Abstract
Curcumin has been shown to have many potentially health beneficial properties in vitro and in animal models with clinical studies on the toxicity of curcumin reporting no major side effects. However, curcumin may chelate dietary trace elements and could thus potentially exert adverse effects. Here, we investigated the effects of a 6 month dietary supplementation with 0.2% curcumin on iron, zinc, and copper status in C57BL/6J mice. Compared to non-supplemented control mice, we observed a significant reduction in iron, but not zinc and copper stores, in the liver and the spleen, as well as strongly suppressed liver hepcidin and ferritin expression in the curcumin-supplemented mice. The expression of the iron-importing transport proteins divalent metal transporter 1 and transferrin receptor 1 was induced, while hepatic and splenic inflammatory markers were not affected in the curcumin-fed mice. The mRNA expression of other putative target genes of curcumin, including the nuclear factor (erythroid-derived 2)-like 2 and haem oxygenase 1 did not differ between the groups. Most of the published animal trials with curcumin-feeding have not reported adverse effects on iron status or the spleen. However, it is possible that long-term curcumin supplementation and a Western-type diet may aggravate iron deficiency. Therefore, our findings show that further studies are needed to evaluate the effect of curcumin supplementation on iron status.
Abbreviations: γ-GCS, γ-glutamyl cysteine synthetase; DMT1, divalent metal transporter 1; FPN, ferroportin; HO1, haem oxygenase; IL, interleukin; NQO1, NAD(P)H quinone oxidoreductase; NRF2, nuclear factor (erythroid-derived 2)-like 2; qRT-PCR, quantitative real-time polymerase chain reaction; TBS, tris buffered saline; TfR1, transferrin receptor 1; TNFα, tumour necrosis factor α
Keywords: Curcumin, Iron store, Liver minerals, Safety, Enlarged spleen, Toxicity
Graphical abstract
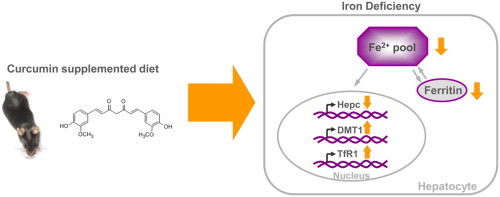
A 6 month dietary supplementation with 0.2% curcumin in C57BL/6J mice led to a significant reduction in iron, but not zinc and copper stores, in the liver and the spleen, and suppressed liver hepcidin and ferritin expression. Furthermore, the expression of the iron-importing transport proteins divalent metal transporter (DMT) 1 and transferrin receptor (TfR) 1 was induced in the curcumin-fed mice. These data suggest that long-term curcumin supplementation and a Western-type diet may aggravate iron deficiency.
Highlights
-
•
0.2% dietary curcumin for 6 months reduced iron stores in murine liver and spleen.
-
•
Curcumin chelated iron but not zinc and copper in vivo.
-
•
Liver hepcidin and ferritin expression was strongly suppressed in curcumin-fed mice.
-
•
Curcumin induced expression of hepatic iron transporters DMT1 and TfR1.
-
•
Curcumin did not affect hepatic and splenic inflammatory and oxidative markers.
Introduction
The phytochemical curcumin (diferuloylmethane) is found in the rhizome of Curcuma longa (family Zingiberaceae) and is responsible for the intense yellow pigmentation of turmeric and the curry blends prepared therewith [1]. Curcumin is used in traditional medicine to treat disorders such as anorexia, biliary complaints, cough, hepatic diseases, and sinusitis [2]. Many positive properties have been attributed to curcumin such as antioxidative, anti-proliferative, anti-inflammatory, and anti-amyloidogenic effects in vitro and in animal models [1], [3]. Therefore, interest in the use of curcumin as a potentially health-beneficial agent has been increasing.
Curcumin preparations have been “generally recognised as safe” (GRAS) by the FDA (http://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000460.pdf) and in patients with pre-malignant lesions, high-dose supplementation with 4–8 g/d for up to 3 months was without adverse effects [4]. In a recent cell culture experiment using endothelial cells, however, concentrations as low as 100 nmol/L induced mitotic catastrophe [5]. Another property of the phytochemical that may lead to potential adverse effects is its iron chelating activity [6], [7], [8], which is particularly effective for ferric ions [7]. Although it has been shown that meals spiced with turmeric (0.5 g/meal) did not affect iron absorption in women [9], long-term supplementation with higher concentrations of curcumin (2% by weight) induced systemic iron depletion and further aggravated iron deficiency symptoms in young mice when fed with diets containing low iron concentrations (5 mg/kg diet) [10]. In addition to chelating iron and thereby limiting iron release, curcumin has an anti-coagulant activity [11], [12] and may increase bleeding time [13], [14]. As internal bleeding or haemorrhaging can affect iron status, anti-coagulant activities of curcumin might also contribute to iron deficiency.
Native curcumin has very low bioavailability [15] and, hence, limited therapeutic efficacy. In many intervention studies, the phytochemical is consequently administered at gram doses [4], [16], [17]. If curcumin indeed impairs iron status, this high-dose supplementation may harbour potential risks that need to be studied in more detail. In order to explore whether long-term oral supplementation with curcumin affects iron stores, we fed female, 12 month old C57BL/6J mice with 0.2% dietary curcumin for 6 months and determined iron status and the expression of genes involved in iron homoeostasis at mRNA and protein levels.
Materials and methods
Mice and diet
The animal study was performed according to German animal welfare laws and regulations, and with permission from the responsible authorities. Thirty female, 12 month old C57BL/6J mice were purchased from Charles River Germany (Sulzfeld, Germany). The mice were housed in groups of 5 in macrolon cages under controlled environmental conditions of 55% relative humidity, 22-24 °C, and a 12-hour light-dark cycle. The mice were maintained on a semi-synthetic Western-type diet (C1000 mod., Altromin, Lage, Germany) containing 20% milk fat, 1.25% cholesterol, and 10% sugar. The concentrations of iron, zinc, and copper in the diet were 178, 28, and 5 mg/kg respectively. The mice were given free access to tap water. For the first 4 weeks, all mice were fed a Western-type diet without curcumin ad libitum to acclimatize them to the new diet. Subsequently, the mice were randomly assigned to two body weight-matched diet groups, namely the control and the curcumin-supplemented groups. Curcumin supplementation was at 0.2% (by weight) in the diet for 28 weeks, during which curcumin-supplemented mice were given ad libitum access to chow. Since food intake slightly decreased upon curcumin supplementation (most likely due to its bitter taste), the control mice were pair fed. We lost four mice in the control group and one mouse in the curcumin group during the supplementation period so that 11 and 14 mice were available for analyses in the control and curcumin groups, respectively. At the end of the trial, the mice were anaesthetised with carbon dioxide and killed by cervical dislocation. Liver and spleen were weighed, snap-frozen in liquid nitrogen, and stored at −80 °C until analysis. One part of the tissues was stored in RNAlater™ (Qiagen, Hilden, Germany) at −20 °C for RNA isolation and qRT-PCR analysis.
Measurement of trace elements in the liver and the spleen
Iron, zinc, and copper were determined using flame atomic absorption spectrometry (AAS, Thermo S2 AA System, Thermo Electron Corporation, Germany) as previously described [18]. Weighed liver and spleen samples were ashed and dissolved in 2.1 mol/L HNO3 prior to AAS analysis. Standard curves were determined with at least 4 different concentrations. Absorption wavelengths used for iron, zinc, and copper were 248.4 nm, 214 nm, and 324.8 nm respectively.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA of liver and spleen was isolated using the NucleoSpin® kit according to the manufacturer׳s protocol (Macherey & Nagel, Düren, Germany). RNA concentration was determined by measuring the absorbance at 260 nm on a spectrophotometer (Beckmann Instruments, Munich, Germany) and RNA purity was determined by calculating 260/280 nm and 260/230 nm ratios. RNA aliquots were then stored at −80 °C until qRT-PCR analysis. The qRT-PCR primers were designed using Primer3 Input software (version 0.4.0; Table 1) and purchased from Eurofins MWG (Ebersberg, Germany). One-step quantitative reverse transcriptase PCR was carried out with the SensiMix™ SYBR No-ROX one step kit (Bioline, Luckenwalde, Germany) and with SybrGreen detection using the Rotorgene 6000 cycler (Corbett Life Science, Sydney, Australia). An external standard curve was applied. The mRNA concentrations of the target genes were normalised for the mRNA concentrations of the respective housekeeping genes. The mean value of the relative mRNA expression in the control group was set to an arbitrary unit of 1 and that in the curcumin group is expressed as a multiple of the control value.
Table 1.
Nucleotide sequences (5׳-3׳) of murine primers used for the quantitative real-time PCR.
| Gene | Gene ID | Primer sequence forward | Primer sequence reverse |
|---|---|---|---|
| β-actin | 11461 | GACAGGATGCAGAAGAGATTACT | TGATCCACATCTGCTGGAAGGT |
| γGCS | 14629 | GTGGAGGCCAATATGAGGAA | GGGTGCTTGTTTATGGCTTC |
| DMT1 | 18174 | GAGCAGTGGCTGGATTTAAG | CGGTGACATACTTCAGCAAG |
| Ferritin L | 14325 | CTTCCAGGATGTGCAGAAG | ATCCAAGAGGGCCTGATT |
| FPN | 53945 | CAAACTACCTGACCTCAGCA | TCCACCAGAAACACAGACAC |
| GAPDH | 14433 | CCGCATCTTCTTGTGCAGT | GGCAACAATCTCCACTTTGC |
| HEPC | 84506 | GCACCACCTATCTCCATCA | GGGGAAGTTGGTGTCTCTC |
| HO1 | 15368 | GAGCCTGAATCGAGCAGAAC | AGCCTTCTCTGGACACCTGA |
| IL1β | 16176 | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| IL6 | 16193 | AGTTGCCTTCTTGGGACTGA | CAGAATTGCCATTGCACAAC |
| NQO1 | 18104 | TTCTCTGGCCGATTCAGAGT | TCCAGACGTTTCTTCCATCC |
| NRF2 | 18024 | GCAACTCCAGAAGGAACAGG | GCAATGTCTCTGCCAAAAGC |
| TfR1 | 22042 | AAGCCAGATCAGCATTCTCT | CGGCATTTTCTTCTTCATCT |
| TNFα | 21926 | TCGTAGCAAACCACCAAGTG | AGATAGCAAATCGGCTGACG |
Western blotting
Cytosolic fractions of liver tissue lysed in RIPA buffer were used to investigate ferritin light chain protein levels. Protein concentrations were determined with the BCA assay (BioRad, Munich, Germany) and 60 μg protein of each sample was mixed with loading buffer, denatured at 95 °C for 5 min, and separated on 4–20% MINI PROTEAN® TGX-Stain-Free™ gels (BioRad, Munich, Germany). Subsequently, proteins were activated by UV-exposure for 5 min and then transferred onto a PVDF membrane using the Trans Blot® Turbo™ System (BioRad, Munich, Germany). The membrane was blocked with 3% skim milk dissolved in TBS+0.05% Tween-20 (TBS/T) for at least 2 h and probed with a ferritin light chain (ab69090 Abcam, Cambridge, UK; 1:1000) antibody at 4 °C overnight. After incubation with a horseradish-peroxidase-conjugated secondary anti-rabbit antibody, the bands were visualised by using ECL reagent (Thermo Scientific, Schwerte, Germany) in a ChemiDoc XRS system (BioRad, Munich, Germany). Normalisation was carried out with reference to the total lane protein as determined using the stain-free technology by Bio-Rad.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (SPSS GmbH Software, Munich, Germany). Normal distribution of data was tested by Kolmogorov–Smirnov and Shapiro–Wilk tests and normally distributed data were analysed using Student׳s t-test. When data were not normally distributed, a Mann–Whitney U test was performed. Results are presented as means+SEM and P-values<0.05 were considered statistically significant.
Results
Long-term feeding with 0.2% curcumin does not affect body weight development but induces splenomegaly in C57BL/6J mice
The weight of the mice in the control group and those receiving oral supplementation of 0.2% curcumin over 28 weeks did not differ significantly (Fig. 1). The 12 month old mice had an average body weight of 28.2±0.4 g at the beginning of the supplementation period and had an average body weight of 37.1±1.0 g at the end of the study. Despite similar weight development curves between control and curcumin-supplemented mice, we observed an enlargement of the spleen (P<0.05) in the curcumin group (Fig. 2). On average, spleen mass of the curcumin-supplemented mice was 40% higher (Fig. 2a) than that of the control mice. In 4 out of 14 curcumin-fed mice, however, we noted splenomegaly with spleen mass of more than double the average of the control group. The mass of the liver (Fig. 2b) and other organs such as lung, heart, and kidney (data not shown) was also measured, but did not differ significantly between the groups. The livers of our mice showed signs of hepatic steatosis known to be caused by high-fat diet [19].
Fig. 1.
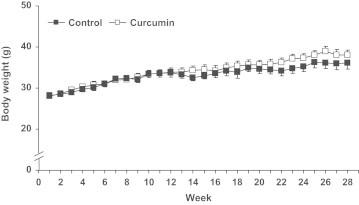
Weight development of control and curcumin-supplemented C57BL/6J mice over the 28 week supplementation period did not show significant differences between control and curcumin-supplemented mice. Values are expressed as means+SEM.
Fig. 2.
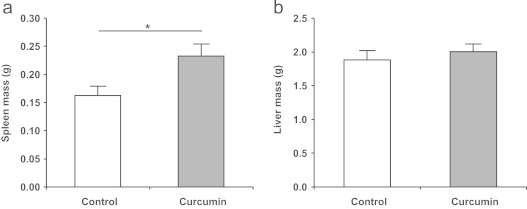
The mass of (a) spleen and (b) liver of control and curcumin-supplemented C57BL/6J mice after the 28 week supplementation period. Curcumin-supplemented mice had significantly higher spleen mass compared to control mice. No significant differences could be observed for liver mass. Values are expressed as means+SEM. ⁎P<0.05.
Curcumin supplementation reduces iron stores in the liver and spleen but does not affect zinc and copper content in either tissue
Iron stores in the liver and spleen were significantly (P<0.001 and P<0.05, respectively) reduced by ca. 50% in the curcumin-fed compared to the control mice (Fig. 3a and b). Tissue concentrations of zinc and copper were determined in the liver and spleen because these elements are known to interact with iron during absorption and trafficking [20], [21]. Furthermore, curcumin has been reported as chelating zinc and copper [22], [23]. In the liver, neither zinc nor copper stores were affected by curcumin supplementation (Fig. 3c and e). Zinc concentration in the spleen did not differ between control and curcumin-supplemented mice (Fig. 3d) and copper concentrations in the spleen were below the limit of detection (2 mg/kg) in both groups.
Fig. 3.
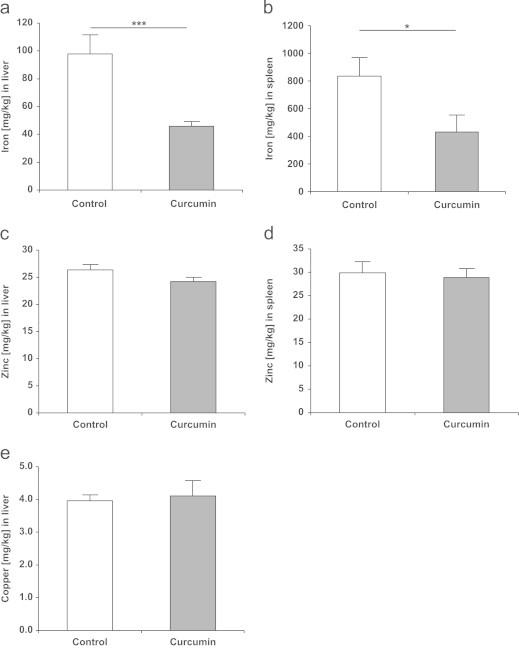
(a) The iron concentration in the liver, (b) iron concentration in the spleen, (c) zinc concentration in the liver, (d) zinc concentration in the spleen, and (e) copper concentration in the liver of control and curcumin-supplemented C57BL/6J mice. The concentrations of trace elements are expressed in mg per kg tissue (wet mass). Iron concentration was significantly lower in curcumin-supplemented mice in both the liver and the spleen. The concentrations of zinc and copper were not significantly regulated. Copper levels in the spleen were below the level of detection which was ~2 mg/kg. Values are expressed as means+SEM. ⁎P<0.05, ⁎⁎⁎P<0.001.
Curcumin supplementation reduced mRNA expression and protein expression of the iron storage protein ferritin in the liver
Relative mRNA of ferritin light (L) chain 1, one of the two subunits of the intracellular iron storage protein ferritin, was significantly down-regulated (P<0.05) by dietary curcumin (Fig. 4a). The expression of ferritin heavy (H) chain 1 mRNA did not differ between the groups (data not shown). Furthermore, the protein concentrations of ferritin L were significantly reduced in the liver of curcumin-fed mice (P<0.001; Fig. 4b).
Fig. 4.
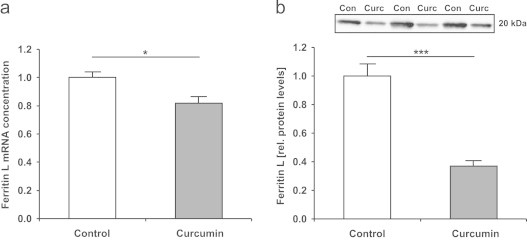
(a) Ferritin light (L) chain 1 mRNA levels in the liver of control and curcumin-supplemented C57BL/6J mice. Relative mRNA concentration was assessed using qRT-PCR and related to the average of two housekeeping genes (β-actin and GAPDH). Mean ferritin L concentration in the control group was set to be 1. The mRNA expression of ferritin L was significantly lower in curcumin-supplemented mice. Values are expressed as means+SEM. ⁎P<0.05. (b) Relative protein levels of ferritin L in the liver of control and curcumin-supplemented C57BL/6J mice as determined by Western blotting of the cytosolic fraction. Relative intensities of bands were quantified by densitometry and total lane protein was used as a loading control. Mean band intensity in the control group was set to be 1. Relative protein levels of ferritin L were significantly lower in curcumin-supplemented mice. Con, control group; Curc, curcumin group. Values are expressed as means+SEM. ⁎⁎⁎P<0.001.
Curcumin supplementation reduced mRNA expression of iron regulatory protein hepcidin and altered mRNA expression of iron transport proteins in the liver
In the liver of curcumin-supplemented mice, we observed a 65% reduction in hepcidin mRNA levels in comparison to the controls (P<0.01; Fig. 5a), reflecting the depleted endogenous iron stores in the curcumin-supplemented mice. Ferroportin (FPN), a known receptor of hepcidin [24], was not significantly regulated at the transcriptional level (Fig. 5b). The mRNA of two iron transporters: divalent metal transporter (DMT) 1 and transferrin receptor (TfR) 1 (Fig. 5c and d) was also significantly up-regulated in response to curcumin supplementation.
Fig. 5.
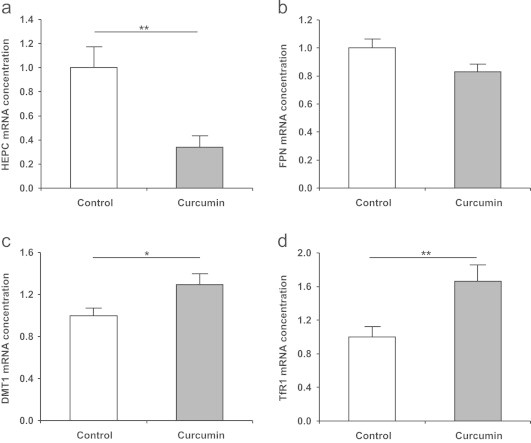
Expression of (a) hepcidin (HEPC), (b) ferroportin (FPN), (c) divalent metal transporter (DMT) 1, and (d) transferrin receptor (TfR) 1 mRNA in the liver of control and curcumin-supplemented C57BL/6J mice. Relative mRNA concentration was assessed using qRT-PCR and related to the average of two housekeeping genes (β-actin and GAPDH). The mean expression levels of genes in the control group were set to be 1. The mRNA expression of hepcidin was significantly lower in curcumin-supplemented mice, while the expressions of iron transport proteins DMT1 and TfR1 were significantly increased. FPN was not significantly regulated. Values are expressed as means+SEM. ⁎P<0.05, ⁎⁎P<0.01.
Inflammatory markers in the liver and the spleen were not regulated by curcumin supplementation in mice
The mRNA expression of pro-inflammatory markers, the tumour necrosis factor α (TNFα), interleukin (IL) 1β, and IL6 in the liver and the spleen did not differ between the groups (Table 2), indicating that the observed splenomegaly may not be related to increased systemic inflammation. In contrast to reports found in the literature [25], [26], [27] and despite the long-term supplementation, curcumin did not exert anti-inflammatory effects in our mouse study.
Table 2.
Expression of tumour necrosis factor α (TNFα), interleukin 1β (IL1β), and 6 (IL6) mRNA in the liver and spleen; and of nuclear factor (erythroid-derived 2)-like 2 (NRF2), haem oxygenase 1 (HO1), NAD(P)H quinone oxidoreductase (NQO1), and γ-glutamyl cysteine synthetase (γ-GCS) heavy chain mRNA in the liver of control and curcumin supplemented C57BL/6J mice. Relative mRNA concentration was assessed using qRT-PCR and related to the average of two housekeeping genes (β-actin and GAPDH) in both tissues. The mean expression levels of genes in the control group were set to be 1. No significant differences between the groups were observed. Values are expressed as means±SEM.
| Control | Curcumin | ||
|---|---|---|---|
| Liver | TNFα | 1.00±0.14 | 1.11±0.07 |
| IL1β | 1.00±0.12 | 1.29±0.18 | |
| IL6 | 1.00±0.11 | 1.02±0.10 | |
| NRF2 | 1.00±0.09 | 1.12±0.08 | |
| HO1 | 1.00±0.12 | 1.17±0.10 | |
| NQO1 | 1.00±0.11 | 1.02±0.10 | |
| γ-GCS | 1.00±0.14 | 0.89±0.09 | |
| Spleen | TNFα | 1.00±0.06 | 1.16±0.09 |
| IL1β | 1.00±0.08 | 0.94±0.13 | |
| IL6 | 1.00±0.05 | 0.86±0.08 |
Curcumin supplementation did not induce nuclear factor (erythroid-derived 2)-like 2 (NRF2) and NRF2-inducible enzymes in the liver
Curcumin has often been reported in the literature as inducing NRF2 and particularly NRF2-dependent enzyme haem oxygenase (HO) 1 [28], [29], [30]. Therefore, we measured NRF2 and HO1, as well as two other NRF2-dependent enzymes, NAD(P)H quinone oxidoreductase (NQO1) and γ-glutamyl cysteine synthetase (γ-GCS) heavy chain in the liver (Table 2). The mRNA expressions of all four genes were not significantly different between the curcumin-supplemented and control groups.
Discussion
Our study aimed at investigating the effects of a 6 month 0.2% dietary curcumin supplementation on iron status and iron transport proteins in the liver and the spleen of adult mice. As the iron concentrations in the liver and the spleen of our curcumin-fed mice were significantly reduced compared to the controls, the present study shows that long-term oral supplementation with curcumin may promote iron deficiency even under conditions of sufficient iron intake. Consistent with depleted hepatic iron stores in our curcumin-supplemented mice, mRNA and protein levels of the L subunit of the iron storage protein ferritin were reduced in the liver. In partial agreement with our results, liver ferritin was decreased in mice after a 12 week 2% curcuminoids (1.5% curcumin) dietary supplementation, but not in mice fed 0.5% curcuminoids (0.4% curcumin) [31]. Additionally, in a model of iron depletion (5 mg iron/kg diet), 2% curcumin supplementation for 6 months induced alterations in haematological parameters, reduced iron stores in the spleen, and reduced ferritin concentrations in the liver. However, contrary to the observations made in our study, none of these parameters were changed in mice fed iron at ≥12 mg/kg diet, and lower curcumin doses including the dose we used (0.2% curcumin) had no adverse effects on iron status in these mice [10]. On the other hand, the mice used in this study were only 5 weeks old at the beginning of the experiment and may therefore have had different dietary iron requirements compared to our mice which were 12 months old.
In our model, middle-aged mice were fed a Western-type diet (20% fat and 10% sugar) to simulate human dietary habits, where curcumin supplements are often taken for potential health promoting effects. Supplement users may have multiple disease-risk factors, including advanced age, obesity, and unbalanced diets rich in fat and sugars. Interestingly, a high-fat diet has been suggested to promote iron deficiency by reducing duodenal iron absorption and by inducing inflammation [32], [33]. Although our mice were supplied with adequate dietary iron, curcumin supplementation markedly depleted iron stores in the presence of the Western-type diet (Fig. 3).
To provide further evidence of the observed iron deficiency, hepcidin mRNA expression was measured in the liver. Hepcidin is an antimicrobial peptide that is synthesised in the liver and is involved in the regulation of iron homoeostasis by inhibiting the iron exporter FPN [24]. Hepcidin is a reliable marker that reflects iron requirements of the body as its expression is induced when iron stores are high and suppressed when iron stores are low [34]. However, hepcidin expression can also be altered during inflammation, erythropoiesis, and hypoxia [35]. In our study, the expression of hepcidin was significantly reduced in the liver of curcumin-fed mice along with depleted iron stores in the liver and the spleen. As the expression of inflammatory markers did not differ between control and curcumin-supplemented mice in our study, a possible effect of inflammation on hepcidin expression may be excluded.
FPN is the only known mammalian iron exporter and the main receptor for hepcidin [36], but its hepatic mRNA levels did not differ between groups in our study (Fig. 5b). Although transcriptional regulation of FPN in hepatocytes by hepcidin has been shown [37], hepcidin mainly stimulates the degradation of FPN protein [24], thereby modulating its activity. Additionally, curcumin supplementation induced the expression of the two hepatic iron importers DMT1 and TfR1 (Fig. 5c and d).
Curcumin has been described as possessing anti-inflammatory properties [25], [26], [27] and as an inducer of NRF2 and therefore NRF2-controlled genes, such as HO1 [28], [29], [30]. In our study, however, curcumin did not significantly regulate the pro-inflammatory markers TNFα, IL1β, and IL6 in the liver and the spleen (Table 2). Likewise, curcumin had no effect on NRF2, and the NRF2-dependent enzymes HO1, NQO1, and γGCS in the liver (Table 2). These findings are in agreement with our observations in the hearts of SAMP8 mice fed curcumin for 5 months [38], [39], but are in contrast to cell culture [40] and mouse studies [41] that suggest NRF2-induction by curcumin. A reason for the absence of NRF2-induction by curcumin in our mice could be its low oral bioavailability and subsequent low hepatic concentrations. Total curcumin concentrations in the liver of our mice (data not shown) were below the limit of detection of our analytical method [15]. Nevertheless, given the observed effects on iron status, it seems possible that the relatively high concentrations of unabsorbed curcumin in the intestinal tract may have exerted local biological effects. The β-diketone moiety of the curcumin molecule has been shown to be the active site involved in the chelation of iron, suggesting the formation of curcumin:iron complex species in the ratio of 1:1 [6]. The molar ratio of curcumin to iron in the diet used in our experiments was 1.7–1. Thus, based on the iron-chelating properties of curcumin [6], the curcumin present in our diet may have been sufficient to chelate most of the iron in the diet and might thereby have induced the iron deficiency observed in our mice. It should also be kept in mind that anti-coagulant activities of curcumin [11], [12], which may lead to gastrointestinal bleeding, could not be excluded in our study and might be an alternative mechanism that may have contributed to the observed iron deficiency in our mice.
We further observed a 40% increase in spleen mass in the curcumin-supplemented mice, which was not associated with increased inflammation in the spleen, since the expression of pro-inflammatory cytokines TNFα, IL1β, and IL6 was not induced (Table 2). The spleen is one of the most sensitive organs with regard to nutritional iron deficiency [42]. It has previously been shown that iron deficiency can increase spleen mass in mice [43], [44]. A possible explanation for iron deficiency-induced increase in spleen mass could be the sequestering of an increased number of abnormal and damaged erythrocytes, resulting from iron deficiency, by the reticuloendothelial system in the spleen [45]. In our previous studies, however, we did not observe spleen enlargement when 0.05% curcumin was fed for 5 months as part of a Western-type diet to 2 month old SAMP8 mice [38], [39], [46] or at higher concentrations of 0.4% in a standard diet for 4 weeks to rats [47]. On the other hand, mice infected with Plasmodium berghei developed splenomegaly, which could be reversed by treatment with a single injection of arteether combined with oral doses of 5 mg curcumin per day for 3 days [48]. Therefore, it remains uncertain if the enlargement of the spleen observed in this experiment was caused by the curcumin induced iron deficiency. It is further unclear whether an unknown interaction between curcumin and environmental factors or the Western-type diet, which was used, could have contributed to this observation. Further experiments are required to confirm or refute the curcumin-induced enlargement of the spleen observed in our mice.
In conclusion, the presented data suggest a potentially adverse biological activity of high-dose curcumin supplementation that might lead to impaired iron absorption and status. It may hence be worthwhile monitoring the iron status in subjects participating in clinical trials during which high doses of native curcumin are administered. Since complexation of iron might be the underlying mechanism, encapsulation of curcumin (e.g., incorporation into micelles or liposomes) may help to reduce its direct interaction with transition metals and thereby their chelation. Micelles and liposomes have been used as vehicles to improve the bioavailability of curcumin [49], [50], [51] and provide the additional advantage that lower oral doses can be administered, which would lead to a smaller number of potential curcumin:iron complexes and thus a reduced potential for adverse effects on iron status.
Acknowledgements
We are grateful to the German Federal Ministry of Education and Research (BMBF) for financial support (grant # 0315679).
References
- 1.Esatbeyoglu T., Huebbe P., Ernst I.M., Chin D., Wagner A.E., Rimbach G. Curcumin—from molecule to biological function. Angew. Chem. Int. Ed. Engl. 2012;51:5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 2.Eigner D., Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 3.Chin D., Huebbe P., Pallauf K., Rimbach G. Neuroprotective properties of curcumin in Alzheimer׳s disease—merits and limitations. Curr. Med. Chem. 2013;20:3955–3985. doi: 10.2174/09298673113209990210. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 5.Jackson S.J., Murphy L.L., Venema R.C., Singletary K.W., Young A.J. Curcumin binds tubulin, induces mitotic catastrophe, and impedes normal endothelial cell proliferation. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;60C:431–438. doi: 10.1016/j.fct.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borsari M., Ferrari E., Grandi R., Saladini M. Curcuminoids as potential new iron-chelating agents: spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorg. Chim. Acta. 2002;328:61–68. [Google Scholar]
- 7.Bernabe-Pineda M., Ramirez-Silva M.T., Romero-Romo M.A., Gonzalez-Vergara E., Rojas-Hernandez A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin–Fe(III)–water and Curcumin–Fe(II)–water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004;60:1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- 8.Dairam A., Fogel R., Daya S., Limson J.L. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. J. Agric. Food Chem. 2008;56:3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 9.Tuntipopipat S., Judprasong K., Zeder C., Wasantwisut E., Winichagoon P., Charoenkiatkul S. Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J. Nutr. 2006;136:2970–2974. doi: 10.1093/jn/136.12.2970. [DOI] [PubMed] [Google Scholar]
- 10.Jiao Y., Wilkinson J.T., Di X., Wang W., Hatcher H., Kock N.D. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113:462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava K.C., Bordia A., Verma S.K. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fatty Acids. 1995;52:223–227. doi: 10.1016/0952-3278(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 12.Shah B.H., Nawaz Z., Pertani S.A., Roomi A., Mahmood H., Saeed S.A. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999;58:1167–1172. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 13.Prakash P., Misra A., Surin W.R., Jain M., Bhatta R.S., Pal R. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb. Res. 2011;127:111–118. doi: 10.1016/j.thromres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim D.C., Ku S.K., Bae J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012;45:221–226. doi: 10.5483/bmbrep.2012.45.4.221. [DOI] [PubMed] [Google Scholar]
- 15.Schiborr C., Eckert G.P., Rimbach G., Frank J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2010;397:1917–1925. doi: 10.1007/s00216-010-3719-3. [DOI] [PubMed] [Google Scholar]
- 16.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M. Oral curcumin for Alzheimer׳s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S.C., Kismali G., Aggarwal B.B. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 18.Siebert F., Luhken G., Pallauf J., Erhardt G. Mutation in porcine Zip4-like zinc transporter is associated with pancreatic zinc concentration and apparent zinc absorption. Br. J. Nutr. 2013;109:969–976. doi: 10.1017/S0007114512002772. [DOI] [PubMed] [Google Scholar]
- 19.Lieber C.S., Leo M.A., Mak K.M., Xu Y., Cao Q., Ren C. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 20.Lonnerdal B. Dietary factors influencing zinc absorption. J. Nutr. 2000;130:1378S–1383SS. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 21.Matak P., Zumerle S., Mastrogiannaki M., El Balkhi S., Delga S., Mathieu J.R. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2alpha and altered expression of iron absorption genes in mice. PLoS One. 2013;8:e59538. doi: 10.1371/journal.pone.0059538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baum L., Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer׳s disease animal models. J. Alzheimers Dis. 2004;6:367–377. doi: 10.3233/jad-2004-6403. (discussion 443-9) [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Golub L.M., Johnson F., Wishnia A. pKa zinc- and serum albumin-binding of curcumin and two novel biologically-active chemically-modified curcumins. Curr. Med. Chem. 2012;19:4367–4375. doi: 10.2174/092986712802884240. [DOI] [PubMed] [Google Scholar]
- 24.Nemeth E., Tuttle M.S., Powelson J., Vaughn M.B., Donovan A., Ward D.M. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 25.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begum A.N., Jones M.R., Lim G.P., Morihara T., Kim P., Heath D.D. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer׳s disease. J. Pharmacol. Exp. Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013;169:1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 29.Scapagnini G., Colombrita C., Amadio M., D׳Agata V., Arcelli E., Sapienza M. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid. Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 30.McNally S.J., Harrison E.M., Ross J.A., Garden O.J., Wigmore S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 31.Jiao Y., Wilkinson J.T., Christine Pietsch E., Buss J.L., Wang W., Planalp R. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Chung J., Kim M.S., Han S.N. Diet-induced obesity leads to decreased hepatic iron storage in mice. Nutr. Res. 2011;31:915–921. doi: 10.1016/j.nutres.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Sonnweber T., Ress C., Nairz M., Theurl I., Schroll A., Murphy A.T. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. J. Nutr. Biochem. 2012;23:1600–1608. doi: 10.1016/j.jnutbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Pigeon C., Ilyin G., Courselaud B., Leroyer P., Turlin B., Brissot P. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 35.Nemeth E. Iron regulation and erythropoiesis. Curr. Opin. Hematol. 2008;15:169–175. doi: 10.1097/MOH.0b013e3282f73335. [DOI] [PubMed] [Google Scholar]
- 36.Ward D.M., Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim. Biophys. Acta. 2012;1823:1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramey G., Deschemin J.C., Durel B., Canonne-Hergaux F., Nicolas G., Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95:501–504. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiborr C., Eckert G.P., Weissenberger J., Muller W.E., Schwamm D., Grune T. Cardiac oxidative stress and inflammation are similar in SAMP8 and SAMR1 mice and unaltered by curcumin and Ginkgo biloba extract intake. Curr. Pharm. Biotechnol. 2010;11:861–867. doi: 10.2174/138920110793262006. [DOI] [PubMed] [Google Scholar]
- 39.Schiborr C., Schwamm D., Kocher A., Rimbach G., Eckert G.P., Frank J. The senescence-accelerated mouse-prone 8 is not a suitable model for the investigation of cardiac inflammation and oxidative stress and their modulation by dietary phytochemicals. Pharmacol. Res.: Off. J. Ital. Pharmacol. Soc. 2013;74:113–120. doi: 10.1016/j.phrs.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Nishinaka T., Ichijo Y., Ito M., Kimura M., Katsuyama M., Iwata K. Curcumin activates human glutathione S-transferase P1 expression through antioxidant response element. Toxicol. Lett. 2007;170:238–247. doi: 10.1016/j.toxlet.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Garg R., Gupta S., Maru G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 42.Gibson J.N., Jellen L.C., Unger E.L., Morahan G., Mehta M., Earley C.J. Genetic analysis of iron-deficiency effects on the mouse spleen. Mamm. Genome. 2011;22:556–562. doi: 10.1007/s00335-011-9344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helyar L., Sherman A.R. Moderate and severe iron-deficiency lowers numbers of spleen lymphocyte-T and lymphocyte-B subsets in the C57/B16 mouse. Nutr. Res. 1992;12:1113–1122. [Google Scholar]
- 44.Kuvibidila S.R., Velez M., Gardner R., Penugonda K., Chandra L.C., Yu L. Iron deficiency reduces serum and in vitro secretion of interleukin-4 in mice independent of altered spleen cell proliferation. Nutr. Res. 2012;32:107–115. doi: 10.1016/j.nutres.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Gardenghi S., Grady R.W., Rivella S. Anemia, ineffective erythropoiesis, and hepcidin: interacting factors in abnormal iron metabolism leading to iron overload in beta-thalassemia. Hematol. Oncol. Clin. North Am. 2010;24:1089–1107. doi: 10.1016/j.hoc.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckert G.P., Schiborr C., Hagl S., Abdel-Kader R., Muller W.E., Rimbach G. Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 2013;62:595–602. doi: 10.1016/j.neuint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Kamal-Eldin A., Frank J., Razdan A., Tengblad S., Basu S., Vessby B. Effects of dietary phenolic compounds on tocopherol, cholesterol, and fatty acids in rats. Lipids. 2000;35:427–435. doi: 10.1007/s11745-000-541-y. [DOI] [PubMed] [Google Scholar]
- 48.Vathsala P.G., Dende C., Nagaraj V.A., Bhattacharya D., Das G., Rangarajan P.N. Curcumin-arteether combination therapy of Plasmodium berghei-infected mice prevents recrudescence through immunomodulation. PLoS One. 2012;7:e29442. doi: 10.1371/journal.pone.0029442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kakkar V., Singh S., Singla D., Sahwney S., Chauhan A.S., Singh G. Pharmacokinetic applicability of a validated liquid chromatography tandem mass spectroscopy method for orally administered curcumin loaded solid lipid nanoparticles to rats. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010;878:3427–3431. doi: 10.1016/j.jchromb.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Dadhaniya P., Patel C., Muchhara J., Bhadja N., Mathuria N., Vachhani K. Safety assessment of a solid lipid curcumin particle preparation: acute and subchronic toxicity studies. Food Chem. Toxicol.: Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011;49:1834–1842. doi: 10.1016/j.fct.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Schiborr C., Kocher A., Behnam D., Jandasek J., Toelstede S., Frank J. The oral bioavailability of curcumin from micronized poweder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014;58:516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]


