Abstract
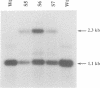
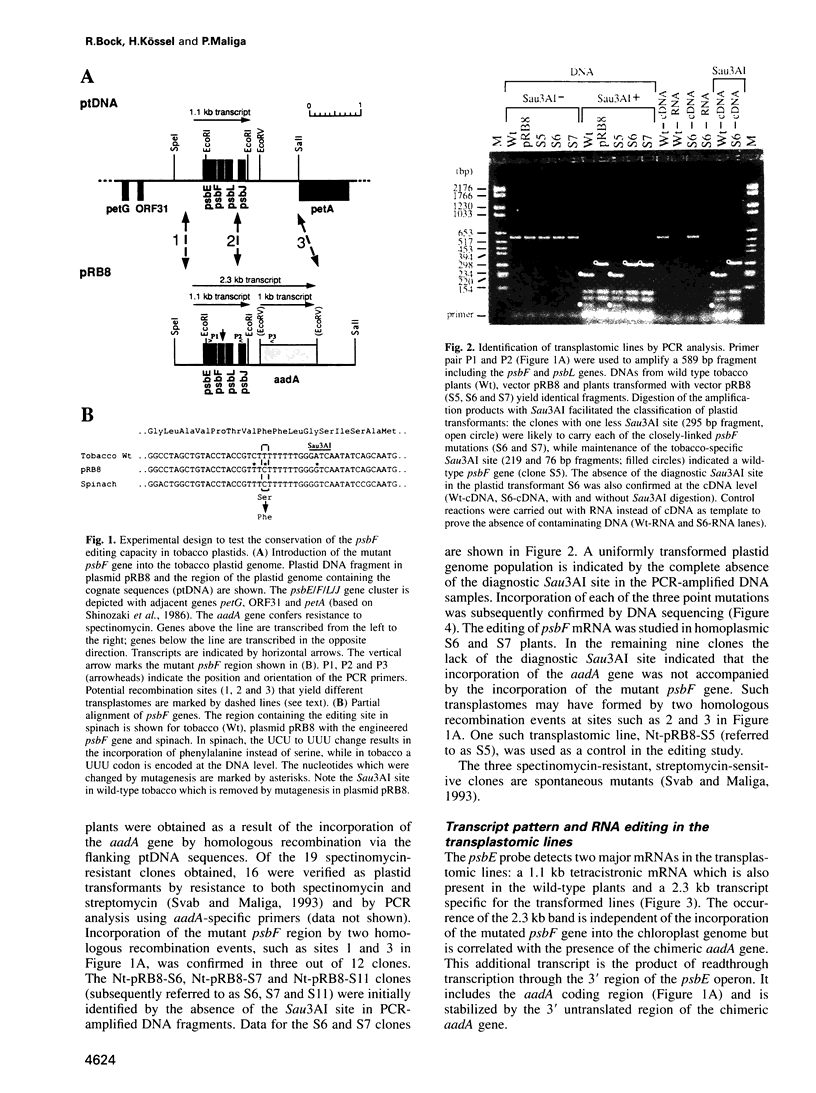
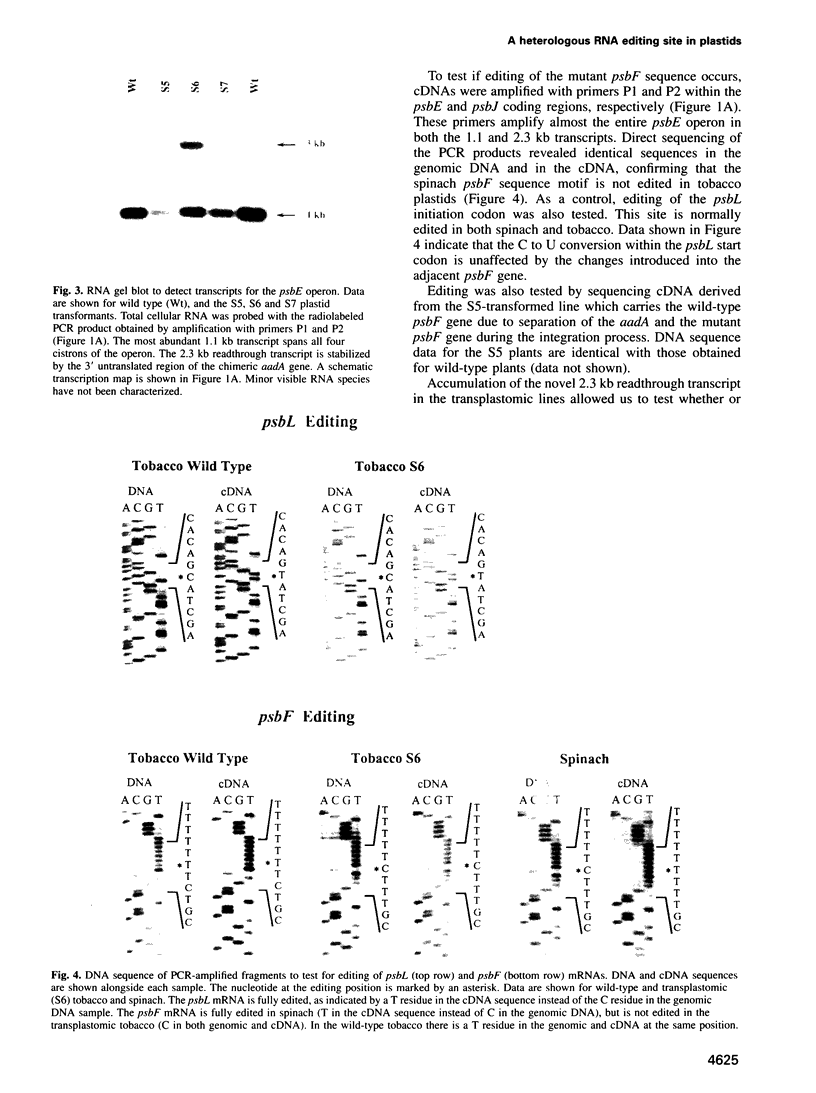
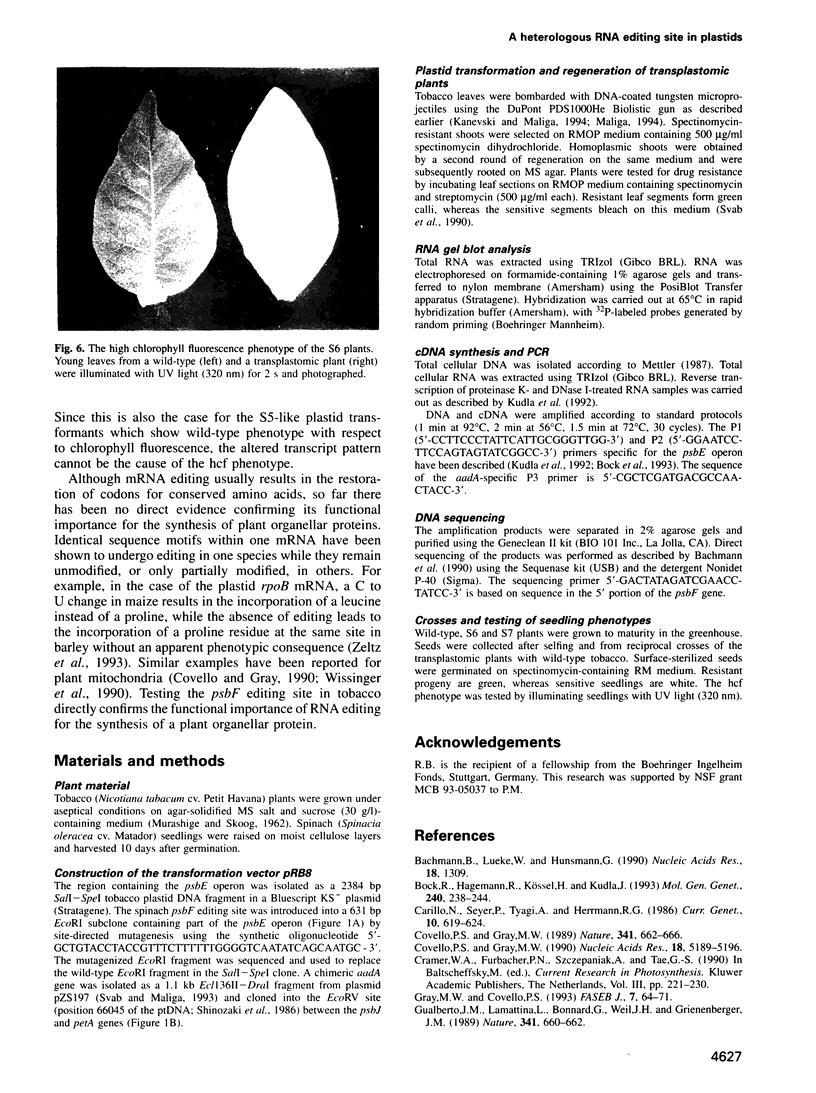
The psbF mRNA is edited in spinach plastids by a C to U conversion, changing a serine to a conserved phenylalanine codon. In tobacco at this position a phenylalanine codon is present at the DNA level, and the psbF mRNA here is not edited. To test if the psbF editing capacity is evolutionarily conserved, the tobacco psbF gene was modified to match the corresponding spinach sequence. The endogenous tobacco gene was replaced with the modified copy using biolistic transformation. We report here that the heterologous editing site remains unmodified in transplastomic tobacco plants. The lack of editing is associated with slower growth, lowered chlorophyll content and high chlorophyll fluorescence, a phenotype characteristic of photosynthetic mutants. This finding confirms that the editing of the psbF mRNA is an essential processing step for protein function and thus provides direct proof for the biological significance of plant organellar RNA editing. Given that a mutant phenotype is associated with the lack of editing, it seems likely that the evolutionary loss of the site-specific capacity for psbF editing was preceded by the mutation that eliminated the editing requirement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R., Hagemann R., Kössel H., Kudla J. Tissue- and stage-specific modulation of RNA editing of the psbF and psbL transcript from spinach plastids--a new regulatory mechanism? Mol Gen Genet. 1993 Aug;240(2):238–244. doi: 10.1007/BF00277062. [DOI] [PubMed] [Google Scholar]
- Carrillo N., Seyer P., Tyagi A., Herrmann R. G. Cytochrome b-559 genes from Oenothera hookeri and Nicotiana tabacum show a remarkably high degree of conservation as compared to spinach. The enigma of cytochrome b-559: highly conserved genes and proteins but no known function. Curr Genet. 1986;10(8):619–624. doi: 10.1007/BF00418129. [DOI] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. Differences in editing at homologous sites in messenger RNAs from angiosperm mitochondria. Nucleic Acids Res. 1990 Sep 11;18(17):5189–5196. doi: 10.1093/nar/18.17.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. RNA editing in plant mitochondria. Nature. 1989 Oct 19;341(6243):662–666. doi: 10.1038/341662a0. [DOI] [PubMed] [Google Scholar]
- Gray M. W., Covello P. S. RNA editing in plant mitochondria and chloroplasts. FASEB J. 1993 Jan;7(1):64–71. doi: 10.1096/fasebj.7.1.8422976. [DOI] [PubMed] [Google Scholar]
- Gualberto J. M., Lamattina L., Bonnard G., Weil J. H., Grienenberger J. M. RNA editing in wheat mitochondria results in the conservation of protein sequences. Nature. 1989 Oct 19;341(6243):660–662. doi: 10.1038/341660a0. [DOI] [PubMed] [Google Scholar]
- Hiesel R., Wissinger B., Schuster W., Brennicke A. RNA editing in plant mitochondria. Science. 1989 Dec 22;246(4937):1632–1634. doi: 10.1126/science.2480644. [DOI] [PubMed] [Google Scholar]
- Hoch B., Maier R. M., Appel K., Igloi G. L., Kössel H. Editing of a chloroplast mRNA by creation of an initiation codon. Nature. 1991 Sep 12;353(6340):178–180. doi: 10.1038/353178a0. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M., Kyozuka J., Shimamoto K. Processing followed by complete editing of an altered mitochondrial atp6 RNA restores fertility of cytoplasmic male sterile rice. EMBO J. 1993 Apr;12(4):1437–1446. doi: 10.1002/j.1460-2075.1993.tb05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevski I., Maliga P. Relocation of the plastid rbcL gene to the nucleus yields functional ribulose-1,5-bisphosphate carboxylase in tobacco chloroplasts. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1969–1973. doi: 10.1073/pnas.91.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Igloi G. L., Metzlaff M., Hagemann R., Kössel H. RNA editing in tobacco chloroplasts leads to the formation of a translatable psbL mRNA by a C to U substitution within the initiation codon. EMBO J. 1992 Mar;11(3):1099–1103. doi: 10.1002/j.1460-2075.1992.tb05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. M., Neckermann K., Hoch B., Akhmedov N. B., Kössel H. Identification of editing positions in the ndhB transcript from maize chloroplasts reveals sequence similarities between editing sites of chloroplasts and plant mitochondria. Nucleic Acids Res. 1992 Dec 11;20(23):6189–6194. doi: 10.1093/nar/20.23.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., De Ciechi P., Whitmarsh J. Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. EMBO J. 1991 Jul;10(7):1619–1627. doi: 10.1002/j.1460-2075.1991.tb07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Hajdukiewicz P., Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissinger B., Schuster W., Brennicke A. Species-specific RNA editing patterns in the mitochondrial rps13 transcripts of Oenothera and Daucus. Mol Gen Genet. 1990 Dec;224(3):389–395. doi: 10.1007/BF00262433. [DOI] [PubMed] [Google Scholar]
- Zeltz P., Hess W. R., Neckermann K., Börner T., Kössel H. Editing of the chloroplast rpoB transcript is independent of chloroplast translation and shows different patterns in barley and maize. EMBO J. 1993 Nov;12(11):4291–4296. doi: 10.1002/j.1460-2075.1993.tb06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]