Abstract
Cortisol is a biomarker of stress reactivity and its diurnal pattern is an indicator of general neuroendocrine health. Despite theories conceptualizing marital dyads as dynamic systems wherein spouses are interdependent in their physiology and stress coping, little is known about the daily processes in which spouses possibly influence each other in biological stress. Nineteen heterosexual couples provided saliva samples containing cortisol 4 times a day for 4 consecutive days. We used multilevel modeling to examine whether one’s cortisol awaking response (CAR) and diurnal cortisol slope (DCS) predict those of the spouse’s on the same day and/or on the next day. We found that spouses synchronize their DCS, such that on days when one experiences faster or slower decline in diurnal cortisol than usual, the spouse also experiences faster or slower decline than usual. For CAR, positive synchrony was only observed in couples reporting high levels of marital strain and disagreement. Cross-lagged regression analysis reveals stability in diurnal cortisol pattern. A steeper cortisol slope on a particular day predicts a steeper slope on the next day within an individual, but no significant cross-lagged relation was found between spouses. Couples reporting more spousal support tend to have stronger stability in CAR. These findings provide evidence that spouses are interdependent in their diurnal cortisol patterns on a day-to-day basis, and that these daily dynamics are associated with marital relationship quality. The study contributes to our understanding of marital processes and biobehavioral health. It also contributes methodologically to the advancement of longitudinal dyadic analysis.
Keywords: cortisol, couples, marriage, stress, dyadic analysis
Being in close relationships is a central experience for human beings. For many adults, living with a romantic partner or spouse provides essential social interactions and defines the basic context of everyday life. Despite many studies indicating the effects of a romantic partner or spouse on individual well-being (Koball, Moiduddin, Henderson, Goesling, & Besculides, 2010; Uchino, 2004), the underlying processes of how partners possibly influence each other remain largely unknown. This study examines the daily dynamics of the stress hormone, cortisol, in married, heterosexual couples1, and investigates how these daily dynamics are associated with characteristics of the marital relationships. The results provide implications for understanding the marital dyad system and the well-being of individuals in close relationships.
Physiological Synchrony and Adult Attachment
Physiological processes play an important role in the formation of attachment bonds. In humans, attachment is thought to emerge when a caregiver consistently soothes an infant in stressful situations (Bowlby, 1958, 1982). Over time, soothing from a caregiver facilitates the infant’s regulation of emotional and physiological arousal. Research has shown that skin-to-skin contact accelerates the autonomic functioning and neurobehavioral maturation in preterm babies (Feldman & Eidelman, 2003). In addition, synchrony in physiology and behaviors appears to be a crucial feature of attachment relationships (Guedeney et al. 2011). Contingency between maternal behavior and infant alertness is observed in the first hours after birth. Parent-child synchrony in infancy is found to be associated with a variety of developmental outcomes, such as children’s emotional self-regulatory ability, IQ scores, and attachment behavior (Feldman, 2007).
Physiological synchrony also is a defining feature of adult attachment in close relationships (Hazan, Gur-Yaish, & Campa, 2004). Researchers argue that partners in close relationships up- or down regulate each other’s psychophysiological arousal, a process known as coregulation (Sbarra & Hazan, 2008). This physiological process is believed to underlie biobehavioral disturbance such as sleep disruption that often occurs when one experiences separation from or loss of a long-term romantic partner (Hofer, 1984; Sbarra & Hazan, 2008). Despite speculation about physiological coregulation in couples, empirical research addressing this issue is limited. Interrelatedness in physiology (e.g., heart rate, skin conductance) was found between spouses during lab-managed conflictual interactions (Levenson & Gottman, 1983). Helm, Sbarra, and Ferrer (2011) also found that couples were able to synchronize their respiration and heart rate during a series of lab tasks designed to elicit coordinated physiological responses. In more natural settings, covariation and transmission of positive and negative affect have often been observed within marital relationships (e.g., Butler, 2011; Goodman & Shippy, 2002; Larson & Almeida, 1999).
Using Cortisol to Study Physiological Synchrony between Spouses
Diamond (2001) identified two biological systems that are particularly important for adult attachment relationships - the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenocortical (HPA) axis. The HPA regulates biobehavioral responses to stress. Its end product, cortisol, has been established as a biomarker of stress responding and an indicator of general neuroendocrine health (Hellhammer et al., 2007; Miller, Chen, & Zhou, 2007). Cortisol increases in response to stressful stimuli and mobilizes energy stores to help meet the physiological demands of responding to the stressor (Sapolsky, Romero, & Munck, 2000). This physiological mechanism is adaptive in the face of acute stress. Chronic exposure to stress and frequent activation of the HPA axis, however, are associated with negative health outcomes such as depression (Belmaker & Agam, 2008; Bhagwagar, Hafizi, & Cowen, 2005) and cognitive decline (Seeman, McEwen, Singer, Albert, & Rowe, 1997). Cortisol levels also change in response to social stimuli. Animal research has found abundant evidence that presence of an attachment figure reduces cortisol secretion (e.g., Mendoza, Lyons, & Saltzman, 1991). In humans, social support from romantic partners has been shown to reduce cortisol reactivity induced by a public speaking task for men (Kirschbaum, Klauer, Filipp, & Hellhammer, 1995). Taken together, this association between cortisol and attachment relationships suggests that cortisol is a promising biomarker for examining physiological synchrony between spouses.
Cortisol has an established diurnal pattern. It typically peaks in the morning soon after waking, and gradually declines through the rest of the day. To our knowledge, only three studies have examined the association of salivary cortisol between spouses, and they all focused on the covariation in cortisol levels at particular time points. Schreiber and colleagues (2006) found a positive correlation between spouses in afternoon basal cortisol levels. Saxbe and Repetti (2010) obtained salivary cortisol samples four times per day from married couples and found a positive association in cortisol levels between husband and wife when controlling for sampling time. A recent study by Papp, Pendry, Simon, and Adam (2012) again found a positive association in cortisol levels assayed from saliva samples taken from married couples seven times per day for two days. These studies importantly suggest that cortisol levels between spouses are interdependent. However, all three studies focused on the synchrony of cortisol levels at particular time points, which is a static measure. It is possible that, besides synchrony in “state”, spouses also covary in their movements in cortisol over time, or synchrony in “change”, which better represents regulation of the HPA axis. Moreover, all three studies only considered concurrent relations between spouses’ cortisol and did not examine lagged relations. An individual’s neuroendocrine stress physiology may not only covary with that of his/her spouse neuroendocrine stress physiology at the same moment, but also depend on the spouse’s neuroendocrine stress physiology at previous time points. These are the research questions addressed in the current study.
Modeling Diurnal Cortisol
Several measures have been developed to quantify different aspects of the diurnal cortisol pattern, including cortisol awakening response (CAR) and diurnal cortisol slope (DCS). CAR refers to the surge in cortisol 30–45 minutes after waking, whereas DCS measures the decline in cortisol from early morning to late evening. In some studies, DCS is modeled from peak cortisol levels (i.e., 30–45 minutes after waking; e.g., Kudielka, Broderick, & Kirschbaum, 2003; Stawski et al., 2011), whereas other studies model DCS from waking cortisol levels (e.g., Adam, Hawkley, Kudielka, & Cacioppo, 2006). Researchers in favor of DCS from waking argue that decline from the peak may depend on the magnitude of CAR. In other words, individuals experiencing a more pronounced cortisol increase soon after waking are likely to experience a more dramatic cortisol decrease from the peak to the evening. Other researchers, however, speculate that cortisol decline from the peak may more accurately capture regulatory functioning of the HPA axis, because it reflects the body’s ability to “recover” from elevated cortisol levels. The present study assesses the daily processes between spouses in CAR and DCS from the peak cortisol values controlling for CAR on the same day. These cortisol measures are considered to be indices of general neuroendocrine health, with blunted CARs and flattened DCSs representing less healthy “profiles” (Adam & Kumari, 2009; Piazza et al., 2010).
Physiological synchrony between spouses also is likely to be moderated by quality of the marital relationships. Levenson and Gottman (1983) found that married couples with lower marital satisfaction tend to show greater physiological concordance (e.g., heart rate, skin conductance) when discussing problem areas in the marriage. They argued that
physiological linkage reflects the ebb and flow of negative affect, the escalation and de-escalation of conflict, and the sense of being “locked into” the interaction and unable to “step back” that can occur when spouses in dissatisfied marriages attempt to solve problems (Levenson & Gottman, 1983, p. 596).
Similarly, Saxbe and Repetti (2010) found that lower marital satisfaction is associated with stronger coregulation in cortisol levels between spouses. In this study, measures of several marital relationship aspects are considered as moderators of synchrony. These measures include perceived support and strain from the spouse and disagreement between spouses.
Research Hypothesis
This study investigates two possible ways that spouses relate to each other in patterns of diurnal salivary cortisol - concurrent relations and lagged relations. Two sets of hypotheses are tested. First, we hypothesize that husbands and wives synchronize their diurnal cortisol patterns over days. Synchrony is operationalized as a within-couple association; that is, on days when an individual experiences a steeper CAR or DCS than his/her average level, the spouse also experiences a steeper CAR or DCS than his/her average level, and vice versa. The moderating effects of three measures of marital relationships (spousal support, strain, and disagreement) on the strength of synchrony are also examined. Poor marital quality (low levels of spousal support, high levels of spousal strain and disagreement) is expected to be associated with stronger synchrony. Second, we hypothesize that lagged relations exist between spouses, such that an individual’s diurnal cortisol pattern (CAR and DCS) on a particular day is predicted by the spouse’s cortisol pattern on the previous day. Again, moderating effects of the three different aspects of marital relationships on the lagged coefficients are investigated.
Method
Participants
Data were drawn from the Penn State Hotel Work and Well-Being Study (Almeida, Davis, O’Neill, & Crouter, 2012), a project investigating connections between work stress, health, and family relations of employees from the hotel industry. Hotel managers completed a baseline interview and provided information about their work and family life. Respondents who were married or in a cohabiting relationship were invited to participate in a daily diary study together with their spouses/partners. The daily diary study aimed to obtain in-depth information about daily work experiences and personal well-being. Specifically, after a baseline interview, hotel managers and their spouses/partners were telephoned on eight consecutive days and interviewed separately about their daily experiences, including time use, physical symptoms, mood, and stressful events. On Day 2 to Day 5, a subset of couples participated in a biomarker study, in which they provided saliva samples for later assessment of cortisol.
Twenty-eight heterosexual couples participated in the biomarker study. Nine couples were excluded because the women were taking medicines known to affect cortisol secretion (e.g., estrogen, Depo-Provera), were pregnant/breastfeeding, or had been pregnant/breastfeeding in the past year. Among the remaining 19 couples, 18 were married and one was cohabiting. Men had an average age of 41.37 years, with a standard deviation (SD) of 9.06, and 52.6% had a college degree or more. Women had an average age of 39.05 years (SD = 9.38), and 63.2% had a college degree or more. Seventy-six percent of the participants were Caucasian, 13% were Hispanic, 5% were Black, and 5% were Asian. The couples had been married or living together for an average of 11.98 years (SD = 11.71) and had an average household income of $90,579 (SD = 32,184). Fifteen couples (79.0%) were raising children together and the average number of children at home for these couples was 1.9.
Procedure
Cortisol was assayed from four saliva samples (at wake up, 30 minutes after wake up, early afternoon, and before bed) collected each day on Days 2, 3, 4, and 5 of the daily diary study, using a saliva kit that was mailed to each spouse. The kits included 16 numbered and color-coded saliva collection tubes (four per day for four days; Salivettes, Sarstedt, Newton, NC); each tube contained a wad of cotton along with an instruction sheet specifying that sample collection should occur at least one hour after consumption of a major meal, that participants should restrict milk and dairy intake for 20 minutes prior to sample collection, and that they should forgo saliva collection if they had a temperature over 102F. A brief questionnaire also was included for participants to record the saliva collection time, medications taken within the past 48 hours, and for women, menstrual cycle information. These collection procedures have been successfully applied in dozens of studies, and do not interfere with the cortisol assay (Granger & Kivlighan, 2003). Instructions for saliva collection and questionnaire completion also were reviewed during the first phone interview, and participants were reminded about the saliva collection on the evenings prior to scheduled collections.
On each collection occasion, participants were instructed to roll the cotton swab in their mouth for two minutes, place the swab back into its tube and cap it, and refrigerate the sample. When all 16 tubes were ready, couples used a pre-addressed, paid courier package for the return mailing to our laboratory where they were frozen at minus 80 degrees until assayed. On assay days, samples were brought to room temperature and centrifuged for five minutes at 1500 x g to separate mucin from clear saliva. Salivary cortisol levels were evaluated in duplicate with sensitivity at 0.08 ng/mL by commercially available enzyme linked immunosorbent assay (EIA) kits (DSL, Webster, TX) in General Clinical Research Center core laboratory. Samples from each participant were kept together on a single assay plate.
In total, the 19 couples contributed 152 person-days2 of saliva samples, among which four person-days were excluded because the participants woke up after 12 pm on that day. In addition, samples with cortisol > 60 nmol/L were considered invalid. Hence, among the 570 remaining cortisol values, four were top coded to 60 nmol/L. Descriptive statistics of cortisol values and the collection time of saliva samples are shown in Table 1.
Table 1.
Descriptive Statistics of Cortisol and Time of Collection
| Occasion | N | Mean (nmol/L) | SD | Mean Collection Time |
|---|---|---|---|---|
| 1 (upon wake) | 144 | 27.21 | 11.24 | 6:43 a.m. |
| 2 (30 min after wake) | 147 | 32.00 | 11.50 | 7:20 a.m. |
| 3 (afternoon) | 138 | 14.57 | 7.54 | 12:58 p.m. |
| 4 (before bed) | 141 | 6.49 | 5.57 | 10:28 p.m. |
| Total | 570 | - | - | - |
Measures
Cortisol slopes
CAR was computed by subtracting sample 2 from sample 1 on the same day and dividing the difference by the time lag between the two samples (Almeida, Piazza, & Stawski, 2009). Because CAR is known to be sensitive to participant compliance (Kudielka, Broderick, & Kirschbaum, 2003), it was excluded if the time lag between the first and second samples was smaller than 15 minutes or larger than 60 minutes. These excluded values made up 2.1% of all CAR values. DCS was computed by subtracting sample 4 from sample 2 and dividing the difference by the time lag. Values were excluded if there was an increase between sample 2 and 3 that was larger than 10 nmol/L, as this indicates that cortisol assessed in sample 2 did not reflect peak value of the day. As a result of this criterion, 0.7% of all DCS values were excluded. The means of valid CAR and DCS, broken down by gender, are presented in Table 2.
Table 2.
Descriptive Statistics of Cortisol Awakening Response (CAR) and Diurnal Cortisol Slope (DCS), Broken Down by Gender
| Male | Female | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | N | Mean | SD | N | Mean | SD |
| CAR | 74 | 5.22 | 24.90 | 66 | 13.58 | 23.09 |
| DCS | 71 | −1.70 | 0.75 | 62 | −1.78 | 0.78 |
Note. Values represent changes in cortisol (nmol/L) per hour.
Spousal support, strain, and disagreement
Hotel managers and their spouses/partners answered questions separately about their perception of spousal support, strain, and disagreement in the baseline interview prior to the daily diary study. The three scales used were adapted from the Midlife in the United States (MIDUS) study (Schuster, Kessler, & Aseltine, 1990; Whalen & Lachman, 2000). These measures have been used in a number of studies on marital relationships and are valid predictors of a variety of psychological well-being and health outcomes (Grzywacz & Marks, 1999; Whalen & Lachman, 2000). The spousal support scale had seven items, such as “How much does your spouse or partner really care about you?” The spousal strain scale had six items, such as “How much do you feel your spouse or partner makes too many demands on you?” The spousal disagreement scale had five items, including “How much do you and your spouse or partner disagree on money matters such as how much to spend, save, or invest?” They were answered on a 4-point scale from “not at all” to “a lot”. Item scores in each scale were summed for each individual. Cronbach’s alphas were computed based on the original sample of the study, which included 302 men and 274 women. The spousal support scale had an alpha of .80 for both men and women. The spousal strain scale had an alpha of .83 for men and .80 for women. The spousal disagreement scale had an alpha of .72 for men and .65 for women. In this study, husband’s and wife’s scores on the same scale were averaged within each family to indicate the overall level of spousal support, strain, and disagreement for that family3.
Analysis
Because the data were nested at multiple levels (across days and within dyads), multilevel modeling (MLM) was the appropriate statistical framework. To test the first hypothesis, cortisol slopes (i.e., CAR or DCS) for each person on a particular day was modeled as an intercept (a person-mean over the four days) plus deviation from the intercept. The deviation was then predicted by the spouse’s deviation from his/her person-mean. The coefficient thus represents strength of synchrony in diurnal cortisol patterns. The basic model is:
| (1) |
where Yti represents the cortisol slope (CAR or DCS) of couple i on day t, HUSBAND and WIFE are dummy coded indicators for husband and wife, and SPOUSEti is the spouse’s person-centered cortisol slope of couple i on day t. Hypothetical data for one couple are provided in the Appendix to illustrate the data structure. The use of two dummy codes at level 1 to identify the individuals allows the estimation of separate intercepts for husband and wife, a technique commonly adopted in dyadic analysis (Kenny, Kashy, & Cook, 2006; Laurenceau & Bolger, 2005; Raudenbush, Brennan, & Barnett, 1995). Therefore, β0i and β1i represent the average cortisol slope for the husband and wife in couple i. Because SPOUSEti is the difference between the spouse’s cortisol slope on day t and his/her person-mean, and it is used to predict the residual of an individual’s cortisol slope on day t after partialling out the intercept, β2i represents strength of synchrony between the husband and wife in couple i. At level 2, the level 1 coefficients are modeled as a sample mean (π) plus a random component (υ) representing the difference between the couple and the whole sample. The random components for the intercepts are allowed to be correlated, and the correlation represents the between-couple association between husbands and wives. The variance for the synchrony coefficient can also be estimated. For CAR, wake time was controlled for because it was likely to affect CAR (William, Magid, & Steptoe, 2005). CAR was controlled for in the model of DCS. These were done by adding person-means of the control variables to level 2 and within-person deviations to level 1. For example, person-means of wake time were constructed by taking the average of wake time for each individual over the four days, and were used to predict the intercept of the individual (level 2). Within-person deviations of wake time were used to predict daily variations in CAR (level 1). The analysis was conducted in SAS and syntax for the basic model (with no control variable) is provided in the Appendix.
After the basic model, measures of marital relationships were added into level 2 to test their moderating effects on strength of synchrony. For example, the equation where spousal support moderated the synchrony coefficient can be written as:
| (2) |
To test the second hypothesis of lagged relations, cross-lagged regression analysis was conducted in the MLM framework. The cross-lagged models tested whether an individual’s cortisol slope was predicted by the spouse’s cortisol slope on the previous day, after controlling for the individual’s own cortisol slope on the previous day. The basic model can be written as:
| (3) |
In this model, Yh(t-1)i and Yw(t-1)i represent cortisol slopes for husband and wife in couple i on day t-1, where t is an integer from 3 to 5. β0i and β1i are intercepts for the husband and wife in couple i. β2i and β3i are lag-1 autoregressive coefficients representing stability of cortisol slopes for the husband and wife in couple i. β4i and β5i are cross-lagged coefficients representing the influence of the spouse’s cortisol slopes on a previous day on one’s cortisol slopes, after controlling for one’s own cortisol slopes on a previous day in couple i. At level 2, the variances and covariances of these coefficients can be estimated. Gender differences are tested to examine whether men and women differ in their stability and in their susceptibility to their spouses. If gender difference is not significant, one single coefficient is estimated for both men and women. Finally, spousal support, strain, and disagreement are added to level 2 to examine whether they moderate the stability or cross-lagged coefficients. An example of the multilevel lagged regression model was given by Rovine and Walls (2006), wherein a univariate case was considered.
Results
Synchrony in Cortisol Awakening Response (CAR)
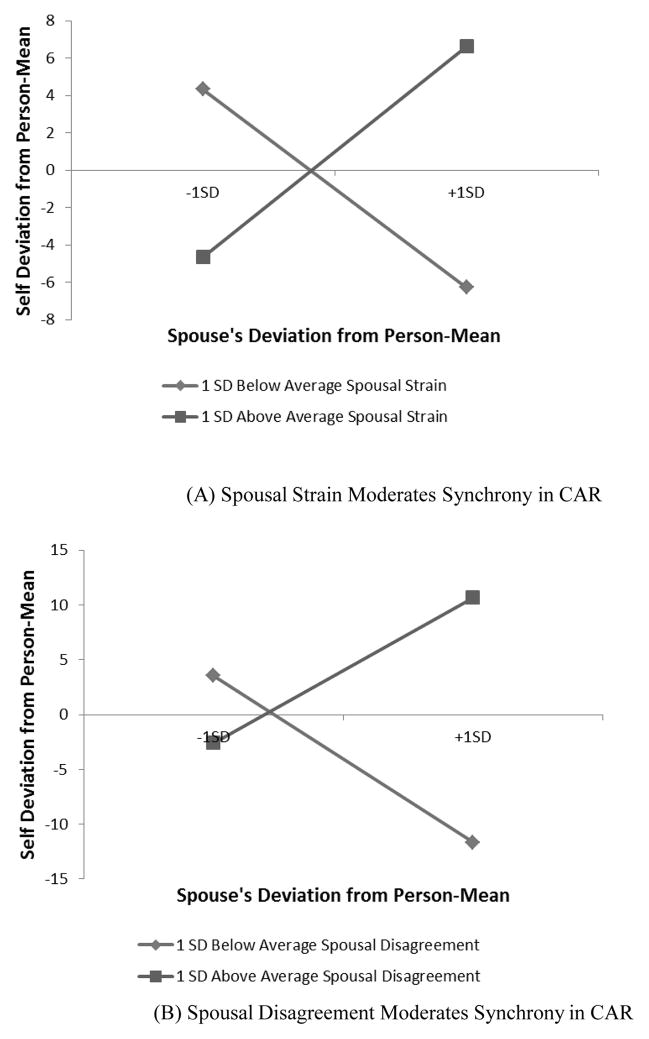
Results from the models examining synchrony in CAR between spouses and the moderating effects of marital relationships are summarized in Table 3. The estimated intercepts for men and women in the sample were 4.19 and 15.68, respectively, in the baseline model (with no moderator). A post-hoc comparison indicated that gender difference in the intercept was significant (p < .05). Hence, women in the sample tended to have more pronounced CARs than men. This gender difference is consistent with findings of Almeida, Piazza, and Stawski (2009) using a national sample of 1,143 adults. Wake time did not predict CAR either at the between-person or within-person level. The coefficient associated with spouse’s deviation from person-mean was also not significant, indicating couples did not show synchrony in CAR when averaged over the whole sample. However, tests of moderating effects of the three measures of marital relationships revealed that spousal strain and disagreement significantly predicted degree of synchrony (p < .01). Figure 1 shows the plots of the moderations. Couples with greater spousal strain and disagreement (one standard deviation above the average) showed a positive association in CAR, whereas couples with lower than average spousal strain and disagreement showed a negative association. In other words, couples characterized by high levels of marital tension travelled up and down together in their CAR, whereas couples with low levels of marital tension showed a complementary pattern, such that when one had a steeper rise in cortisol after waking than usual, his/her spouse showed a less pronounced response than usual.
Table 3.
Synchrony in the Cortisol Awakening Response (CAR) between Spouses
| Parameters | No Moderator | Moderator: Spouse Strain | Moderator: Spouse Disagreement |
|---|---|---|---|
| Intercept for Husband | 4.19 (3.94) | 3.83 (3.92) | 0.23 (3.96) |
| Intercept for Wife | 15.68*** (3.85) | 14.92** (3.96) | 11.50* (4.31) |
| Wake Time (Between-Person) | 3.38 (2.81) | 3.64 (2.92) | 3.07 (3.10) |
| Wake Time (Within-Person) | 2.54 (2.09) | 2.26 (2.08) | −0.52 (2.30) |
| Spouse’s Deviation from Person-Mean | 0.19 (0.14) | 0.01 (0.11) | −0.03 (0.12) |
| Moderator | - | 0.51 (1.73) | 1.68 (1.37) |
| Moderator × Spouse’s Deviation from Person-Mean | - | 0.16** (0.05) | 0.16** (0.06) |
|
| |||
| N | 124 | 124 | 96 |
Note. Wake time (between-person), spouse strain, and spouse disagreement were centered by sample mean.
p < .05;
p < .01;
p < .001.
Figure 1.
Marital relationship moderates synchrony in cortisol awakening response (CAR)
Synchrony in Diurnal Cortisol Slope (DCS)
Table 4 summarizes the results from the models looking at synchrony in DCS between spouses. Both men and women had negative intercepts (p < .001), indicating that cortisol did show a declining pattern throughout the day. Post-hoc analysis indicated no significant gender difference in average DCS. CAR was negatively related to DCS, both at the between- and within-person levels. Individuals who showed more pronounced CARs, on average, had steeper declines in their cortisol for the rest of the day than did individuals with less pronounced CARs. In addition, on days when an individual experienced a more pronounced CAR than his/her usual level, he or she also tended to experience a steeper decline in cortisol. The coefficient associated with spouse’s deviation from person-mean was positive and significant (p < .05). This suggests that spouses synchronized their DCSs. On days when an individual experienced faster or slower cortisol decline compared to his/her usual level, the spouse also experienced faster or slower decline compared to his/her usual level. None of the measures of marital relationships significantly moderated strength of synchrony. Thus, synchrony in DCS between spouses did not vary in a systematic way depending on spousal support, strain, or disagreement.
Table 4.
Synchrony in the Diurnal Cortisol Slope (DCS) between Spouses
| Parameters | |
|---|---|
| Intercept for Husband | −1.81*** (0.14) |
| Intercept for Wife | −1.77*** (0.10) |
| CAR (Between-Person) | −0.02** (0.01) |
| CAR(Within-Person) | −0.02*** (0.00) |
| Spouse’s Deviation from Person-Mean | 0.18* (0.09) |
|
| |
| N | 112 |
Note. CAR (between-person) was centered by sample mean.
p < .05;
p < .01;
p < .001.
Cross-Lagged Relations in Cortisol Awakening Response and Diurnal Cortisol Slope
Results from the cross-lagged regression models for CAR and DCS are shown in Table 5. A series of models were tested for each dependent variable as outlined in the method section, but only the final models are presented. For CAR, there was no gender difference in the lag-1 autoregressive coefficient or the cross-lagged coefficient. Spousal support significantly moderated the lag-1 autoregressive coefficient (p < .05). For couples with spousal support one standard deviation above the average, their estimated lag-1 autoregressive coefficient was 0.66, whereas for couples whose perceived spousal support was one standard deviation below the average, the estimated coefficient was 0.18. Thus, higher levels of spousal support were associated with higher stability in CAR. The cross-lagged coefficient, however, was not significant and not moderated by any measure of marital relationships. This lack of an effect indicates that spouse’s CAR on a previous day did not net any significant influence on one’s CAR, after controlling for one’s own CAR on the previous day.
Table 5.
Cross-Lagged Models of Cortisol Awakening Response (CAR) and Diurnal Cortisol Slope (DCS) between Spouses
| Parameters | CAR | DCS |
|---|---|---|
| Intercept for Husband | 0.90 (3.54) | −1.09** (0.34) |
| Intercept for Wife | 6.86† (3.57) | −1.33*** (0.33) |
| Lag-1 Autoregressive | 0.42*** (0.11) | 0.25† (0.13) |
| Spouse Support | −0.42 (1.68) | - |
| Spouse Support×Lag-1 Autoregressive | 0.16* (0.06) | - |
| Cross-Lagged | 0.16 (0.10) | 0.08 (0.13) |
|
| ||
| N | 90 | 84 |
Note. Spouse support was centered by sample mean.
p < .1;
p < .05;
p < .01;
p < .001.
For DCS, the lag-1 autoregressive coefficient was marginally significant (p < .1), and the cross-lagged coefficient was not significant. There were no gender differences in these coefficients. The moderating effects of marital relationship measures were also not significant.
Discussion
This study examined the daily processes in diurnal salivary cortisol patterns between spouses in a naturalistic setting. Diurnal cortisol pattern was conceptualized as consisting of an awakening response (CAR), characterized by an increase in cortisol level 30–45 minutes after waking, and a decline from the peak value to the bedtime value (DCS). We found that spouses synchronize their DCS, such that on days when one experiences faster or slower decline in his/her cortisol level than usual, the spouse also experiences faster or slower decline than usual. For CAR, the positive association was only observed in couples reporting high levels of strain and disagreement with their partners. Couples with low levels of spousal strain and disagreement showed a negative association, reflecting a complementary pattern in their CARs.
These results are consistent with previous work by Saxbe and Repetti (2010) and by Papp et al. (2012), which found synchrony in cortisol levels between spouses after taking into account its diurnal rhythm4. We extended these previous studies by not using static measures of cortisol levels at particular time points, but instead focusing on synchrony between spouses in diurnal cortisol pattern, which more directly taps onto the concept of stress regulation. Together, these studies provide strong evidence that individuals in marriage/cohabiting relationships are interdependent in their endocrine responses to stress.
The use of multilevel models in the present study has allowed us to examine synchrony as a within-couple phenomenon, thus ruling out shared chronic stressors, such as financial strain, as possible causes of spousal covariation in diurnal cortisol pattern. Specifically, our study shows that when one has a more or less dramatic decline in salivary cortisol as compared to his/her usual level, the spouse also shows a more or less dramatic decline than his/her usual level. Because the standard of comparison is an individual’s usual level, synchrony between spouses cannot be possibly explained by stable contextual factors. Rather, the within-couple synchrony indicates either physiological coregulation, i.e., partners influencing each other’s stress physiology directly, or shared daily environment that affects diurnal cortisol, e.g., a fight between spouses on a particular day. The design of this study does not allow us to identify the mechanism of within-couple synchrony. However, given that diurnal cortisol patterns are associated with a large variety of psychosocial and health outcomes, such as immunologic function, coronary calcification (Matthews, Schwartz, Cohen, & Seeman, 2006), and cognitive functioning (Stawski et al., 2011), our study provides important implications to understanding how marital processes contribute to the physical and mental well-being of individuals.
The finding that couples characterized by higher levels of spousal strain and disagreement tend to show stronger synchrony in their CARs also is consistent with previous research. Saxbe and Repetti (2010) found that individuals who were less satisfied with their marriage were more reactive to fluctuations in their spouses’ cortisol. When discussing problem areas in their marriage, more distressed couples tended to show higher degree of “interrelatedness” in their physiology and emotions, reflecting escalated negative interactions (Levenson & Gottman, 1983). The stronger positive physiological linkage among distressed couples can be explained in two ways - perhaps poor marital quality exacerbates the impact of spouse’s stress on an individual, or perhaps individuals who are easily affected by their spouses’ stress tend to report poor marital quality.
Our results also suggest that couples reporting low levels of spousal strain and disagreement actually showed negative covariation in their CARs. In these dyads, when an individual experienced a higher than usual cortisol increase upon waking, his/her spouse tends to display a dampened cortisol awaking response. Interestingly, the differential pattern in synchrony is only evident in CAR, but not in DCS. Although the physiological significance of CAR and DCS is not completely understood, some researchers have argued that they may reflect distinct biological and neuroendocrine processes (Clow, Thorn, Evans, Hucklebridge, 2004). Some evidence suggests that CAR is sensitive to the anticipation of a potentially stressful day (Kunz-Ebrecht, Kirschbaum, Marmot, & Steptoe, 2004; McHale et al., 2012). It is possible that distressed spouses travel together in their anticipated stress on a day-to-day basis because of shared anticipation of family stressors (e.g., marital conflict). In low marital tension couples, in contrast, perhaps when an individual is stressed, his/her partner is able to keep calm and sooth the individual. Due to limited sample size, there is not enough power to test whether the negative coefficient in low marital tension couples is statistically significant. Future research with a larger sample size is needed to investigate this interesting and potentially important phenomenon.
Cross-lagged regression analysis indicated that an individual’s diurnal cortisol pattern on a particular day is not associated with the spouse’s diurnal cortisol pattern on the previous day, if the individual’s own diurnal pattern on the previous day is taken into account. There is, however, considerable stability in diurnal cortisol pattern. Averaging across individuals, both CAR and DCS have a positive lag-1 autoregressive coefficient, indicating that a steeper slope on day t is predictive to a steeper slope on day t+1. In addition, stability in CAR is found to be stronger in couples characterized by higher levels of spousal support. Given that CAR may be linked to anticipated stress of the day, this relation may indicate that individuals who receive more support from their spouses tend to have higher regularity in their anticipated stress from day to day. On the other hand, it may also indicate that high levels of spousal support buffer HPA axis responses to stressful daily events.
This study contributes to the literature in a number of important ways. Despite theories and empirical evidence linking physiological coregulation and synchrony and adult attachment (Sbarra & Hazan, 2008), few studies have explicitly examined the interrelatedness between spouses at the physiological level, and fewer have focused on the HPA axis which plays a significant role in health and aging. This study fills a gap in the literature and suggests that research of this type is promising. Similar studies may help us better understand the effects of marriage or cohabitation on individuals’ psychological and physical well-being. Researchers may also extend the current framework to explore relations between health disparity across families and their exposure to differential levels of stress by conducting longitudinal studies with a longer time span. In addition, this study highlights the utility of family system theory, which conceptualizes marital dyads as self-organizing dynamic systems in which spouses mutually influence each other. According to this perspective, family processes evolve in a reciprocal way such that short-term interactions both depend on and reinforce relatively stable characteristics of relationships. Results from this study are in line with this notion. Specifically, the strength of synchrony between spouses in their stress was associated with characteristics of the marital relationships, including spousal strain and disagreement, and the degree of stability in CAR was linked to the general level of spousal support in the relationships.
Methodologically, this study demonstrates a way to capture covariation of a construct within the unit of analysis (e.g., family, group) using relatively few data points. The methods used represent an extension to the standard multilevel dyadic model (Laurenceau & Bolger, 2005; Raudenbush, Brennan, & Barnett, 1995), where covariation between dyad members is modeled as covariance. In this study, covariation between spouses is modeled as a fixed effect, instead of a random effect. This extension greatly increases the flexibility of the model in studying within-family processes, because it allows the strength of covariation to be predicted by between-family covariates, such as measures of marital relationships used in this study. In other words, whereas the standard multilevel dyadic model provides a way to examine whether dyad members are interdependent, the extended approach presented in this study enables us to further investigate what factors explain the variation in the degree of interdependence. The same technique has been applied in Butner, Diamond, and Hicks (2007) to look at coregulation of positive and negative affect between spouses and the moderating effects of attachment style.
It should be noted that the models presented in this study also differ from the analysis in Saxbe and Repetti’s (2010) earlier work, although the data in the two studies have similar structures. In Saxbe and Repetti’s (2010) study, cortisol data measured over days were collapsed and treated as measures obtained on one single day. The assumption behind the analysis is that the day-to-day variation is negligible when looking at synchrony in cortisol at a particular time point. This averaging strategy is common in diurnal cortisol research (Adam, 2006; Adam & Gunnar, 2001; Saxbe, Repetti, & Nishina, 2008). In contrast, day-to-day variation plays a key role in the current study. In fact, synchrony was conceptualized as husbands and wives moving up or down together in their day-to-day variation. The differences in the model set-up have allowed us to address the same research question in different ways, which is illuminating.
Finally, there are several caveats to results from the current study. First, this study has a small sample size and participants were recruited from a single industry. Statistically, the small sample size has limited the power of between-family analysis. With a larger sample, we may be able to reveal significant effects of other between-family covariates. The relatively high homogeneity of the sample has also prevented us from generalizing the findings to a larger population. Given a more diverse sample, we may be able to investigate some other interesting questions, such as whether the strength of physiological synchrony depends on the stage of the relationships (e.g., people who just started dating vs. people who have been together for a long time) or sexual orientation (i.e., same-sex couples).
Second, this study is cross-sectional and does not allow us to disentangle the direction of effects between strength of synchrony and characteristics of the marital relationships. Although measures of marital relationships were obtained prior to the cortisol data, they are likely to be relatively stable over time and may result from the history of how spouses relate to each other in their physiology. Or, there may be bidirectional influences between characteristics of the marital relationships and physiological synchrony. Longitudinal data would help us determine the direction of the relation and gain deeper insight to spousal regulation.
Finally, although the repeated measurement of salivary cortisol over several days is intensive compared to most behavioral research and field studies in the neuroendocrine health area, the number of measures from each marital dyad and the span of the study do not allow us to model synchrony as a time-varying phenomenon. It is possible, for example, that degree of synchrony changes with the occurrence of some important life events (e.g., the birth of a child). More intensive data collection (i.e., more collection time points or a multiple bursts design) over a longer time period will allow us to investigate this interesting question. Further, inclusion of data collection surrounding the context of the relationship (e.g., depression, anxiety, sleep deprivation) that might link neuroendocrine biomarkers, such as cortisol, with quality of marital functioning could help provide insight into potential intervention or prevention opportunities.
In sum, this study provides empirical support for synchrony in diurnal cortisol pattern in couples. These data contribute both to our knowledge of marital processes and biobehavioral health, and to the advancement of longitudinal dyadic analysis. Future research with a larger and more diverse sample and/or more intensive measurements appears to be very promising.
Acknowledgments
This study was supported by the Alfred P. Sloan Foundation and the General Clinical Research Center of The Pennsylvania State University (NIH grant MO1-RR-10732). It was conducted as part of the Work, Family and Health Network (www.WorkFamilyHealthNetwork.org), which is funded by a cooperative agreement through the National Institutes of Health and the Centers for Disease Control and Prevention: National Institute of Child Health and Human Development (Grant # U01HD051217, U01HD051218, U01HD051256, U01HD051276), National Institute on Aging (Grant # U01AG027669), Office of Behavioral and Science Sciences Research, and National Institute for Occupational Safety and Health (Grant # U010H008788). Special acknowledgement goes to Extramural Staff Science Collaborator, Rosalind Berkowitz King, Ph.D. and Lynne Casper, Ph.D. for design of the original Workplace, Family, Health and Well-Being Network Initiative.
Appendix
Hypothetical Data for One Couple and SAS Syntax for Multilevel Dyadic Synchrony Model
| CoupleID | ID | Day | DCS | Husband | Wife | Person Mean | DCS_Self | DCS_Spouse |
|---|---|---|---|---|---|---|---|---|
| 1 | 101 | 1 | −1 | 1 | 0 | −1.5 | 0.5 | 0 |
| 1 | 101 | 2 | −2 | 1 | 0 | −1.5 | −0.5 | −0.5 |
| 1 | 101 | 3 | −2 | 1 | 0 | −1.5 | −0.5 | 0.5 |
| 1 | 101 | 4 | −1 | 1 | 0 | −1.5 | 0.5 | 0 |
| 1 | 102 | 1 | −2 | 0 | 1 | −2 | 0 | 0.5 |
| 1 | 102 | 2 | −2.5 | 0 | 1 | −2 | −0.5 | −0.5 |
| 1 | 102 | 3 | −1.5 | 0 | 1 | −2 | 0.5 | −0.5 |
| 1 | 102 | 4 | −2 | 0 | 1 | −2 | 0 | 0.5 |
Note: DCS = Diurnal Cortisol Slope. DCS_Self = DCS - Person Mean. It represents deviation of the individual’s DCS on a particular day from the person mean. DCS_Spouse (denoted as SPOUSEti in Equation 1) is the spouse’s person-centered cortisol slope.
SAS Syntax for Multilevel Dyadic Synchrony Model (Equation 1):
PROC MIXED COVTEST METHOD=REML;
CLASS CoupleID Day;
MODEL DCS = Husband Wife DCS_Spouse / NOINT S DDFM=sat;
RANDOM Husband Wife / TYPE=un SUB=CoupleID G GCORR;
RANDOM DCS_Spouse / TYPE=un SUB=CoupleID G;
RUN;
Footnotes
The sample in this study includes one unmarried but cohabiting couple. For the ease of presentation, we refer to the participants as “married couples” and in a “marital relationship”. Terms such as “husband”, “wife”, and “spouse” are also used to refer to the men and women in the sample.
Each individual contributed one person-day each day when he or she provided at least one saliva sample.
To examine the relation between average scores and concordance in ratings, we constructed measures of discrepancy by computing the absolute values of the difference scores between spouses. Discrepancy in spousal support was highly negatively correlated with averaged spousal support within family (r = −.90, p < .001). However, there was no significant correlation between discrepancy scores and averaged scores for the other two variables (r = −.15 for strain; r = .04 for disagreement). Hence, results presented in this paper that involve the overall level of spousal support should be interpreted taking into account its negative correlation with discrepancy in ratings.
In Saxbe and Repetti’s (2010) study, cortisol was measured early morning upon wakening, late morning just before lunch, afternoon just before leaving work, and evening before going to bed. These measures do not capture CAR. Papp et al. (2012)’s study includes saliva samples that capture CAR.
Contributor Information
Siwei Liu, University of California, Davis.
Michael J. Rovine, The Pennsylvania State University
Laura Cousino Klein, The Pennsylvania State University.
David M. Almeida, The Pennsylvania State University
References
- Adam EK. Transactions among trait and state emotion and adolescent diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar M. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/S0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience - cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Davis KD, O’Neill JW, Crouter AC. Translational research on work and family: Daily stress processes in hotel employees and their families. In: Wethington E, Dunifon RE, editors. Research for the public good: Applying the methods of translational research to improve human health and well-being. Washington, DC: American Psychological Association; 2012. pp. 127–146. [DOI] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24(4):819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. The New England Journal of Medicine. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen P. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss: Vol. 1: Attachment. Vol. 2. New York: Basic Books; 1982. [Google Scholar]
- Bowlby J. The nature of the child’s tie to his mother. International Journal of Psychoanalysis. 1958;39:350–373. [PubMed] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems: The “TIES” that form relationships. Personality and Social Psychology Review. 2011;15(4):367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Butner J, Diamond LM, Hicks AM. Attachment style and two forms of affect coregulation between romantic partners. Personal Relationships. 2007;14(3):431–455. doi: 10.1111/j.1475-6811.2007.00164.x. [DOI] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7(1):29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Diamond LM. Contributions of psychophysiology to research on adult attachment: Review and recommendations. Personality and Social Psychology Review. 2001;5(4):276–295. doi: 10.1207/S15327957PSPR0504_1. [DOI] [Google Scholar]
- Feldman R. Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007;16:340–345. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [Google Scholar]
- Feldman R, Eidelman A. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Developmental Medicine & Child Neurology. 2003;45:274–281. doi: 10.1111/j.1469-8749.2003.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Goodman CR, Shippy RA. Is it contagious? Affect similarity among spouses. Aging & Mental Health. 2002;6:266–274. doi: 10.1080/13607860220142431. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT. Integrating biological, behavioral, and social levels of analysis in early child development research: Progress, problems, and prospects. Child Development. 2003;74:1058–1063. doi: 10.1111/1467-8624.00590. [DOI] [PubMed] [Google Scholar]
- Grzywacz JG, Marks NF. Family solidarity and health behaviors: Evidence from the National Survey of Midlife Development in the United States. Journal of Family Issues. 1999;20(2):243–268. doi: 10.1177/019251399020002004. [DOI] [Google Scholar]
- Guedeney A, Guedeney N, Tereno S, Dugravier R, Greacen T, Welniarz B, Tubach F. Infant rhythms versus parental time: Promoting parent-infant synchrony. Journal of Physiology-Paris. 2011;105(4–6):195–200. doi: 10.1016/j.jphysparis.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Hazan C, Gur-Yaish N, Campa M. What does it mean to be attached? In: Rholes WS, Simpson JA, editors. Adult attachment: New directions and emerging issues. New York: Guilford Press; 2004. pp. 55–85. [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Helm JL, Sbarra D, Ferrer E. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion. 2011 doi: 10.1037/a0025036. [DOI] [PubMed] [Google Scholar]
- Hofer M. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. Retrieved from http://www.wisebrain.org/papers/RelsRegGrieving.pdf. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic data analysis. New York: Guilford; 2006. [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. Retrieved from http://www.psychosomaticmedicine.org/content/57/1/23.short. [DOI] [PubMed] [Google Scholar]
- Koball HL, Moiduddin E, Henderson J, Goesling B, Besculides M. What do we know about the link between marriage and health? Journal of Family Issues. 2010;31(8):1019–1040. doi: 10.1177/0192513X10365834. [DOI] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosomatic Medicine. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/S0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Larson RW, Almeida DM. Emotional transmission in the daily lives of families: A new paradigm for studying family process. Journal of Marriage and Family. 1999;61(1):5–20. Retrieved from http://www.jstor.org/stable/353879. [Google Scholar]
- Laurenceau JP, Bolger N. Using diary methods to study marital and family processes. Journal of Family Psychology. 2005;19(1):86–97. doi: 10.1037/0893-3200.19.1.86. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45(3):587–597. doi: 10.1037/0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic Medicine. 2006;68(5):657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- McHale SM, Blocklin MK, Walter KN, Davis KD, Almeida DM, Klein LC. The role of daily activities in youths’ stress physiology. Journal of Adolescent Health. 2012;51:623–628. doi: 10.1016/j.jadohealth.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SP, Lyons DM, Saltzman W. Sociophysiology of squirrel-monkeys. American Journal of Primatology. 1991;23:37–54. doi: 10.1002/ajp.1350230105. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Simon CD, Adam EK. Spouses’ cortisol associations and moderators: Testing physiological synchrony and connectedness in everyday life. Family Process. 2012 doi: 10.1111/j.1545-5300.2012.01413.x. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva N, Klein LC. Frontiers in the use of biomarkers in research on stress and aging. Journal of Gerontology: Psychological Sciences. 2010;65:513–25. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9(2):161–174. doi: 10.1037/0893-3200.9.2.161. [DOI] [Google Scholar]
- Rovine MJ, Walls TA. Multilevel autoregressive modeling of interindividual differences in the stability of a process. In: Walls TA, Schafer JL, editors. Models for intensive longitudinal data. New York: Oxford University Press; 2006. pp. 124–147. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of Personality and Social Psychology. 2010;98(1):92–103. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Repetti RL, Nishina A. Marital satisfaction, recovery from work, and diurnal cortisol among men and women. Health Psychology. 2008;27(1):15–25. doi: 10.1037/0278-6133.27.1.15. [DOI] [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: An integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12:141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, Goldsmith HH. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster TL, Kessler RC, Aseltine RH. Supportive interactions, negative interactions, and depressive mood. American Journal of Community Psychology. 1990;18:423–438. doi: 10.1007/BF00938116. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. Journal of Clinical Endocrinology & Metabolism. 1997;82(8):2458–2465. doi: 10.1210/jc.82.8.2458. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: Timing is everything. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B(suppl 1):i71–i81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen HR, Lachman ME. Social support and strain from partner, family and friends: Costs and benefits for men and women in adulthood. Journal of Social and Personal Relationships. 2000;17:1, 5–30. doi: 10.1177/0265407500171001. [DOI] [Google Scholar]
- Williams E, Magid K, Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005;30:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Social support and physical health: Understanding the health consequences of relationships. New Haven, CT: Yale University Press; 2004. [Google Scholar]