Abstract
Introduction
The aim of this study was to assess the effects of preoperative pulmonary rehabilitation (PPR) on preoperative clinical status changes in patients with chronic obstructive pulmonary disease (COPD) and non-small cell lung cancer (NSCLC), and net effects of PPR and cancer resection on residual pulmonary function and functional capacity.
Material and methods
This prospective single group study included 83 COPD patients (62 ±8 years, 85% males, FEV1 = 1844 ±618 ml, Tiffeneau index = 54 ±9%) with NSCLC, on 2–4-week PPR, before resection. Pulmonary function, and functional and symptom status were evaluated by spirometry, 6-minute walking distance (6MWD) and Borg scale, on admission, after PPR and after surgery.
Results
Following PPR significant improvement was registered in the majority of spirometry parameters (FEV1 by 374 ml, p < 0.001; VLC by 407 ml, p < 0.001; FEF50 by 3%, p = 0.003), 6MWD (for 56 m, p < 0.001) and dyspnoeal symptoms (by 1.0 Borg unit, p < 0.001). A positive correlation was identified between preoperative increments of FEV1 and 6MWD (r s = 0.503, p = 0.001). Negative correlations were found between basal FEV1 and its percentage increment (r s = –0.479, p = 0.001) and between basal 6MWD and its percentage change (r s = –0.603, p < 0.001) during PPR. Compared to basal values, after resection a significant reduction of most spirometry parameters and 6MWD were recorded, while Tiffeneau index, FEF25 and dyspnoea severity remained stable (p = NS).
Conclusions
Preoperative pulmonary rehabilitation significantly enhances clinical status of COPD patients before NSCLC resection. Preoperative increase of exercise tolerance was the result of pulmonary function improvement during PPR. The beneficial effects of PPR were most emphasized in patients with initially the worst pulmonary function and the weakest functional capacity.
Keywords: lung cancer, lung cancer surgery, lung resection, pulmonary rehabilitation, preoperative physiotherapy
Introduction
Coexistence of primary lung cancer and obstructive lung disease is well known, since almost 2/3 males and 1/2 females with newly diagnosed primary lung cancer suffer from chronic obstructive pulmonary disease (COPD) [1, 2]. Resection is currently the most efficient treatment for primary lung cancer. However, COPD presence adds a considerable risk of perioperative pulmonary complications, while serious damage of lung function may render potentially resectable cancer inoperable [3–6].
In COPD and primary lung cancer, resection can have complex effects on residual pulmonary function and result in its deterioration due to the loss of functioning parts of lungs, or vice versa, improvement by eliminating non-functional emphysematous lung areas, especially after upper lobectomy [7, 8]. Preoperative pulmonary rehabilitation (PPR) may improve exercise tolerance, symptomatic status and quality of life in COPD patients subjected to lung volume reduction surgery or lung transplantation [9]. However, currently the contribution of PPR to clinical status of COPD patients undergoing resection of non-small cell lung cancer (NSCLC) is not quite elucidated. Although it was demonstrated that PPR may improve preoperative pulmonary function and functional status, reduce the perioperative pulmonary complication rate and shorten postoperative recovery, the results of the studies were not aligned and the programme of preoperative rehabilitation in the prevailing clinical environment is not standardized due to different protocols in use [10–17]. In practice, many centres do not apply routine PPR in such clinical circumstances due to certain concerns related to postponement of surgical resection and lack of solid evidence related to the benefits of PPR in those patients.
The aims of this study were to determine: (1) effects of PPR on preoperative change in pulmonary function and clinical status of COPD patients with NSCLC, and (2) net effects of PPR and resection of lung cancer on residual postoperative pulmonary function, functional capacity and symptomatic status of such patients.
Material and methods
This prospective, observational, single group study was carried out between January 2007 and December 2009 in the Clinic for Physical Medicine and Rehabilitation, and the Clinic for Thoracic Surgery of the Clinical Centre of Serbia in Belgrade. The study was approved by the ethical committee of the institution and all the patients signed informed written consent. The study included 83 consecutive patients (mean age: 62 ±8 years, 85% male) who met the following criteria: (1) histological diagnosis on NSCLC, (2) associated COPD (Tiffeneau index ≤ 70%), and (3) lung resection performed by open thoracotomy. Table I shows patients’ characteristics on admission.
Table I.
Baseline patients’ characteristics
| Parameter | Results |
|---|---|
| No. of patients | 83 |
| Age [years] | 62 ±8 |
| Gender (male/female) | 71 (85%)/12 (15%) |
| BMI category I/II/III/IV | 24 (29%)/41 (49%)/14 (17%)/4 (5%) |
| Smokers (current/former) | 29 (35%)/42 (51%) |
| COPD stage I/II/III/IV | 14 (17%)/58 (70%)/11 (13%)/0 |
| Extrapulmonary comorbidities | 77 (93%) |
| Hypertension | 38 (46%) |
| Cardiac arrhythmia | 3 (4%) |
| Cardiomyopathy | 6 (7%) |
| Coronary disease | 29 (35%) |
| Diabetes mellitus | 6 (7%) |
| Gastrointestinal disorder | 13 (16%) |
| Previous extrapulm. Malignancy | 5 (6%) |
| Cancer histology | |
| Squamous cell carcinoma | 59 (71%) |
| Adenocarcinoma | 21 (25%) |
| Adenosquamous cell carcinoma | 3 (4%) |
| Cancer stage I/II/III/IV | 17 (20%)/57 (69%)/7 (9%)/2 (2%) |
| Operation procedure | |
| Pneumonectomy | 18 (22%) |
| Sleeve lobectomy | 7 (8%) |
| Bilobectomy | 7 (8%) |
| Lobectomy | 48 (58%) |
| Segmentectomy | 3 (4%) |
BMI – body mass index, BMI categories I–IV: I – underweight (< 18.5 kg/m2), II – normal weight (18.5–24.9 kg/m2), III – overweight (25–29.9 kg/m2), IV – obese (≥ 30 kg/m2), COPD – chronic obstructive pulmonary disease
Preoperative diagnostics and evaluation of local and systemic spread of malignity were done by conventional chest radiography and computed tomography of the thorax, abdomen and head, and by endoscopic bronchoscopy with biopsy and histological analysis of tumour tissue.
On admission, pulmonary function was assessed at rest by spirometry, COPD staging by GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria (stage I – mild COPD: forced expiratory volume in the 1st second (FEV1) ≥ 80%; stage II – moderate COPD: 50% ≤ FEV1 < 80%; stage III – severe COPD: 30% ≤ FEV1 < 50%; and stage IV – very severe COPD: FEV1 < 30%) [18]. Assessment of patients’ functional capacity (i.e. effort tolerance) was conducted by 6-minute walking test (6MWT) according to the recommendations of the American Thoracic Society [19]. Immediately prior to and after 6MWT respiratory and heart rates were measured, while oxygen blood saturation was determined by pulse oximetry. Degree of dyspnoea was assessed on the modified Borg scale and by the visual analogue scale (VAS): the patient was asked to grade the dyspnoea degree on the mentioned scales while at rest (before 6MWT) and after physical exercise (immediately after 6MWT) [20].
Following the initial evaluation, patients started preoperative preparations including: (1) intravenous bronchodilator therapy with theophylline 12.5–15 mg twice a day, without corticosteroids; (2) measures of pulmonary rehabilitation; and (3) measures of general rehabilitation and physiotherapy.
Preoperative rehabilitation lasted 2–4 weeks (5 days a week) with three daily sessions of 45 min. Preoperative pulmonary rehabilitation included aerosol therapy by bronchodilators (solution 0.5 ml salbutamol/3 ml 0.9% NaCl) through an inhalator (jet nebuliser) under 5 kPa pressure from a central O2 supply or mixed air. Aerosol therapy took approximately 10 min. During that time the patient resorted to diaphragmatic breathing under the instructions and supervision of a physiotherapist. In addition, exercises for chest expansion and shoulder girdle mobilization were applied. They were done in front of a mirror, under the physiotherapist's supervision, 10 times per series. In the second week the exercises were carried out under the loading of 1 kg by means of elastic bands, in two series – 10 times each. Finally, preoperative preparation included education and training of patients for exercise in early postoperative pulmonary rehabilitation.
Following the completion of preoperative rehabilitation (immediately prior to surgery) spirometry, 6MWT and evaluation of symptomatic status were repeated – identical as on admission.
After lung resection, postoperative rehabilitation started as early as the first postoperative day, in the intensive care unit (ICU). First, diaphragmatic breathing exercises, peripheral circulation exercises, aerosol therapy with bronchodilators and exercises for chest expansion and shoulder girdle mobilization were introduced. On the first postoperative day the exercises were conducted at the bed level with 75% elevation at the back; on the second day the same exercises were applied in a sitting position at the edge of the bed, while the patients began to walk across the room with the help of a physiotherapist on the third postoperative day and later. Throughout their stay in the ICU, the regimen was repeated 3–4 times a day. Once outside the ICU, the patients were rehabilitated twice a day.
After discharge, patients were clinically followed up and within one month after lung resection, spirometry, 6MWT and evaluation of symptom status were done, according to the same protocol.
Statistical analysis
Descriptive statistics are presented as mean ± SD or mean, with 95% confidence interval (CI) for continuous variables and numbers with percentages for categorical variables. Normal distribution of all continuous variables was tested using the Shapiro-Wilk test. In the case of normally distributed variables, comparisons between different time points (baseline values, after PPR and values after surgery) were performed by one-way repeated measures ANOVA followed by the Bonferroni test. Otherwise, the Friedman test was used with post hoc analysis by the Wilcoxon signed rank test (with Holm-Bonferroni correction for significance level). Comparisons between different subgroups of patients were tested using the Kruskal-Wallis test with post-hoc Mann-Whitney U test (Holm-Bonferroni correction for significance level).
For determination of the correlation between variables, the Pearson correlation test (normal distribution) or Spearman rank correlation test (asymmetrical distribution) was used. Statistical association of spirometric or functional parameter increments with cases of lethal outcome or perioperative complications was examined by univariate logistic regression. A two-tailed p-value of < 0.05 was considered statistically significant. Data were analysed using SPSS for Windows, version 17.0.
Results
Following PPR a significant increase in the majority of pulmonary function parameters was found, including FEV1 (mean increment by 374 ml, p < 0.001), vital lung capacity (VLC) (by 407 ml, p < 0.001), and forced expiratory flow (FEF50) (by 2%, p = 0.006), while the change in FEF25 was insignificant (p = NS), as presented in detail in Table II.
Table II.
Change in pulmonary function after preoperative rehabilitation and after lung resection (n = 83)
| Parameter | Before PPR | After PPR | After resection | Mean difference (after PPR-before PPR) | Mean difference (after resection-after PPR) | Mean difference (after resection-before PPR) | |||
|---|---|---|---|---|---|---|---|---|---|
| n (95% CI) | p | n (95% CI) | p | n (95% CI) | p | ||||
| FEV1 [ml] | 1844 ±618 | 2217 ±654 | 1612 ±580 | 374 (261, 486) | < 0.001 | –605 (–703, –507) | < 0.001 | –231 (–327, –136) | < 0.001 |
| FEV1 pred% | 65 ±14 | 74 ±15 | 54 ±13 | 12 (9, 15) | < 0.001 | –20 (–23, –17) | < 0.001 | –10 (–13, –7) | < 0.001 |
| VLC [ml] | 3267 ±895 | 3674 ±909 | 2803 ±934 | 407 (264, 549) | < 0.001 | –871 (–1030, –711) | < 0.001 | –464 (–646, –281) | < 0.001 |
| VLC pred% | 88 ±17 | 100 ±16 | 76 ±17 | 12 (9, 16) | < 0.001 | –25 (–28, –21) | < 0.001 | –12 (–17, –7) | < 0.001 |
| TI (%) | 54 ±9 | 59 ±10 | 55 ±10 | 5 (2, 7) | < 0.001 | –5 (–7, –2) | < 0.001 | 0 (–2, 3) | NS |
| FEF50 (%) | 26 ±13 | 30 ±14 | 20 ±11 | 3 (–1, 8) | 0.003 | –10 (–15, –5) | < 0.001 | –7 (–11, –2) | 0.002 |
| FEF25 (%) | 23 ±14 | 29 ±14 | 19 ±12 | 6 (0, 11) | NS | –10 (–15, –5) | 0.001 | –4 (–9, 1) | NS |
PPR – preoperative pulmonary rehabilitation, FEV1 – forced expiratory volume in the first second, VLC – vital lung capacity, TI – Tiffeneau index, FEF – forced expiratory flow
After PPR, 6-minute walking distance (6MWD) was significantly enhanced, by 56 m average gain (p < 0.001), as well as blood oxygen saturation, both at rest (on average by 0.4%, p = 0.002) and after physical exercise (by 0.8%, p < 0.001) (Table III). In concordance, a significant reduction in respiratory rate (by 0.7/min at rest, p < 0.001 and by 1.6/min after physical exercise, p < 0.001) and heart rate (by 0.9/min at rest, p = 0.009 and by 2.2/min after 6MWT, p < 0.001) were recorded.
Table III.
Change in functional and symptom status after preoperative rehabilitation and after lung resection (n = 83)
| Parameter | Before PPR | After PPR | After resection | Mean difference (after PPR-before PPR) | Mean difference (after resection-after PPR) | Mean difference (after resection-before PPR) | |||
|---|---|---|---|---|---|---|---|---|---|
| n (95% CI) | p | n (95% CI) | p | n (95% CI) | p | ||||
| 6MWD [m] | 360 ±88 | 416 ±81 | 336 ±81 | 56 (45, 67) | < 0.001 | –80 (–91, –69) | < 0.001 | –24 (–33, –15) | < 0.001 |
| Sat O2% at rest | 96.9 ±0.6 | 97.3 ±0.6 | 97.3 ±0.8 | 0.4 (0.2, 0.6) | 0.002 | 0 (–0.2, 0.2) | > 0.999 | 0.4 (0.1, 0.7) | 0.016 |
| Sat O2% after exerc. | 95.5 ±0.7 | 96.3 ±0.7 | 96.3 ±0.7 | 0.8 (0.5, 1.0) | < 0.001 | 0 (–0.2, 0.2) | NS | 0.8 (0.5, 1.1) | < 0.001 |
| RR at rest | 18.7 ±1.9 | 17.9 ±1.5 | 20.6 ±1.9 | –0.7 (–1.0, –0.4) | < 0.001 | 2.7 (2.2, 3.1) | < 0.001 | 1.9 (1.4, 2.4) | < 0.001 |
| RR after exercise | 22.1 ±3.9 | 20.5 ±2.7 | 24.4 ±2.8 | –1.6 (–2.4, –0.8) | < 0.001 | 3.9 (3.3, 4.5) | < 0.001 | 2.2 (1.3, 3.1) | < 0.001 |
| HR at rest | 68.8 ±3.7 | 67.8 ±3.6 | 70.7 ±3.4 | –0.9 (–1.6, –0.3) | 0.009 | 2.9 (2.3, 3.5) | < 0.001 | 1.9 (1.2, 2.7) | < 0.001 |
| HR after exercise | 73.6 ±4.5 | 71.4 ±4.0 | 75.1 ±3.9 | –2.2 (–3.1, –1.4) | < 0.001 | 3.8 (2.9, 4.7) | < 0.001 | 1.5 (0.5, 2.5) | 0.009 |
| Borg at rest | 2.3 ±0.9 | 1.4 ±0.8 | 2.0 ±0.6 | –1.0 (–1.2, –0.7) | < 0.001 | 0.7 (0.5, 0.9) | < 0.001 | –0.3 (–0.6, 0) | NS |
| Borg after exercise | 3.4 ±0.9 | 2.2 ±0.8 | 3.0 ±0.7 | –1.1 (–1.3, –0.9) | < 0.001 | 0.8 (0.5, 1.1) | < 0.001 | –0.3 (–0.7, 0) | NS |
| VAS at rest | 2.9 ±0.9 | 1.9 ±0.9 | 2.6 ±0.7 | –1.0 (–1.2, –0.8) | < 0.001 | 0.7 (0.4, 0.9) | < 0.001 | –0.4 (–0.7, 0) | NS |
| VAS after exercise | 3.8 ±1.0 | 2.6 ±0.9 | 3.4 ±0.8 | –1.3 (–1.5, –1.0) | < 0.001 | 0.9 (0.5, 1.2) | < 0.001 | –0.4 (–0.8, 0) | NS |
PPR – preoperative pulmonary rehabilitation, 6MWD – 6 minute walking distance, Sat O2 – blood saturation with O2, RR – respiratory rate (1/min), HR – heart rate (1/min), Borg – units on the Borg scale (dyspnoea), VAS – visual analogue scale units (dyspnoea)
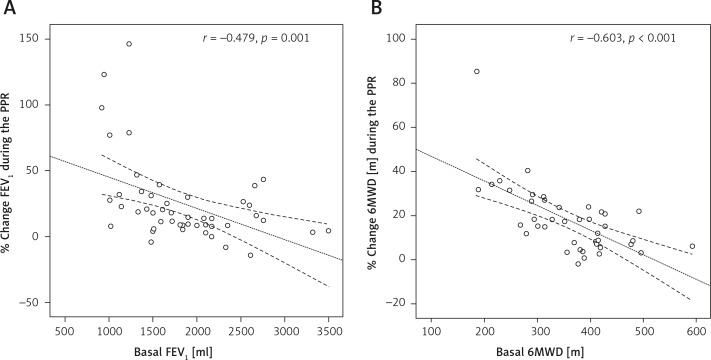
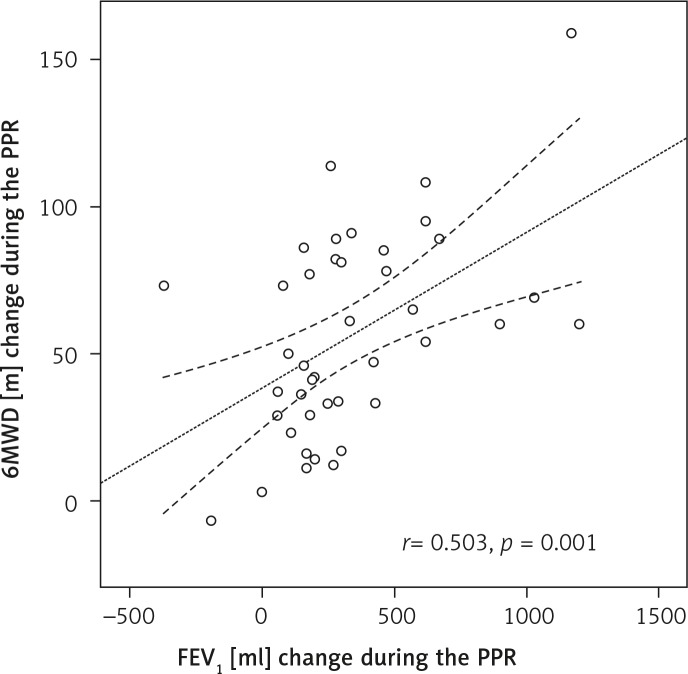
A significant negative correlation was found between FEV1 on admission and percentage change of FEV1 during PPR (r s = –0.479, p = 0.001), and between basal 6MWD and percentage extension of 6MWD after PPR (r s = –0.603, p = 0.001), as illustrated in Figure 1 – panels A and B, respectively. In addition, a strong positive correlation was identified between increment of FEV1 and extension of 6MWD, achieved during PPR (r s = 0.503, p = 0.001), as illustrated in Figure 2.
Figure 1.
Negative linear correlation between basal FEV1 value and percentage change of FEV1 during PPR (A) and negative linear correlation between basal 6MWD and percentage increase of 6MWD during PPR (B)
FEV1 – forced expiratory volume in the 1 s, 6MWD – 6-minute walking distance, PPR – preoperative pulmonary rehabilitation. Dotted lines represent mean values with 95% confidence interval
Figure 2.
Positive linear correlation between change in FEV1 and change in 6MWD during PPR
FEV1 – forced expiratory volume in the 1 s, 6MWD – 6-minute walking distance, PPR – preoperative pulmonary rehabilitation. Dotted lines represent mean values with 95% confidence interval
After PPR significant relief of dyspnoea symptoms was observed both at rest (by 1.0 unit of the Borg scale, p < 0.001, and by 1.0 unit of the VAS, p < 0.001), and after physical exercise (by 1.1 Borg units, p < 0.001, and by 1.3 VAS units, p < 0.001) (Table III).
Net effect of PPR and lung resection on pulmonary function, and functional and symptomatic status, is represented by the difference between the value of parameters after lung resection and before PPR.
Compared to the values on admission, after lung resection a significant reduction of the majority of spirometry parameters was recorded (FEV1, VLC and FEF50), while the values of the Tiffeneau index and FEF25 showed no significant change (p = NS). Moreover, in 6 patients (7.2%) a relative increase in FEV1 by as much as 22% (95% CI: 10–35%), FEF50 by 60% (4–116%) and FEF25 by 72% (1–146%), relative to basal values, was recorded postoperatively.
Among the candidates for different types of lung resection (segmentectomy (n = 3), upper lobectomy (n = 24), middle or lower lobectomy (n = 1 + 23), bilobectomy (n = 7), sleeve lobectomy (n = 7) and pneumonectomy (n = 18)), there was no significant difference in basal values of FEV1 and VC at hospital admission (p = NS), as well as in the degree of change of those parameters after completion of the PPR programme (p = NS). However, after different types of lung resection there was a significant difference in the degree of net change of spirometric parameters (FEV1, p < 0.001 and VC, p < 0.001), in comparison with the values at admission. Actually, post-hoc analysis showed that after pneumonectomy average reductions of FEV1 (for 572 ml) and VC (for 1097 ml) were significantly higher in comparison with average changes of those parameters after segmentectomy (increase of FEV1 for 260 ml, p < 0.001 and VC for 140 ml, p < 0.001), upper lobectomy (reduction of FEV1 for 82 ml and VC for 207 ml, p = 0.002), middle or lower lobectomy (reduction of FEV1 for 216 ml, p < 0.001 and VC for 298 ml, p < 0.001), bilobectomy (increase of FEV1 for 200 ml, p = 0.004 and VC for 443 ml, p = 0.015), but it was not the case after sleeve lobectomy (reduction of FEV1 for 320 ml, p = NS and VC for 390 ml, p = NS).
Although significant postoperative shortening of 6MWD was seen as well as acceleration of respiratory and heart rates, oxygen blood saturation was significantly higher after lung resection compared to the value on admission, both at rest (p = 0.016) and after physical activity (p < 0.001), as shown in Table III.
Compared to the value on admission, there was no significant deterioration of dyspnoea symptoms, either at rest (p = NS) or after physical exercise (p = NS).
Overall hospitalization took 35 ±16 days, while preoperative and postoperative stay in hospital was 18 ±11 days and 17 ±8 days, respectively. Perioperative mortality (within 30 days after surgery) was 7%. Perioperative complications were found in 39 (47%) patients, and 33 (40%) had one or more respiratory complications: prolonged air leak 16%, pneumonia 12%, atelectasis 7%, sputum retention 4% and empyema 1%. No significant association was found between the rate of change of examined spirometric and functional parameters (FEV1, FEV1% predicted, VLC, VLC% predicted, Tiffeneau index, FEF50, FEF25 and 6MWD) during PPR and appearance of perioperative complications and/or lethal outcome.
Discussion
Our results indicated that PPR significantly enhances preoperative pulmonary function and symptom status in COPD patients with NSCLC. During the PPR programme, yield of FEV1 directly correlates with better exercise tolerance on 6MWT. We observed that after PPR, the percentage increase in FEV1 and percentage extension of 6MWD were more emphasized in patients with more serious damage of pulmonary function on admission and weaker basal effort tolerance, respectively. It should be stated as well that PPR partly mitigated the expected postoperative reduction of the majority of lung volumes and functional capacity, and completely neutralized the effects of resection on the Tiffeneau index, function of small airways (FEF25), oxygen blood saturation and dyspnoea symptoms.
Poor exercise tolerance, damaged pulmonary function and presence of COPD are important determinants of postoperative morbidity and mortality after lung cancer resection [3–6]. Hence, preoperative modification of those factors could improve the surgery outcome. In COPD patients, respiratory rehabilitation increases the lung inflation and pulmonary volumes, maintains small airways potency, strengthens the weakened inspiratory muscles, increases the efficiency of respiratory muscles work and raises patient motivation for further treatment [21–23]. A “respiratory conditioning” programme under supervision of a physical therapist in COPD patients improves the flexibility of the chest with the correction of posture as well as stretching and mobilization of the rib cage [24].
Several studies involving COPD patients with associated lung cancer have shown that preoperative rehabilitation considerably enhances effort tolerance, not necessarily coupled with the improvement of pulmonary function [10–17]. Using 2-week pre-surgical exercise training in 13 patients with primary lung cancer and mild to moderate COPD, Jones et al. demonstrated considerable increase in effort tolerance but without a significant change of lung function or symptomatic status (dyspnoea, leg discomfort) [12]. Similarly, following 4-week preoperative respiratory rehabilitation coupled with aerobic peripheral muscle exercise training in 12 COPD patients with NSCLC and high surgical risk (basal FEV1 = 1.23 l, VO2max < 15 ml/kg/min), Bobbio et al. reported a significant improvement in effort tolerance (increase of VO2max by 2.8 ml/kg/min), not associated with significant change of spirometry parameters of lung function. Importantly, the authors suggested that such improvement of functional capacity enabled surgery even on patients initially considered inoperable [13].
On the other hand, Cesario et al. found in 8 patients with primary lung cancer and severe COPD (basal FEV1: 0.56–1.29 l), applying a similar programme of preoperative physiotherapy, significantly enhanced respiratory function (increase of VLC by 0.44 l and FEV1 by 0.12 l), along with a parallel increase of effort tolerance (extension of 6MWD by 79 m), where the highest preoperative benefit was experienced by patients with initially the worst respiratory function [14]. In addition, Subotić et al. transferred a significant number of lung cancer patients from severe-to-moderate to mild stages of COPD (increase of FEV1 by 417 ml, and FEF50/FEF25 by 7.4%/5.7%) by measures of PPR [15].
Our study confirmed the mentioned findings in the group of 83 patients with NSCLC and moderate COPD. After 2–4-week PPR almost all the parameters of pulmonary function and parameters of effort tolerance significantly improved (FEV1 by 374 ml, VLC by 407 ml, FEF50 by 2% and 6MWD by 56 m), and the feeling of dyspnoea was considerably mitigated. For the first time we determined a direct correlation between the increase in FEV1 and extension of 6MWD during preoperative physiotherapy. This finding suggests that preoperative increase of effort tolerance was largely the consequence of improvement of respiratory function, achieved by measures of PPR. We found that there is an inverse correlation between basal values of FEV1 and 6MWD and percentage increase in these parameters during preoperative rehabilitation. Namely, relative increase in FEV1 and 6MWD was most pronounced in the patients with initially the worst respiratory function and functional capacity, who carry the highest surgical risk.
Extension of resection of functional parts of lungs is an independent predictor of perioperative complications in patients with lung cancer. On the other hand, bullectomy and resection of non-functional emphysematous parts of the lung (“lung volume reduction surgery”) improve pulmonary function in COPD patients [25]. Moreover, in selected COPD patients with primary lung cancer, lobectomy for cancer resection could result in improvement of the relation FEV1/VLC (Tiffeneau index) [7, 8].
Weiner et al. demonstrated that in COPD patients with primary lung carcinoma, subjected to 2-week preoperative training of respiratory muscles, postoperative reduction in FEV1 and VLC after lobectomy and pneumonectomy was smaller than predicted, by 570 ml and 680 ml, respectively [26]. Sekine et al., with PPR in COPD patients with primary lung carcinoma, achieved a protective effect against deterioration of FEV1 after lobectomy: in the group of patients subjected to preoperative rehabilitation, compared to the control group without rehabilitation, postoperative reduction of FEV1 was considerably less and the ratio of actual and predicted postoperative FEV1 was significantly higher [17].
In our study, as well, almost all lung volumes and effort tolerance were significantly lower postoperatively than on admission, as expected. Pneumonectomy led to a significantly more pronounced deterioration of pulmonary function in comparison with less voluminous types of resection, and the type of lung resection in our study was not associated with lung volume reduction effect after the operation. Nevertheless, pulmonary function in our patients was evaluated within the first month after the operation, while postoperative restoration of the elastic recoil of pulmonary parenchyma and improvement of thoracic motion could require a longer period (over 6 months) [7]. However, significant preoperative improvement of respiratory function, achieved by PPR, exerted a protective effect on the Tiffeneau index, small airways function and blood oxygen saturation after the operation. Although it failed to fully annul unfavourable effects of lung resection, preoperative rehabilitation did place such patients in a better starting position immediately prior to surgery (due to preoperative improvement of their respiratory function and functional capacity), and partly mitigated postoperative reduction of the majority of respiratory volumes and effort tolerance. Moreover, in 7.2% of the patients, a relative increase of FEV1 by 22% with parallel improvement of the small airways function (FEF50/25) by 60–72% was recorded following resection. In addition, PPR completely eliminated postoperative deterioration of dyspnoea symptoms, which is in contrast with the results of previous studies [12].
Previous studies have suggested that preoperative rehabilitation may result in reducing the rate of pulmonary complications and shortening postoperative hospitalization, but controversies continue [16, 17]. Due to the design of our study (single group) and absence of a control group (without PPR) it was not possible to determine direct effects of PPR on perioperative mortality and morbidity. The rate of perioperative complications in our group (47%) was in line with earlier studies, but the mortality rate was only 7%, which is half the findings of other researchers [3]. We did not identify significant predictive value of increments of individual spirometric and functional parameters during PPR for the appearance of perioperative complications or lethal outcome.
In conclusion, the programme of 2–4 weeks of PPR significantly improves pulmonary function, functional capacity and symptomatic status of patients with moderate COPD prior to resection of NSCLC by open thoracotomy. In addition, preoperative increase of effort tolerance to 6MWT predominantly was the result of higher FEV1 that is enhanced respiratory function, accomplished by PPR measures. Beneficial effects of PPR were most emphasized in patients with the worst basal respiratory function and the weakest basal functional capacity, who were chiefly at risk from perioperative pulmonary complications. The PPR may considerably relieve the effects of lung resection on the residual pulmonary function and clinical status of COPD patients with NSCLC.
References
- 1.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129:1305–12. doi: 10.1378/chest.129.5.1305. [DOI] [PubMed] [Google Scholar]
- 2.Ciatkowska-Rysz A, Kowalczyk M, Gottwald L, Kazmierczak-Tukaszewicz S. The comparison of common cancer types and the coincidence of concomitant chronic diseases between palliative home care patients in Lodz Voivodeship and the general Polish population. Arch Med Sci. 2012;8:496–503. doi: 10.5114/aoms.2012.29406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer. 2002;37:95–101. doi: 10.1016/s0169-5002(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 4.Magdeleinat P, Seguin A, Alifano M, Boubia S, Regnard JF. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg. 2005;27:1099–105. doi: 10.1016/j.ejcts.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Algar FJ, Alvarez A, Salvatierra A, Baamonde C, Aranda JL, Lopez-Pujol FJ. Predicting pulmonary complications after pneumonectomy for lung cancer. Eur J Cardiothorac Surg. 2003;23:201–8. doi: 10.1016/s1010-7940(02)00719-4. [DOI] [PubMed] [Google Scholar]
- 6.Agostini P, Cieslik H, Rathinam S, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors. Thorax. 2010;65:815–8. doi: 10.1136/thx.2009.123083. [DOI] [PubMed] [Google Scholar]
- 7.Kushibe K, Takahama M, Tojo T, Kawaguchi T, Kimura M, Taniguchi S. Assessment of pulmonary function after lobectomy for lung cancer – upper lobectomy might have the same effect as lung volume reduction surgery. Eur J Cardiothorac Surg. 2006;29:886–90. doi: 10.1016/j.ejcts.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Sekine Y, Iwata T, Chiyo M, et al. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2003;76:356–62. doi: 10.1016/s0003-4975(03)00489-2. [DOI] [PubMed] [Google Scholar]
- 9.Ries AL, Make BJ, Lee SM, et al. The effects of pulmonary rehabilitation in the National Emphysema Treatment Trial. Chest. 2005;128:3799–809. doi: 10.1378/chest.128.6.3799. [DOI] [PubMed] [Google Scholar]
- 10.Shannon VR. Role of pulmonary rehabilitation in the management of patients with lung cancer. Curr Opin Pulm Med. 2010;16:334–9. doi: 10.1097/MCP.0b013e32833a897d. [DOI] [PubMed] [Google Scholar]
- 11.Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13:300–2. doi: 10.1510/icvts.2010.264507. [DOI] [PubMed] [Google Scholar]
- 12.Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110:590–8. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 13.Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:95–8. doi: 10.1016/j.ejcts.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Cesario A, Ferri L, Galetta D, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57:118–9. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Subotić DR, Mandarić DV, Eminović TM, et al. Influence of chronic obstructive pulmonary disease on postoperative lung function and complications in patients undergoing operations for primary non small cell lung cancer. J Thorac Cardiovasc Surg. 2007;134:1292–9. doi: 10.1016/j.jtcvs.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74:441–5. doi: 10.1016/j.lungcan.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine Y, Chiyo M, Iwata T, et al. Perioperative rehabilitation and physiotherapy for lung cancer patients with chronic obstructive pulmonary disease. Jpn J Thorac Cardiovasc Surg. 2005;53:237–43. doi: 10.1007/s11748-005-0032-8. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Guidelines for the six minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Nici L, Donner C, Wouter E, et al. the American Thoracic Society/European Respiratory Society Pulmonary Rehabilitation Writting Committee. ATS/ERS Statement on Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 21.Pardy RL, Rivington RN, Despas PJ, Macklem PT. The effects of inspiratory muscle training on exercise performance in chronic airflow limitation. Am Rev Respir Dis. 1981;123:426–33. doi: 10.1164/arrd.1981.123.4.426. [DOI] [PubMed] [Google Scholar]
- 22.Jaworski A, Goldberg SK, Walkenstein MD, Wilson B, Lippmann ML. Utility of immediate postlobectomy fiberoptic bronchoscopy in preventing atelectasis. Chest. 1988;94:38–43. doi: 10.1378/chest.94.1.38. [DOI] [PubMed] [Google Scholar]
- 23.O'Donohue WJ., Jr National survey of the usage of lung expansion modalities for the prevention and treatment of postoperative atelectasis following abdominal and thoracic surgery. Chest. 1985;87:76–80. doi: 10.1378/chest.87.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimi K, Ueki J, Seyama K, et al. Pulmonary rehabilitation program including respiratory conditioning for chronic obstructive pulmonary disease (COPD): improved hyperinflation and expiratory flow during tidal breathing. J Thorac Dis. 2012;4:259–64. doi: 10.3978/j.issn.2072-1439.2012.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna RJ, Jr, Gelb A, Brenner M. Lung volume reduction surgery for chronic obstructive pulmonary disease: where do we stand? World J Surg. 2001;25:231–7. doi: 10.1007/s002680020023. [DOI] [PubMed] [Google Scholar]
- 26.Weiner P, Man A, Weiner M, et al. The effect of incentive spirometry and inspiratory muscle training on pulmonary function after lung resection. Thorac Cardiovasc Surg. 1997;113:552–7. doi: 10.1016/S0022-5223(97)70370-2. [DOI] [PubMed] [Google Scholar]