Abstract
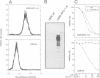
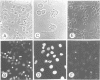
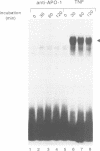
Tumor necrosis factor receptor (TNF-R) and APO-1/Fas (CD95) are members of the tumor necrosis factor/nerve growth factor receptor superfamily involved in various forms of physiological cell death. Due to the structural homology between these receptors and their ligands, it has been suggested that APO-1/Fas and TNF-R kill cells by similar mechanisms. Here, we compared the killing pathways mediated by each receptor molecule in TNF-sensitive L929 cells stably transfected with APO-1/Fas cDNA. Morphological analysis revealed that TNF-induced cell death resembles necrosis, while APO-1/Fas-mediated cell killing shows an apoptotic pattern, evident by the appearance of membrane blebbing, nuclear condensation and non-random DNA degradation. Studies with inhibitors of several intracellular pathways further demonstrate that the mechanisms of TNF- and APO-1/Fas-mediated cell killing are substantially different. TNF cytotoxicity is mediated by reactive oxygen intermediates generated during mitochondrial respiration. However, these mediators are not involved in APO-1/Fas-mediated cell death as neither mitochondrial inhibitors nor antioxidants exert a protecting effect. Moreover, several inhibitors of calcium metabolism, ADP ribosylation and phospholipase action suppress TNF cytotoxicity, but not APO-1/Fas-mediated apoptosis. Additional differences between the two molecules were observed at the transcriptional level. Whereas transcription factor NF-kappa B was readily activated by TNF, activation was not induced by triggering APO-1/Fas. These data suggest that the two molecules, though structurally related, utilize distinct signal transduction pathways, even in a single cell type. Hence, cells may undergo different programs of cell death depending on the activating stimulus.
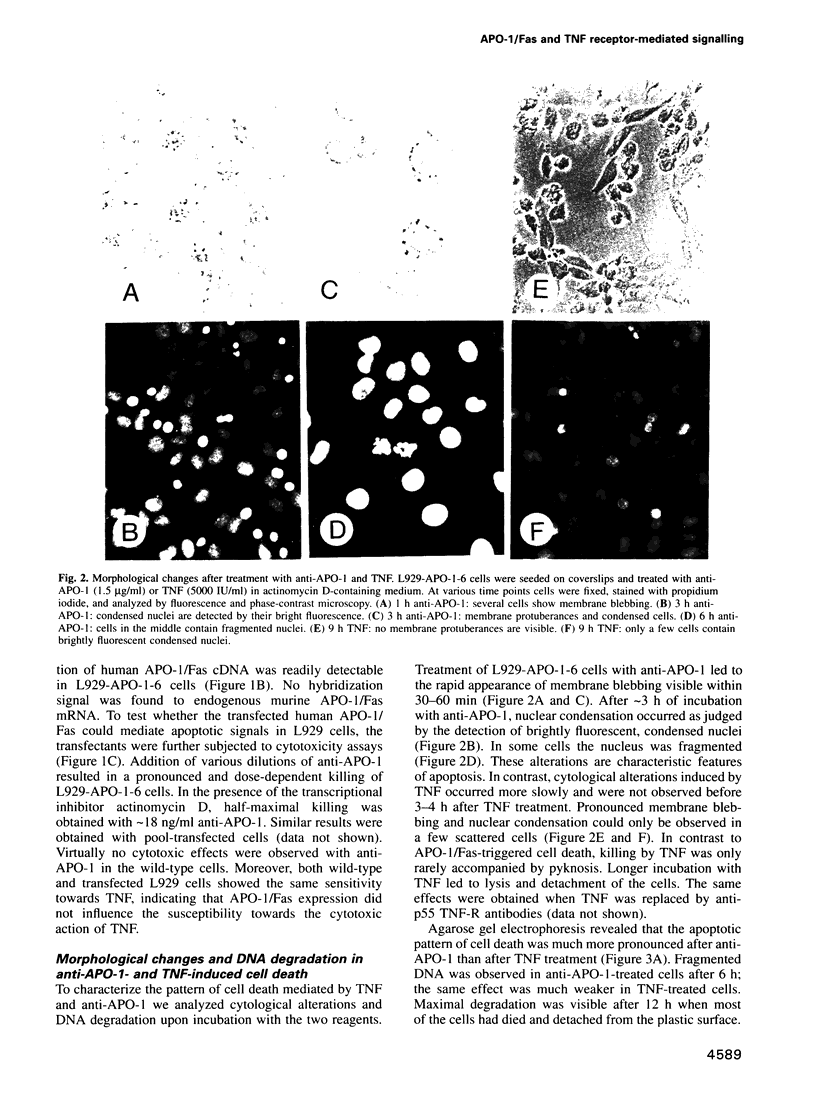
Full text
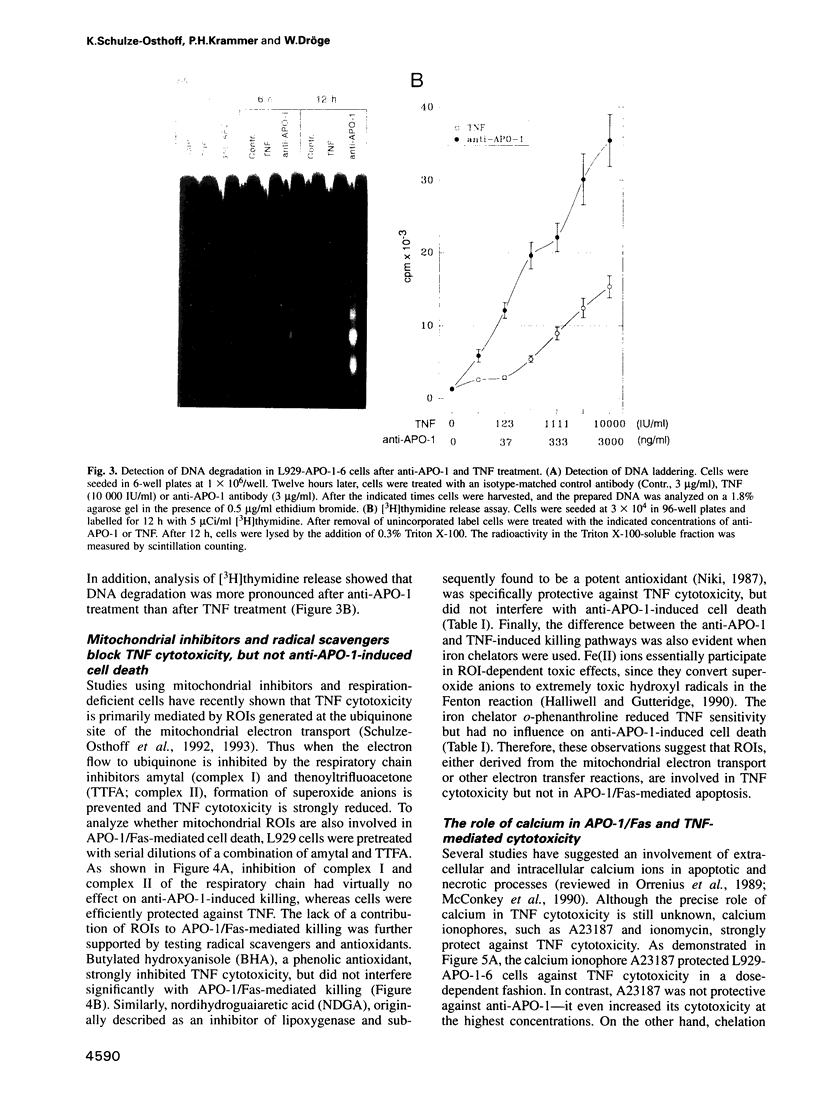
PDF
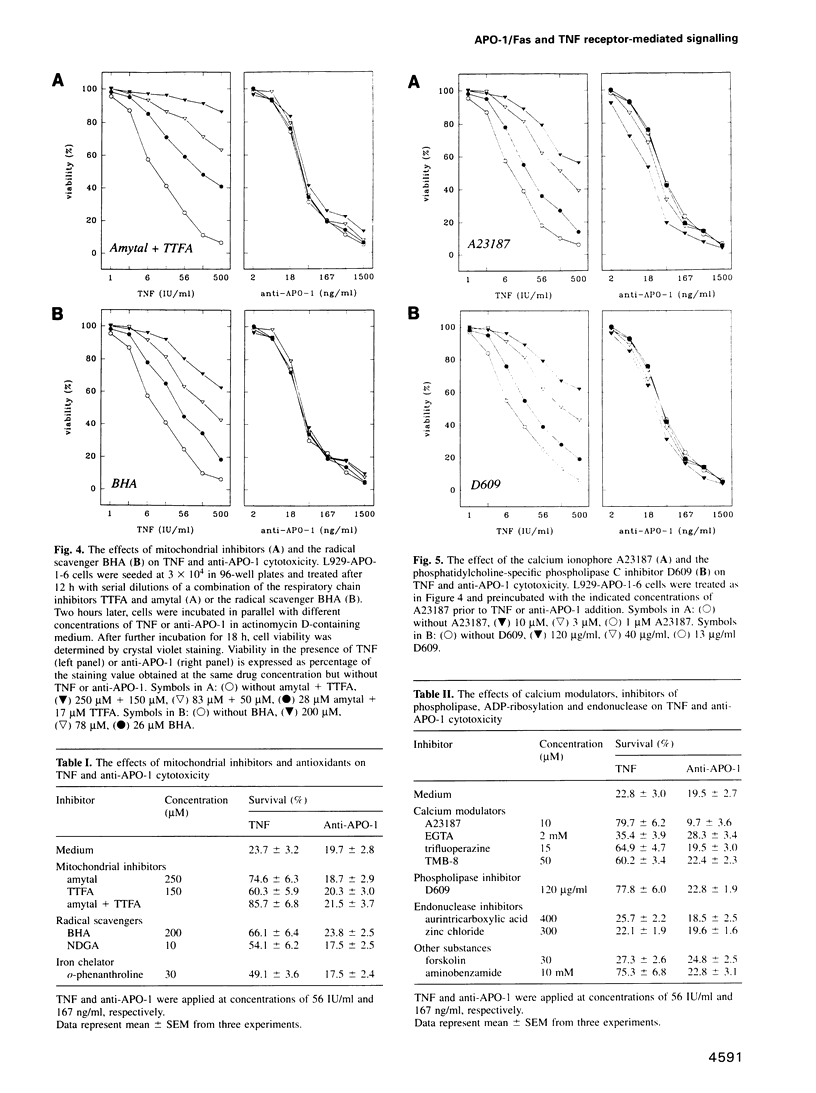




Images in this article
Selected References
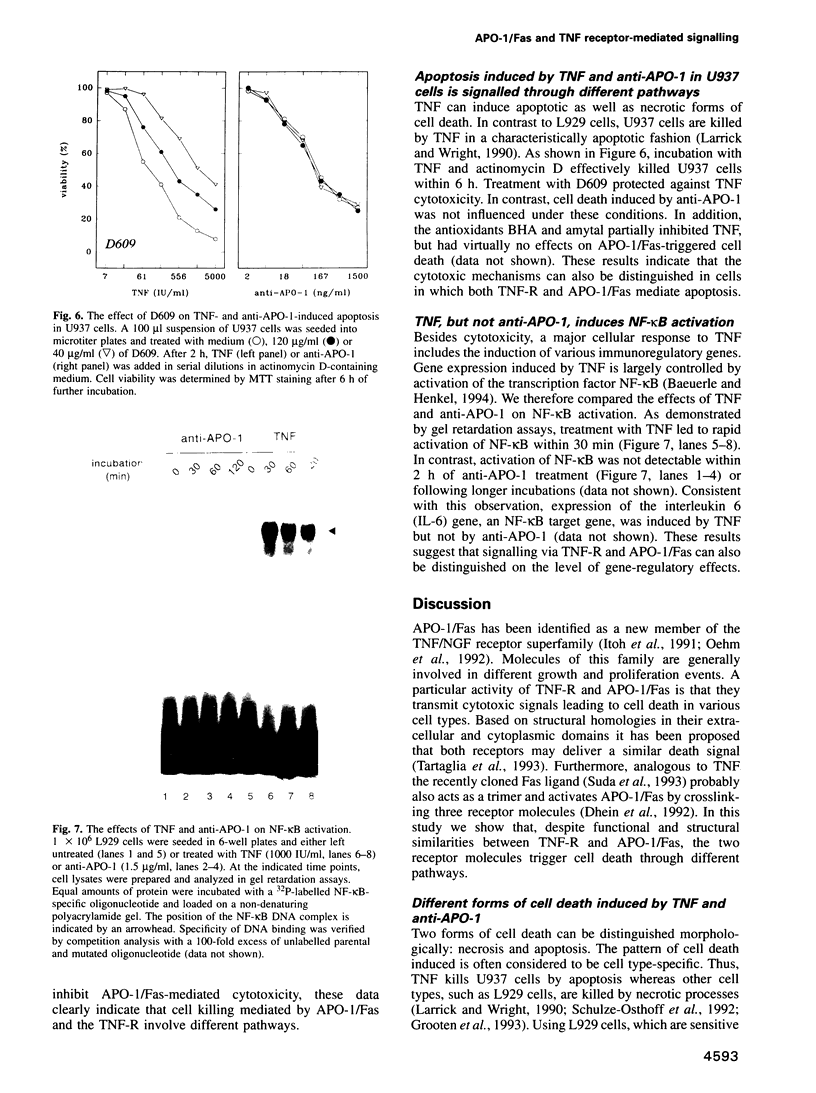
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Perotti M., Taddei F., Mirabelli F., Finardi G., Nicotera P., Orrenius S. Tumor necrosis factor alpha induces apoptosis in mammary adenocarcinoma cells by an increase in intranuclear free Ca2+ concentration and DNA fragmentation. Cancer Res. 1992 Mar 1;52(5):1342–1346. [PubMed] [Google Scholar]
- Beyaert R., Heyninck K., De Valck D., Boeykens F., van Roy F., Fiers W. Enhancement of tumor necrosis factor cytotoxicity by lithium chloride is associated with increased inositol phosphate accumulation. J Immunol. 1993 Jul 1;151(1):291–300. [PubMed] [Google Scholar]
- Buttke T. M., Sandstrom P. A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994 Jan;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Dealtry G. B., Naylor M. S., Fiers W., Balkwill F. R. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol. 1987 May;17(5):689–693. doi: 10.1002/eji.1830170517. [DOI] [PubMed] [Google Scholar]
- Dhein J., Daniel P. T., Trauth B. C., Oehm A., Möller P., Krammer P. H. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992 Nov 15;149(10):3166–3173. [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcieri E., Martelli A. M., Bareggi R., Cataldi A., Cocco L. The protein kinase inhibitor staurosporine induces morphological changes typical of apoptosis in MOLT-4 cells without concomitant DNA fragmentation. Biochem Biophys Res Commun. 1993 May 28;193(1):19–25. doi: 10.1006/bbrc.1993.1584. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Fransen L., Müller R., Marmenout A., Tavernier J., Van der Heyden J., Kawashima E., Chollet A., Tizard R., Van Heuverswyn H., Van Vliet A. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985 Jun 25;13(12):4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P., Ojcius D. M., Young J. D. Cell death mechanisms and the immune system. Immunol Rev. 1991 Jun;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Grooten J., Goossens V., Vanhaesebroeck B., Fiers W. Cell membrane permeabilization and cellular collapse, followed by loss of dehydrogenase activity: early events in tumour necrosis factor-induced cytotoxicity. Cytokine. 1993 Nov;5(6):546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hennet T., Richter C., Peterhans E. Tumour necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993 Jan 15;289(Pt 2):587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Behrmann I., Daniel P., Dhein J., Debatin K. M. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994 Apr;6(2):279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Debatin K. M. When apoptosis fails. Curr Biol. 1992 Jul;2(7):383–385. doi: 10.1016/0960-9822(92)90084-n. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Wright S. C. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990 Nov;4(14):3215–3223. doi: 10.1096/fasebj.4.14.2172061. [DOI] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Masutani H., Nakamura H., Miyajima S., Yamauchi A., Yonehara S., Uchida A., Irimajiri K., Horiuchi A., Yodoi J. Protective activity of adult T cell leukemia-derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991 Dec 1;147(11):3837–3841. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Amador-Pérez J. F., Orrenius S., Jondal M. Calcium-dependent killing of immature thymocytes by stimulation via the CD3/T cell receptor complex. J Immunol. 1989 Sep 15;143(6):1801–1806. [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Agents that elevate cAMP stimulate DNA fragmentation in thymocytes. J Immunol. 1990 Aug 15;145(4):1227–1230. [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Cellular signalling in programmed cell death (apoptosis). Immunol Today. 1990 Apr;11(4):120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Neale M. L., Fiera R. A., Matthews N. Involvement of phospholipase A2 activation in tumour cell killing by tumour necrosis factor. Immunology. 1988 May;64(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Niki E. Antioxidants in relation to lipid peroxidation. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):227–253. doi: 10.1016/0009-3084(87)90052-1. [DOI] [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Orrenius S., McConkey D. J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989 Jul;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Matsuyama T., Kündig T. M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P. S., Krönke M., Mak T. W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993 May 7;73(3):457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Richter C., Frei B. Ca2+ release from mitochondria induced by prooxidants. Free Radic Biol Med. 1988;4(6):365–375. doi: 10.1016/0891-5849(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M., López-Rivas A. Regulation of apoptosis in interleukin-3-dependent hemopoietic cells by interleukin-3 and calcium ionophores. EMBO J. 1990 Sep;9(9):2997–3002. doi: 10.1002/j.1460-2075.1990.tb07492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Hornung R., McGrath K. M., Paul N., Ruddle N. H. Target cell DNA fragmentation is mediated by lymphotoxin and tumor necrosis factor. Lymphokine Res. 1987 Summer;6(3):195–202. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Schulze-Osthoff K., Beyaert R., Vandevoorde V., Haegeman G., Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993 Aug;12(8):3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992 Nov 27;71(5):765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Suffys P., Beyaert R., De Valck D., Vanhaesebroeck B., Van Roy F., Fiers W. Tumour-necrosis-factor-mediated cytotoxicity is correlated with phospholipase-A2 activity, but not with arachidonic acid release per se. Eur J Biochem. 1991 Jan 30;195(2):465–475. doi: 10.1111/j.1432-1033.1991.tb15727.x. [DOI] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Ayres T. M., Wong G. H., Goeddel D. V. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993 Sep 10;74(5):845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Ucker D. S., Obermiller P. S., Eckhart W., Apgar J. R., Berger N. A., Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992 Jul;12(7):3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J Immunol. 1994 Feb 15;152(4):1751–1755. [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Chan A., Leadon S. A. Oxidative damage in murine tumor cells treated in vitro by recombinant human tumor necrosis factor. Cancer Res. 1989 Apr 1;49(7):1644–1648. [PubMed] [Google Scholar]