | 2318 (4.67) | 1.05 (0.99, 1.12) | 1.02 (0.96, 1.08) | ||
| 270.3–333.7 mg | 2329 (4.81) | 1.05 (0.99, 1.11) | 1.01 (0.95, 1.07) | ||
| 333.8–422.4 mg | 2351 (5.14) | 1.05 (0.99, 1.11) | 1.00 (0.94, 1.06) | ||
| ≥422.5 mg | 2367 (5.37) | 1.08 (1.02, 1.14) | 1.01 (0.95, 1.08) | ||
| Falls (≥2 in past year) | <0.001 | <0.001 | |||
| <206.5 mg | 4277 (4.57) | 1.00 | 1.00 | ||
| 206.5–270.2 mg | 4481 (4.69) | 1.02 (0.98, 1.07) | 1.01 (0.97, 1.06) | ||
| 270.3–333.7 mg | 4604 (4.81) | 1.05 (1.01, 1.10) | 1.04 (1.00, 1.09) | ||
| 333.8–422.4 mg | 4903 (5.14) | 1.12 (1.08, 1.17) | 1.11 (1.06, 1.16) | ||
| ≥422.5 mg | 5016 (5.37) | 1.18 (1.14, 1.23) | 1.15 (1.10, 1.20) | ||
Fully adjusted model included the following covariates: age, race-ethnicity, parental history of fracture, fracture at ≥55 y of age, BMI, history of coronary heart disease, treated diabetes, self-reported health, hormone therapy use, alcohol intake, total calcium intake, current and past smoking, and physical activity. HRs (95% CIs) were obtained from Cox proportional hazard models. P values are from tests for linear trend. Magnesium values include the combined intake from food and supplements. Ann, annual.
Although physical activity did not modify the association of magnesium intake to fracture outcomes, women in the highest 2 quintiles of magnesium intake were more active. Because there is a greater potential for falls with increased activity, we included ≥2 falls in the past year as an endpoint in the analysis (Table 2). HRs for falls mirrored the pattern seen for lower-arm and wrist fractures, with a significantly increased fall risk in women who consumed >333.8 mg Mg/d (quintile 4 HR: 1.11 (95% CI: 1.06, 1.16); quintile 5 HR: 1.15 (95% CI: 1.10, 1.20); P-trend < 0.001).
Magnesium and other bone-active nutrients
We examined calcium and vitamin D intakes for an effect modification and showed no interaction with consumption on the association of magnesium to fracture risk. In addition, because foods that are high in magnesium are often high in potassium, we tested for interaction between these 2 minerals but showed no modification of the effect of magnesium on fracture risk at any site (data not shown).
DISCUSSION
This prospective cohort study of 73,684 postmenopausal women examined magnesium intake and how it relates to BMD and fracture incidence. Higher hip and whole-body BMD were noted with a higher consumption of magnesium at baseline. We showed no detrimental association of low magnesium intake to hip fracture or total fracture risk; lower-arm and wrist fractures and falls increased in women who consumed >333.7 mg Mg/d, which was a value only slightly greater than the current RDA.
Significantly higher BMD of the hip and whole body compared with the referent were noted in women with a higher daily magnesium intake at baseline. This result is in agreement with data from several large studies including the Health, Aging and Body Composition study, which showed a positive association of total body BMD with magnesium intake in white men and women (32), and cross-sectional data from the Framingham study, which showed higher BMD at the hip in postmenopausal women who consumed greater than two-thirds of the current RDA for magnesium (9). In addition, recent data from Nielsen et al (33) suggest that magnesium intake >237 mg/d is associated with higher total body BMD in postmenopausal women. As with the Framingham study, we showed no longitudinal association between magnesium intake and BMD in WHI women.
Lower magnesium intake was not associated with increased fracture risk at any site in our cohort. This finding was surprising in light of the evidence that magnesium stabilizes amorphous calcium phosphate and slows its transformation to hydroxyapatite (34), and with a greater magnesium content in bone, the hydroxyapatite crystal content decreases and forms smaller but sturdier crystals (35–38), which make bones stronger. The opposite situation of low magnesium and high hydroxyapatite has been more commonly shown in the trabecular bone of osteoporotic women (37).
Rates of lower-arm and wrist fractures in the WHI Observational Study cohort were 15% and 23% higher in women with the greatest magnesium intake (333.8–422.4 and >422.5 mg/d, respectively) than in women with the lowest magnesium intake. This result was most likely attributable to the greater physical activity and risk of falls in this group; however, it possible that regional differences in cortical compared with trabecular bone could also have been a contributing factor. The lower arm and wrist have a greater ratio of cortical to trabecular bone compared with other clinically pertinent sites, including the hip. Previous studies have shown that the magnesium content was normal or increased in cortical bone samples of postmenopausal women with osteoporosis (39–41); thus, increased intake could further increase the magnesium content in the already replete cortical bone–containing regions, which might possibly contribute to the regional susceptibility to a fracture. In addition, cortical bone may be more sensitive than trabecular bone to the diet. This hypothesis is supported by observational research that suggested less loss of cortical bone, but not trabecular bone, from the tibia in elderly women who consumed a diet higher in several macronutrients and micronutrients, including magnesium (42).
The investigation into the relation of falls to magnesium consumption in WHI participants revealed a 15% increase in risk of ≥2 falls in the past year with magnesium intake >422.5 mg/d. This result may have been related to the increased physical activity in women in the highest quintile; however, it is biologically plausible that excess magnesium intake from supplements may increase fall risk related to hypotension, muscle weakness, and changes in deep-tendon reflexes associated with hypermagnesemia (43–45). Although possible, this seems an unlikely explanation because hypermagnesemia, which usually occurs in the presence of renal insufficiency plus excessive magnesium intake from dietary supplements or medications (46), would not be expected in such a large number of women (n = 5016 in quintile 5) in our sample. A more likely explanation is that falls might have been a surrogate marker of women who were more active and, therefore, had a higher potential to fall and fracture a wrist. Indeed, the highest quintile of magnesium intake also had the greatest percentage of women (52%) who were extremely physically active (>12 METs/wk) and the greatest percentage of women (20%) who walked ≥150 min/wk outside the home. Despite these findings, we showed no significant interaction between physical activity and magnesium intake by quintile for any of the 3 fracture variables. More physical activity has been associated with greater risk of wrist fractures that result from falls in other large cohorts of postmenopausal women (47, 48).
In a subgroup analysis, a significant interaction between the highest magnesium intake, lower-arm and wrist fracture, and age at screening was noted such that women <65 y old were more likely to have a wrist fracture if their magnesium intake was >422.5 mg/d. In contrast, results of the National Osteoporosis Risk Assessment trial showed that postmenopausal women <65 y old were less likely to sustain a wrist fracture than were women ≥65 y old (3.9% compared with 5.8%, respectively) (49).
Because food sources of magnesium are frequently high in other nutrients that are beneficial to bone such as potassium and calcium, it is difficult to separate effects of individual nutrients. In the Framingham Heart Study, greater potassium plus magnesium consumption significantly correlated with greater BMD of the radius (9). In this study, potassium, calcium, and vitamin D consumption did not modify the association of magnesium to fracture risk at any site. Women in the highest quintile of magnesium intake also consumed the highest amounts of calcium and vitamin D, which are nutrients known to benefit bone; this result lends support to the idea that increased falls and wrist fractures in this group may have been related to factors other than nutrient intake, such as lower body weight combined with increased physical activity.
One of the primary strengths of this study was the large sample of postmenopausal women in the WHI Observational Study cohort, which made it feasible to examine a biomarker of fracture risk, such as BMD, and actual fracture outcomes. In addition, hip fractures were confirmed by a medical record review, and the validity of self-reported fractures was high. The availability of data on important covariates associated with osteoporosis was also an advantage of this analysis.
This research was limited because of the observational nature of the study, and results should only be generalized to healthy, postmenopausal women. The measurement error associated with a self-report was also a limitation, although the reliability of several self-reported variables, including physical activity, has been shown to be high in the WHI (50). Food intake was obtained from one administration of a baseline FFQ. The FFQ has been shown to correlate highly with food recalls and intake records in this study population, but no biological markers of nutrient intake, including magnesium, were available to confirm self-reported food intake. Dietary magnesium is also highly correlated with other nutrients including potassium and calcium, which may act independently to modify fracture risk. We attempted to address this relation by including calcium intake in our models and testing for an interaction between magnesium, calcium, vitamin D, potassium, and fracture. In addition, this study could not entirely account for previous magnesium intake or stores. Additional research is required to better define adequate compared with excess magnesium intake and its impact on osteoporosis and fracture.
In conclusion, in this large prospective cohort study of postmenopausal women, lower baseline BMD of the total hip and whole body were noted in participants with lower daily magnesium intake. However, lower magnesium intake was not associated with greater risk of hip fracture or total fractures. Magnesium consumption slightly greater than the RDA was correlated with increased falls and increased lower-arm and wrist fractures, which were possibly related to more physical activity in this group of women.
Acknowledgments
We acknowledge the following WHI investigators—program office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD); clinical coordinating center: Ross Prentice, Garnet Anderson, Andrea LaCroix, and Charles L Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA), Evan Stein (Medical Research Laboratories, Highland Heights, KY), and Steven Cummings (University of California at San Francisco, San Francisco, CA); clinical centers: Sylvia Wassertheil-Smoller (Albert Einstein College of Medicine, Bronx, NY), Haleh Sangi-Haghpeykar (Baylor College of Medicine, Houston, TX), JoAnn E Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), Charles B Eaton (Brown University, Providence, RI), Lawrence S Phillips (Emory University, Atlanta, GA), Shirley Beresford (Fred Hutchinson Cancer Research Center, Seattle, WA), Lisa Martin (George Washington University Medical Center, Washington, DC), Rowan Chlebowski (Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles Medical Center, Torrance, CA), Erin LeBlanc (Kaiser Permanente Center for Health Research, Portland, OR), Bette Caan (Kaiser Permanente Division of Research, Oakland, CA), Jane Morley Kotchen (Medical College of Wisconsin, Milwaukee, WI), Barbara V Howard (MedStar Research Institute/Howard University, Washington, DC), Linda Van Horn (Northwestern University, Chicago/Evanston, IL), Henry Black (Rush Medical Center, Chicago, IL), Marcia L Stefanick (Stanford Prevention Research Center, Stanford, CA), Dorothy Lane (State University of New York at Stony Brook, Stony Brook, NY), Rebecca Jackson (The Ohio State University, Columbus, OH), Cora E Lewis (University of Alabama at Birmingham, Birmingham, AL), Cynthia A Thomson (University of Arizona, Tucson/Phoenix, AZ), Jean Wactawski-Wende (University at Buffalo, Buffalo, NY), John Robbins (University of California at Davis, Sacramento, CA), F Allan Hubbell (University of California at Irvine, CA), Lauren Nathan (University of California at Los Angeles, Los Angeles, CA), Robert D Langer (University of California at San Diego, La Jolla/Chula Vista, CA), Margery Gass (University of Cincinnati, Cincinnati, OH), Marian Limacher (University of Florida, Gainesville/Jacksonville, FL), J David Curb (University of Hawaii, Honolulu, HI), Robert Wallace (University of Iowa, Iowa City/Davenport, IA), Judith Ockene (University of Massachusetts/Fallon Clinic, Worcester, MA), Norman Lasser (University of Medicine and Dentistry of New Jersey, Newark, NJ), Mary Jo O'Sullivan(University of Miami, Miami, FL), Karen Margolis (University of Minnesota, Minneapolis, MN), Robert Brunner (University of Nevada, Reno, NV), Gerardo Heiss (University of North Carolina, Chapel Hill, NC), Lewis Kuller (University of Pittsburgh, Pittsburgh, PA), Karen C Johnson (University of Tennessee Health Science Center, Memphis, TN), Robert Brzyski (University of Texas Health Science Center, San Antonio, TX), Gloria E Sarto (University of Wisconsin, Madison, WI), Mara Vitolins (Wake Forest University School of Medicine, Winston-Salem, NC), and Michael S Simon (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI); and Women's Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
The authors’ responsibilities were as follows—RDJ, AZL, ZC, JW-W, and JAC: designed the research; JCL: performed statistical analyses; TSO, NA, and SB-T: wrote the manuscript; RDJ and TSO: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. RDJ is a coinvestigator for studies on stroke and electronic health records sponsored by Pfizer and a consultant for educational materials for a clinical research module sponsored by Merck. AZL, ZC, JW-W, JAC, JCL, TSO, NA, and SB-T reported no conflicts of interest.
Footnotes
Abbreviations used: BMD, bone mineral density; CAD, coronary artery disease; FFQ, food-frequency questionnaire; HT, hormone therapy; MET, metabolic equivalent task; RDA, Recommended Dietary Allowance; WHI, Women's Health Initiative.
REFERENCES
- 1.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev 2012;70:153–64. [DOI] [PubMed] [Google Scholar]
- 2.Laires MJ, Monteiro CP, Bicho M. Role of cellular magnesium in health and human disease. Front Biosci 2004;9:262–76. [DOI] [PubMed] [Google Scholar]
- 3.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Kilburn J. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone 2005;37:211–9. [DOI] [PubMed] [Google Scholar]
- 4.Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr 2009;28:131–41. [DOI] [PubMed] [Google Scholar]
- 5.Welsh JJ, Weaver VM. Adaptation to low dietary calcium in magnesium-deficient rats. J Nutr 1988;118:729–34. [DOI] [PubMed] [Google Scholar]
- 6.Rude RK, Gruber HE, Norton HJ, Wei LY, Frausto A, Mills BG. Bone loss induced by dietary magnesium reduction to 10% of the nutrient requirement in rats is associated with increased release of substance P and tumor necrosis factor-alpha. J Nutr 2004;134:79–85. [DOI] [PubMed] [Google Scholar]
- 7.Griffin TP, Murphy M, Coulter J, Murphy MS. Symptomatic hypocalcaemia secondary to PTH resistance associated with hypomagnesaemia after elective embolisation of uterine fibroid. BMJ Case Rep . 2013;2013:pii: bcr2013008708. [DOI] [PMC free article] [PubMed]
- 8.Freitag JJ, Martin KJ, Conrades MB, Bellorin-Font E, Teitelbaum S, Klahr S, Slatopolsky E. Evidence for skeletal resistance to parathyroid hormone in magnesium deficiency. Studies in isolated perfused bone. J Clin Invest 1979;64:1238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PWF, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater mineral density in elderly men and women. Am J Clin Nutr 1999;69:727–36. [DOI] [PubMed] [Google Scholar]
- 10.New SA, Robins SP, Campbell MK, Martin JC, Garton MJ, Bolton-Smith C, Grubb DA, Lee SJ, Reid DM. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health? Am J Clin Nutr 2000;71:142–51. [DOI] [PubMed] [Google Scholar]
- 11.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr 1985;42:877–88. [DOI] [PubMed] [Google Scholar]
- 12.Aydin H, Deyneli O, Yavuz D, Gozu H, Mutlu N, Kaygusuz I, Akalin S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol Trace Elem Res 2010;133:136–43. [DOI] [PubMed] [Google Scholar]
- 13.Abraham GE, Grewal H. A total dietary program emphasizing magnesium instead of calcium. Effect on the mineral density of calcaneous bone in postmenopausal women on hormonal therapy. J Reprod Med 1990;35:503–7. [PubMed] [Google Scholar]
- 14.Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res 1993;6:155–63. [PubMed] [Google Scholar]
- 15.Freudenheim JL, Johnson NE, Smith EL. Relationships between usual nutrient intake and bone-mineral content of women 35-65 years of age: longitudinal and cross-sectional analysis. Am J Clin Nutr 1986;44:863–76. [DOI] [PubMed] [Google Scholar]
- 16.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes FaNB, Institute of Medicine. Dietary Reference Intakes for calcium. phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: The National Academies Press, 1997. [PubMed] [Google Scholar]
- 17.Takeda R, Nakamura T. Effects of high magnesium intake on bone mineral status and lipid metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 2008;54:66–75. [DOI] [PubMed] [Google Scholar]
- 18.Riond JL, Hartmann P, Steiner P, Ursprung R, Wanner M, Forrer R, Spichiger UE, Thomsen JS, Mosekilde L. Long-term excessive magnesium supplementation is deleterious whereas suboptimal supply is beneficial for bones in rats. Magnes Res 2000;13:249–64. [PubMed] [Google Scholar]
- 19.Wedig KE, Kogan J, Schorry EK, Whitsett JA. Skeletal demineralization and fractures caused by fetal magnesium toxicity. J Perinatol 2006;26:371–4. [DOI] [PubMed] [Google Scholar]
- 20.Tsukahara H, Kobata R, Tamura S, Mayumi M. Neonatal bone abnormalities attributable to maternal administration of magnesium sulphate. Pediatr Radiol 2004;34:673–4. [DOI] [PubMed] [Google Scholar]
- 21.Malaeb SN, Rassi AI, Haddad MC, Seoud MA, Yunis KA. Bone mineralization in newborns whose mothers received magnesium sulphate for tocolysis of premature labour. Pediatr Radiol 2004;34:384–6. [DOI] [PubMed] [Google Scholar]
- 22.Cruikshank DP, Chan GM, Doerrfeld D. Alterations in vitamin D and calcium metabolism with magnesium sulfate treatment of preeclampsia. Am J Obstet Gynecol 1993;168:1170–6, discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 23.The Women's Health Initiative Study Group. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 24.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S98–106. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SAA, Black HR, Blanchette P, et al. Calcium plus vitamin d supplementation and the risk of fractures. N Engl J Med 2006;354:669–83. [DOI] [PubMed] [Google Scholar]
- 26.Caire-Juvera G, Ritenbaugh C, Wactawski-Wende J, Snetselaar LG, Chen Z. Vitamin A and retinol intakes and the risk of fractures among participants of the Women's Health Initiative Observational Study. Am J Clin Nutr 2009;89:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Kooperberg C, Pettinger MB, Bassford T, Cauley JA, LaCroix AZ, Lewis CE, Kipersztok S, Borne C, Jackson RD. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women's Health Initiative observational study and clinical trials. Menopause 2004;11:264–74. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 29.Neuhouser ML, Wassertheil-Smoller S, Thomson C, Aragaki A, Anderson GL, Manson JE, Patterson RE, Rohan TE, van Horn L, Shikany JM, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women's Health Initiative cohorts. Arch Intern Med 2009;169:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc 1988;88:1268–71. [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 32.Ryder KM, Shorr RI, Bush AJ, Kritchevsky SB, Harris T, Stone K, Cauley J, Tylavsky FA. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects.. J Am Geriatr Soc 2005;53:1875–80. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen FH, Lukaski HC, Johnson LK, Roughead ZK. Reported zinc, but not copper, intakes influence whole-body bone density, mineral content and T score responses to zinc and copper supplementation in healthy postmenopausal women. Br J Nutr 2011;106:1872–9. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal NC, Betts F, Posner AS. Stabilization of amorphous calcium phosphate by Mg and ATP. Calcif Tissue Res 1977;23:245–50. [DOI] [PubMed] [Google Scholar]
- 35.Bigi A, Foresti E, Gregorini R, Ripamonti A, Roveri N, Shah JS. The role of magnesium on the structure of biological apatites. Calcif Tissue Int 1992;50:439–44. [DOI] [PubMed] [Google Scholar]
- 36.Bachra BN, Trautz OR, Simon SL. Precipitation of calcium carbonates and phosphates. 3. The effect of magnesium and fluoride ions on the spontaneous precipitation of calcium carbonates and phosphates. Arch Oral Biol 1965;10:731–8. [DOI] [PubMed] [Google Scholar]
- 37.Manicourt DH, Orloff S, Brauman J, Schoutens A. Bone mineral content of the radius: good correlations with physicochemical determinations in iliac crest trabecular bone of normal and osteoporotic subjects. Metabolism 1981;30:57–62. [DOI] [PubMed] [Google Scholar]
- 38.Cohen L, Kitzes R. Infrared spectroscopy and magnesium content of bone mineral in osteoporotic women. Isr J Med Sci 1981;17:1123–5. [PubMed] [Google Scholar]
- 39.Hogervorst EJ, Lips P, de Blieck-Hogervorst JM, van der Vijgh WJ, Netelenbos JC. Bone mineral content of transilial biopsies in patients with hip fracture. Bone 1985;6:297–9. [DOI] [PubMed] [Google Scholar]
- 40.Reginster JY, Strause L, Deroisy R, Lecart MP, Saltman P, Franchimont P. Preliminary report of decreased serum magnesium in postmenopausal osteoporosis. Magnesium 1989;8:106–9. [PubMed] [Google Scholar]
- 41.Burnell JM, Baylink DJ, Chestnut CH, 3rd, Mathews MW, Teubner EJ. Bone matrix and mineral abnormalities in postmenopausal osteoporosis. Metabolism 1982;31:1113–20. [DOI] [PubMed] [Google Scholar]
- 42.Pedone C, Napoli N, Pozzilli P, Rossi FF, Lauretani F, Bandinelli S, Ferrucci L, Antonelli-Incalzi R. Dietary pattern and bone density changes in elderly women: a longitudinal study. J Am Coll Nutr 2011;30:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whang R. Clinical disorders of magnesium metabolism. Compr Ther 1997;23:168–73. [PubMed] [Google Scholar]
- 44.Nick JM. Deep tendon reflexes, magnesium, and calcium: assessments and implications. J Obstet Gynecol Neonatal Nurs 2004;33:221–30. [DOI] [PubMed] [Google Scholar]
- 45.Mordes JP, Wacker WE. Excess magnesium. Pharmacol Rev 1977;29:273–300. [PubMed] [Google Scholar]
- 46.Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol 2009;41:357–62. [DOI] [PubMed] [Google Scholar]
- 47.Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR. Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol 1992;135:477–89. [DOI] [PubMed] [Google Scholar]
- 48.Rikkonen T, Salovaara K, Sirola J, Kärkkäinen M, Tuppurainen M, Jurvelin J, Honkanen R, Alhava E, Kröger H. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res 2010;25:2332–40. [DOI] [PubMed]
- 49.Barrett-Connor E, Sajjan S, Siris E, Miller P, Chen YT, Markson L. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2008;19:607–13. [DOI] [PubMed] [Google Scholar]
- 50.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13(suppl):S107–21. [DOI] [PubMed] [Google Scholar]