Abstract
Background
Properly dosed oral anticoagulation effectively prevents thromboembolic events. It is unclear whether adult patients with an indication for long-term oral anticoagulation can benefit from self-management in terms of patient-oriented endpoints and improved coagulation values.
Methods
We selectively searched the Medline database for high-quality systematic reviews based on randomized controlled trials of self-measurement or self-management of oral anticoagulation, compared to standard treatment.
Results
We identified eight review articles based on overlapping sets of randomized clinical trials. In all of these systematic reviews, patients who performed self-measurement or self-management had a 40% to 50% lower rate of thromboembolic events; in six of them, the mortality was also significantly lower, by 30% to 50%. Subgroup analysis revealed that these effects were present exclusively in patients who performed self-management, and not in those who only performed self-measurement. None of the review articles revealed any difference in the frequency of severe hemorrhagic events. Quality of life and patient satisfaction were rated in five reviews, which, however, used different instruments, with the result that no clear conclusions could be drawn. All of the review articles documented an improvement in coagulation values, but information on statistical significance was mostly lacking.
Conclusion
Adults with an indication for long-term oral anticoagulation benefit from self-management, as compared to standard treatment with management of dosing by a physician. A limitation of this study is that the multiple review articles on which it is based were largely analyses of the same group of clinical trials.
Numerous indications for oral anticoagulation are associated with an increased risk of thromboembolic events, e.g. atrial fibrillation arrhythmia, artificial heart valves, cardiomyopathies, vascular prostheses, and status post thrombosis or embolism (1).
Currently, vitamin K antagonists (coumarin derivatives) are among the most commonly used drugs for long-term anticoagulation (2). The standard model of care for patients receiving oral anticoagulation (OAC) therapy comprises the collection of venous blood samples at regular intervals to determine INR values, followed by INR-guided dose adjustments typically made by general practitioners or other specialists.
The emergence of point-of-care devices (POCDs) paved the way for the development of new models of care, such as patient self-testing (PST) and, as the next step, patient self-management (PSM).
In the PSM model, the patient is responsible for INR self-measurement and the self-adjustment of the medication dosage. To prepare the patients for their new role they have to be educated and instructed in the use of the monitoring device; for structured training, several standardized and evaluated educational programs are available (3, 4). In contrast, patient involvement in the PST model is limited to INR self-monitoring—adjusting the anticoagulant dosage continues to be the responsibility of the physicians providing the care.
Potential advantages of PSM or PST include:
Improved patient adherence with anticoagulation therapy (5)
Enhanced treatment satisfaction (6)
Fewer thromboembolic events along with an unchanged risk of bleeding due to more frequent checks and improved dosage adjustments (7).
It was the aim of this review to answer the question whether adult patients with a long-term indication for oral anticoagulation would benefit from patient self-management (PSM) with regard to patient-relevant endpoints and improved anticoagulation control.
Methods
Study types
Only systematic reviews summarizing analysis results from randomized controlled trials (RCTs) were included (e.g. meta-analyses). According to the methods of the Institute for Quality and Efficiency in Healthcare (IQWiG, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen) (8), such an approach may be regarded as reliable and time-saving as long as certain specific requirements, such as a sufficient number of high-quality reviews with concordant results, are met. The Cochrane Collaboration has recently incorporated this type of review, sometimes referred to as meta-review, too (9). The RCTs on which these results were based had to meet the PICO criteria:
P: Population: adults with long-term indication for OAC (coumarin derivatives)
I: Intervention: either PST or PSM
C: Comparison: monitoring and dose adjustment by healthcare professionals
O: Outcomes: patient-relevant endpoints, such as mortality, thromboembolic complications, major bleeding complications, and quality of life, plus surrogate parameters of anticoagulation control based on INR monitoring.
Collecting information, identifying relevant studies and evaluating quality
A literature search was conducted in Medline. Two experts independently performed abstract and full text screening as well as quality evaluation; subsequently, any discrepancies in evaluation were discussed and reconciled. With regard to methodology, the review had to be of high quality according to the quality index of Oxman and Guyatt (10, 11). Only reviews scoring at least 5 of 7 points when evaluated by two independent experts were included.
The extraction of relevant data was performed by one expert and controlled by a second.
Results
Results of information collection
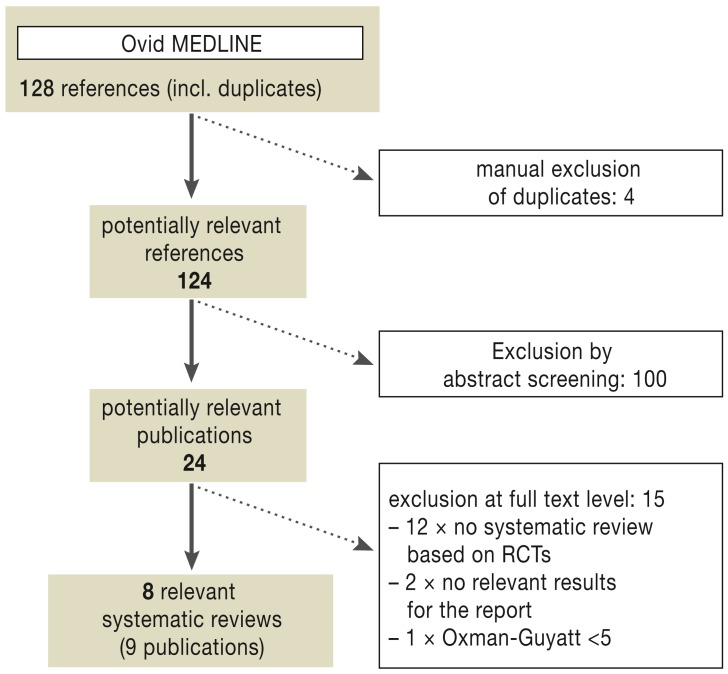
Altogether the database search identified 128 references (Figure 1). Of these, 9 publications about 8 high-quality systematic reviews were included after full text viewing and quality assessment using the Oxman and Guyatt index.
Figure 1.
Systematic reviews: bibliographic literature search and study selection process;
RCTs, randomized controlled trials
Systematic review characteristics
Table 1 provides information about the characteristics of the included reviews. Detailed information is available in the full report of the Federation of Austrian Social Insurance Institutions (HVB, Hauptverband der Österreichischen Sozialversicherungsträger) (12). Four reviews (7, 13– 15) included studies evaluating PST or PSM. In 1 review, only studies on PSM were considered (16), while in another review additional studies with corresponding measurements by a practice nurse (near-patient testing, NPT) were also included (17). The remaining 2 reviews included studies in general in which the anticoagulation measurements were performed using a POCD (18, 19). Study durations ranged from a few months to several years. The number of included RCTs varied between 5 and 22, with each review considering a large proportion of the RCTs (Table 2). The total numbers of patients ranged from 2219 to 8413. Only 2 reviews (7, 13) reported the average age which was 65 years. The proportion of female subjects was stated in only 4 reviews; this was mostly smaller than that of male subjects.
Table 1. Characteristics of reviews, study duration and patient characteristics.
| Systematic review author/year/source | Key inclusion criteria | Search details | Patient number (range)/ duration (median)/ mean age (range)/ percentage females (range) | Indication(s): n studies |
|---|---|---|---|---|
| Bloomfield 2011 (13) |
included: RCT (n=22); English language; adults in ambulatory care; intervention: PST or PSM of OAC therapy; control: standard therapy (GP/hospital) |
MEDLINE (2005 to 2010) CENTRAL (n.s.) Reference lists from Connock M, et al. 2007 (1966 to 2005) |
8413 (50–2922)/ 3 to 57 months (6 months)/ 65 years (42–75)/ 25% (2–57) |
MHVR: 6; AF: 2; MHVR or AF: 1; mixed indic.: 14*1 |
| Christensen 2007 (16) |
included: RCT (n=10); adults (>18 years); OAC therapy >6 months; intervention: PSM of OAC therapy; control: standard therapy (GP/hospital) or PST or computer-assisted dosing |
PubMed (1951 to Dec 2005) CENTRAL (2005 Issue 4) Reference lists of relevant publications Personal files |
2724 (49–649)/ 4.6 to 24 months (7.25 months)/ n.s./ n.s. |
MHVR: 2; AF: 1; mixed indic.: 7*1 |
| Connock 2007 (17) |
included: RCT (n=16) und non RCT; Intervention: PST or PSM of OAC therapy or corresponding measurements at GP (NPT); control: standard therapy (GP/hospital) |
MEDLINE (1966 to Sep 2005) EMBASE (1980 to week 38, 2005) CINAHL (1982 to Sep 2005) CENTRAL (2005 Issue 3) Reference lists of relevant publications Study register (National Research Register) |
4283*2 (50–1155)/ 2 to >24 months (6 months)/ n.s. (42–75)/ n.s. (24–57) |
MHVR: 3; AF: 2; MHVR or AF: 1; mixed indic.: 10*1 |
| Garcia Alamino 2010 (14) |
included: RCT (n=18); adults and children; OAC therapy >2 months; intervention: PST and PSM of OAC therapy; control: standard therapy (GP/hospital) |
MEDLINE (1966 to Nov. 2007) EMBASE (1980 to Nov 2007) CINAHL (1982 to Nov 2007) CENTRAL (2007, Issue 4) Reference lists of all relevant publications Manufacturer enquiry at Roche Diagnostics Study register (UK National Research Register, Trials Central, Current Controlled Trials) |
4723 (50–1155)/ 2 to >24 months (12 months)*3 / n.s. (42–75)/ n.s. |
MHVR: 3; AF: 2; mixed indic.: 13*1 |
| Heneghan 2012 (7) |
included RCT (n=11); adults; intervention: PST or PSM of OAC therapy control: standard therapy (GP/hospital) |
MEDLINE (1966 to 2009) EMBASE (1980 to 2009) CINAHL (1982 to 2009) CENTRAL (2009, Issue 2) Reference lists of all relevant publications Study register (UK National Research Register, Trials Central) |
6417 (49–2922)/ 3 to 36 months (12 months)/ 65 years (42.3–74.7)/ 22% (2–57) |
MHVR: 1; AF: 1; mixed indic.: 9*1 |
| Ontario HTA 2009 (18) |
included: RCTs (n=15) with min. 50 subjects; ≥3 months follow-up; English language; intervention: anticoagulation measurement using point-of-care INR device (POCD) incl. PST, PSM; control: standard care with venous INR measurement (GP/hospital) |
MEDLINE (1998 to 2008) EMBASE (1998 to 2008) CINAHL (to 2009) Cochrane databases (to 2008) INAHTA (to 2008) Reference lists of all relevant publications |
5221*2 (50–1155)/ 3 to 38 months (6 months)/ n.s. (42–70)/ n.s. (29–57) |
MHVR: 3; AF: 2; mixed indic.: 10*1 |
| Wells 2007 (19) |
included: RCT (n=14); OAC therapy ≥3 months; intervention: anticoagulation measurement using point-of-care device (POCD); control: usual care |
MEDLINE (to July 2005) EMBASE (to July 2005) DIALOG (to July 2005) BIOSIS Previews (to July 2005) PASCAL (to July 2005) Alerts for MEDLINE, EMBASE and BIOSIS Previews to March 2007 Pubmed (n.s.) Cochrane Library (n.s.) Web sites of regulatory authorities, HTA organisations and near-technology assessment organisations Latin American and Caribbean Center on Health Sciences Information (LILACS) |
4496 (79–834)/ 3 to ≥ 51 months (6 months)/ n.s./ n.s. |
MHVR: 3; AF: 2; mixed indic.: 9*1 |
| Xu 2012 (15) |
included: RCT (n=5); mechancal heart valves; OAC therapy ≥ 6 months; intervention: PST or PSM of OAC therapy control: standard therapy (GP/hospital) |
MEDLINE (1966 to Dec 2010) EMBASE (1980 to Dec 2010) CENTRAL, CDSR, DARE (to Dec 2010) CNKI (1966 to Dec 2010) Wanfangdata (1998 to Dec 2010) CQVIP (1989 to Dec 2010) Reference lists of all relevant publications Study register (ClinicalTrials.gov) |
2219 (48–1155)/ 12 to 51 months (24 months)/ n.s./ n.s. |
MHVR: 5 |
*1Patients with oral anticoagulation therapy regardless of indication. No details about indications provided.
*2Calculated
*3Mean
mixed indic., mixed indications; GP, general practitioner; n.s., not specified; MHVR, mechanical heart valve replacement; NPT, near patient testing; POCD, point-of-care device; PST, patient self-testing; PSM, patient self-management; OAC, oral anticoagulation; RCT, randomized controlled trial; AF, atrial fibrillation
Table 2. RCTs included in the various reviews (study overview).
| SR RCT |
Bloomfield 2011 (13) |
Connock 2007 (17) |
Christensen 2007 (16) |
Garcia-Alamino 2010 (14) |
Heneghan 2012 (7) |
Ontario HTA 2009 (18) |
Wells 2007 (19) |
Xu 2012 (15) |
Type of intervention |
|---|---|---|---|---|---|---|---|---|---|
| White 1989 (e1) | x | x | PST | ||||||
| Horstkotte 1998 (e2) | x | x | x | x | x | x | PST | ||
| Sawicki 1999 (3) | x | x | x | x | x | x | PSM | ||
| Beyth 2000 (e3) | x | x | x | x | x | x | PST | ||
| Cromheecke 2000 (e4) | x | x | x | x | x | x | x | PSM | |
| Kaatz 2001 (e5) | x | x | PST | ||||||
| Körtke 2001 (e6) | x | x | x | x | x | x | x | x | PSM |
| Sidhu and O’Kane 2001 (e7) | x | x | x | x | x | x | x | PSM | |
| Fitzmaurice 2002 (e8) | x | x | x | x | PSM | ||||
| Gadisseur 2003 (e9) | x | x | x | x | x | x | PST, PSM | ||
| Khan 2004 (e10) | x | x | x | x | x | PST | |||
| Sunderji 2004 (e11) | x | x | x | x | x | x | x | PSM | |
| Claes 2005 (e12) | x | x | PST | ||||||
| Fitzmaurice 2005 (e13) | x | x | x | x | x | x | x | PSM | |
| Gardiner 2005 (e14) | x | x | x | PST | |||||
| Menéndez-Jándula 2005 (e15) | x | x | x | x | x | x | x | PSM | |
| Völler 2005 (e16) | x | x | x | x | x | x | x | PSM | |
| Christensen 2006 (e17) | x | x | x | x | PSM | ||||
| Gardiner 2006* (e18) | x | x | PSM | ||||||
| Dauphin 2008 (e19) | x | PST | |||||||
| Eitz 2008 (e20) | x | x | PSM | ||||||
| Siebenhofer 2008 (20) | x | x | x | x | x | PSM | |||
| Ryan 2009 (e21) | x | PSM | |||||||
| Soliman Hamad 2009 (e22) | x | x | PSM | ||||||
| Matchar 2010 (e23) | x | x | PST |
*This study compares PSM versus PST. It was included in 2 systematic reviews, but not taken into consideration in the meta-analyses. PSM, patient-self management; PST, patient self testing; RCT, randomized controlled trial; SR, systematic review
Results for relevant endpoints
Patient-relevant endpoints
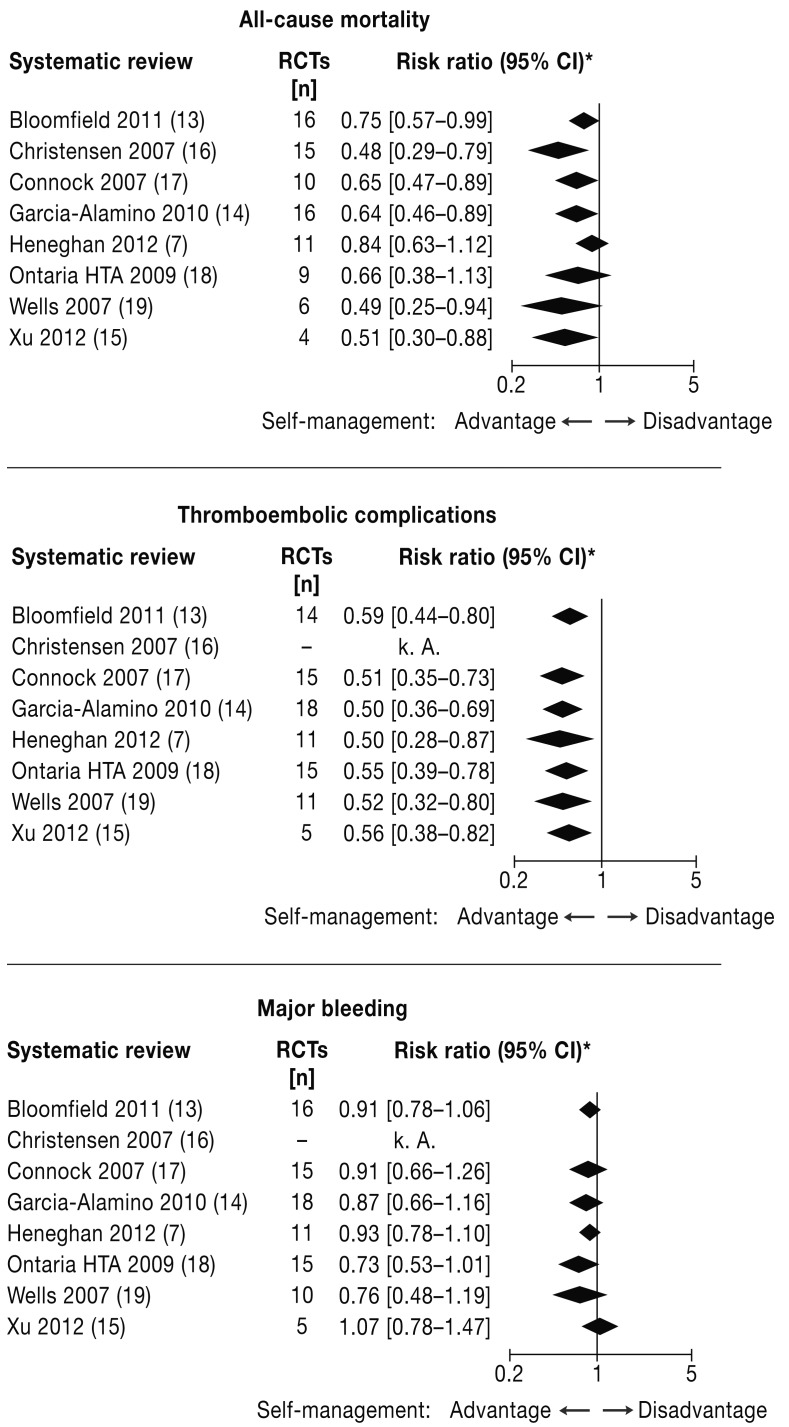
All-cause mortality—All 8 reviews report all-cause mortality results and also perform meta-analyses. In 6 reviews, statistically significant advantages of PST/ PSM over standard therapy were revealed regardless of the number of RCTs included in the calculations (4 to 16), with relative risk reductions between 25% and 52% (calculated from the data in Table 3 (Figure 2). The reviews by Heneghan 2012 and the Ontario HTA Group in 2009 showed only a numeric advantage of 16% and 34%, respectively, for the intervention group; however, these differences did not reach statistical significance (Table 3). Statistical heterogeneity was found increased in those 2 reviews (7, 13) with the highest number of patients included in the analyses (I2 = 51% and 37%, respectively).
Figure 2.
Comparison of the results of the meta-analyses:
all-cause mortality, thromboembolic events, major bleeding
*To facilitate comparability the relative risk point estimator for each of the reviews was determined from the respective published numbers of events or participants in the individual studies.
Table 3. Results of the systematic reviews for the endpoints all-cause mortality, thromboembolic complications, and major bleeding.
| Systematic review | All-cause mortality Events/group (IG vs CG); Effect measure—group difference |
Major thromboembolic events Events/group (IG vs CG); Effect measure—group difference |
Major bleeding Events/group (IG vs CG); Effect measure—group difference |
|
|---|---|---|---|---|
| Bloomfield 2011 (13) | 298/3247 vs 369/3123; OR (95% CI): 0.74 (0.63–0.87)I2 = 51%; p<0.001 |
95/4004 vs 149/3755; OR (95% CI): 0.58 (0.45 to 0.75) I2 = 27%; p < 0.001 |
283/4061 vs 300/3806; OR (95% CI): 0.89 (0.75–1.05) I2 = 2%; p = 0.169 |
|
| Christensen 2007 (16) | 20/1367 vs 45/1357; RR (95% CI): 0.48 (0.29–0.79) I2 = 0%; p = 0.004 |
51/1367 vs 90/1357; (95% CI): 0.58 (0.42–0.81)*1 I2 = 0%; p<0.001 |
||
| Connock 2007 (17) | 59/2028 vs 92/1952; Risk difference (95% CI): –0.0170 (−0.0287 to −0.0053) I2 = 13%; p = 0.004 *2 |
41/2028 vs 86/1952; Risk difference (95% CI): –0.0224 (−0.0334 to −0.0115) I2 = 26%; p<0.0001*2 |
68/2028 vs 74/1952; Risk difference (95% CI): −0.0039 (−0.0154 to 0.0077) I2 = 0%; p = 0.54*2 |
|
| Garcia-Alamino 2010 (14) | 53/2181 vs 84/2124; RR (95% CI): 0.64 (0.46–0.89) I2 = 0%; p = 0.007 |
48/2329 vs 98/2394; RR (95% CI): 0.50 (0.36–0.69)*3 I2 = 0%; p<0.0001 |
79/2329 vs 93/2394; RR (95% CI): 0.87 (0.66–1.16) I2 = 0%; p = 0.34 |
|
| Heneghan 2012 (11) | 247/3266 vs 274/3251; HR (95% CI): 0.82 (0.62–1.09) I2 = 37%; p =0.18 |
114/3266 vs 152/3151; HR (95% CI): 0.51 (0.31–0.85) I2 = 52.6%; p<0.010 |
230/3216 vs 244/3101; HR (95% CI): 0.88 (0.74–1.06) I2 = 0%; p = 0.18 |
|
| Ontario HTA 2009 (18) | 43/1278 vs 63/1215; OR (95% CI): 0.63 (0.36–1.12) I2 = 31%*2; p = 0.11*2 |
47/2249 vs 99/2441; OR (95% CI): 0.53 (0.37–0.76)*3 I2 = 0%*2; p = 0.0006*2 |
59/2249 vs 87/2441; OR (95% CI): 0.72 (0.51–1.02) I2 = 0% *2; p = 0.06 *2 |
|
| Wells 2007 (19) | 16/1015 vs 33/953; OR (95% CI): 0.48 (0.24–0.94) I2 = 8.8%; p = 0.03 |
26/1573 vs 54/1610; OR (95% CI): 0.49 (0.30–0.79) I2 = 0%; p<0.003 |
32/1498 vs 41/1535; OR (95% CI): 0.75 (0.47–1.20) I2 = 0%; p = 0.23 |
|
| Xu 2012 (15) | 19/724 vs 39/728; OR (95% CI): 0.50 (0.29–0.86) I2 = 0%; p = 0.0115 |
41/1194 vs 68/1023; OR (95% CI): 0.52 (0.35–0.77)*3 I2 = 0%; p<0.0012 |
81/1194 vs 65/1023; OR (95% CI): 1.07 (0.77–1.50) I2 = 0%; p = 0.68 |
|
*1 Major complications (thromboembolic and hemorrhagic events combined)
*2Calculated
*3All thromboembolic events
HR, hazard ratio; IG, intervention goup; n.s., not specified; CG, control group; CI, confidence interval; OR, odds ratio; RR, risk ratio
In the context of subgroup analyses, 6 reviews (7, 14, 15, 17– 19) reported separate results for PSM compared with standard therapy. In another review (16), the general focus was exclusively on PSM as an intervention. All in all, statistically significant all-cause mortality advantages of PSM were demonstrated in 5 reviews. In 2 reviews (7, 18), the reported advantage was not significant. On the other hand, 4 reviews (7, 14, 17, 18) failed to demonstrate a statistically significant positive effect for PST alone compared with standard therapy. Only the review of Xu in 2012 (15), which exclusively included patients with mechanical heart valve replacements, also found a statistically significant advantage for PST.
Thromboembolic events—Analyses for thromboembolic events were reported in 7 reviews, while 1 paper (16) presented a combined meta-analysis of severe complications (thromboembolic and hemorrhagic events) only. All of the 7 meta-analyses for thromboembolic events and the one for severe complications demonstrated a statistically significant positive effect in favor of PST/PSM with relative risk reductions between 41% and 50% (calculated from the data in Table 3 (Figure 2). Statistical heterogeneity ranged from I2 = 0% to 53%.
All of the subgroup analyses comparing PSM with standard therapy reported in 6 reviews (7, 14, 15, 17– 19) showed statistically significant advantages for the intervention groups.
For PST alone, this was the case only in 1 review (15), while the other 4 reviews (7, 14, 17, 18) showed no statistically significant positive effect.
Major bleeding—A definition of major bleeding was provided in 6 reviews. Results regarding major hemorrhages were reported in 7 reviews. None of the meta-analyses showed a statistically significant advantage or disadvantage for PST/PSM compared with standard therapy, in the presence of no or very low statistical heterogeneity (Table 3, Figure 2).
In the context of subgroup analyses, 6 reviews (7, 14, 15, 17– 19) reported separate results for PSM compared with standard therapy, but none of these reviews showed any statistically significant group difference. Results for PST alone were presented in 5 reviews (7, 14, 15, 17, 18). These were mixed. A statistically significant advantage over standard therapy was found in 2 reviews (14, 17), while the other 3 reviews showed no statistically significant difference.
Quality of life—In 5 reviews information about patient satisfaction or quality of life (QoL) was reported (13, 14, 17– 19). Because different instruments were used in the various RCTs, none of these trials provided a quantitative summary. All 5 reviews reported data on perceived treatment quality, which in 3 RCTs were collected using a questionnaire developed by Sawicki (3). Here the comparison revealed a significant advantage of PSM over standard care for 4 or all 5 categories of the questionnaire. With PST, a significant difference was only demonstrated in the “self-efficacy” category. Several other patient satisfaction instruments also indicated advantages for PST/PSM.
QoL was assessed using a variety of instruments (EuroQoL/EQ-5D, SF-36, SEIQoL, and a questionnaire specifically designed for patients on anticoagulation) and the corresponding results were only incompletely published. As far as reported, the differences between the groups were generally not significant. The available data did not allow to draw definite conclusions about the impact of PST/PSM on quality of life.
Surrogate parameters
Anticoagulation control—In the reviews, data on anticoagulation control were reported as INR measurements in the target range or as the percentage of time in the target range. Pooled data of the time in the target range were reported in 4 reviews (13, 17– 19) and ranged between 56% and 66% in the control groups, while for PST/PSM comparatively better results were found, ranging from 64% to 73%. However, most of these reviews do not provide information about the statistical significance of these differences. Regarding the percentage of INR values in the target range, only 1 review reported pooled results (13); the percentages were 59% for the control group and 71% for PST/PSM. The weighted difference between the two groups was not statistically significant.
Detailed information about QoL and anticoagulation control findings is available in the full report of the Federation of Austrian Social Insurance Institutions (HVB) (12).
Discussion
Altogether 8 high-quality systematic reviews concerned with patient self-testing or self-management (PST/ PSM) versus standard care were identified. All systematic reviews consistently showed a reduction in thromboembolic events with PST/PSM; in the majority of reviews significantly lower mortality rates were also reported. The incidence of major bleeding events remained unchanged in all reviews. A significant advantage for PST/PSM with regard to treatment satisfaction was observed in most reviews and also found in a study among patients older than 60 years (6). When the various strategies for the assumption of responsibility by patients are considered separately, PSM appears to be the superior strategy.
Likewise, the authors of the 2010 Cochrane Review (14) on PST/PSM with oral anticoagulation, which was included in this review, arrive at the conclusion that patients can with the help of PST/PSM improve the quality of therapy compared with standard care. For both the number of deaths (relative risk: 0.64 [0.46–0.89]) and the number of thromboembolic events (relative risk: 0.50 [0.36–0.69]) a statistically significant reduction was achieved without any additional harm (Table 3). In addition, the Cochrane Review showed that for about half of all patients long-term therapy with PST or PSM is a viable option. This was confirmed by the SPOG 60 + study (20) especially for elderly patients. It goes without saying that, for example, elderly bedridden patients and patients with dementia or major visual impairment who have nobody to take over the care are excluded from self-management.
At present, no studies are available that directly compare therapy with coumarin derivatives under PST or PSM with the new antithrombotic agents (currently approved: dabigatran, rivaroxaban, apixaban). Thus, the difference between the two therapies can only be estimated by comparing the effects of each of the therapeutic options with standard treatment (physician-managed coumarin therapy). In addition, further considerations regarding their use under real-life conditions are to be taken into account as well. Inasmuch as significant advantages have been observed in the studies evaluating the new antithrombotic agents (21– 23) to date, the estimated annual numbers needed to treat (NNTs) were above 200 for all-cause mortality, at least 130 for thromboembolic events such as stroke and myocardial infarction, and above 100 for major bleeding. However, many studies did not find significant differences for these endpoints. In contrast, for PST/PSM, especially if PSM is evaluated alone, annual NNTs for all-cause mortality and thromboembolic events of under 100 can be assumed.
Heneghan (2012) calculated the NNTs on the basis of individual patient data and described positive effects of PST and PSM on thromboembolic events, with an NNT of 78 after 1 year and an NNT of 27 for a period of 5 years (7).
A limitation of this review may be that the systematic research undertaken was limited to only 1 electronic database. However, since a validated filter was used, it can be assumed that the most important papers were identified. These include recent publications in peer-reviewed journals, a Cochrane Review and health technology assessment (HTA) reports, most of which used the same RCTs as evidence basis and arrived at essentially similar results when evaluating the efficacy of PST/PSM. This was an interesting observation since at the start of our research we had expected major differences between the reviews with regards to the included RCTs and, consequently, their results. The selected method of using secondary literature as the basis for the evaluation allows to save the steps on the primary literature level already undertaken by the authors of high-quality reviews, without compromising on the robustness of the result of our research while avoiding redundant analyses. However, this robustness advantage postulated by us also has the aspect of a limitation as it is to be expected that reviews based on the same studies will arrive at similar results.
Finally, it should be noted that there is a general need for care optimization in the area of anticoagulation management. The paper of Saal (2009) shows that, for example, in Germany significant safety gaps exist among physicians, especially regarding documentation, patient information, awareness of side effects, and drug interactions (24). This situation will not change with the prescription of the new antithrombotic agents, which, because of their mechanism of action, naturally have a comparable side effect profile, in particular with regard to the risk of bleeding events. This was documented in the study evaluating dabigatran in patients with mechanical heart valves which was terminated early (25). In addition, costs should be taken into consideration. Even when the annual costs associated with self-management (test sticks and a coumarin-type anticoagulant) are added up, the new antithrombotic agents are still about EUR 1000 more expensive on an annual basis (26). Nevertheless, the number of new prescriptions, e.g. of rivaroxaban, rose to a 2-digit million figure within just a few months (27). The use of such new blockbuster drugs adds a further enormous burden on the German health system, especially since the majority of patients with a long-term indication for anticoagulation can be adequately treated with conventional coumarin therapy (28). Even though not all health insurance providers reimburse the costs of the devices as a rule, these costs are in most cases covered if conclusive reasons are given (Table 4). Therefore, a large health services research study (PICANT) has recently been initiated (29) in Germany with the aim of improving the care of patients with a long-term indication for anticoagulant medication and of reducing the incidence of anticoagulation-associated complications, by using a best practice approach.
Table 4. Possible reasons for prescribing point-of-care monitoring devices.
| No medical reason required for suitable patients with artificial heart valve replacement not later than 3 months after surgery | Evidence for reduction of complication rate is available*1 | |
Statutory health insurance providers in Germany have a medically justified reimbursement obligation for patient self-monitoring of the disease course with immediate dose adjustments in the following cases, among others:
|
Need for anticoagulation monitoring and self-adjustment of dosage is only met by self-management of anticoagulation therapy | |
| “We recommend that you explain in each case the reasons why anticoagulation self-testing is an absolute medical requirement in great detail to ensure, to the extent possible, that your patient’s application for cost coverage is accepted by the health insurance provider without problems.“ *2 | ||
*1Koertke, H. et al.: INR self-management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation 2003; 108 (Suppl 1): II75–78
*2According to the recommendation of the Arbeitsgemeinschaft Selbstkontrolle der Antikoagulation e. V. (ASA) for the updating of the medical technical aids reimbursement list
Altogether it was shown that adult patients with a long-term indication for oral anticoagulation benefit from patient self-management compared with standard therapy with physician-managed anticoagulation. However, for patient self-testing alone this was not confirmed.
Key Messages.
Self-management of oral anticoagulation therapy reduces the incidence of major thromboembolic events and fatalities compared with physician-managed anticoagulation therapy alone.
Patient self-management appears to be superior to patient self-testing alone with regard to providing a clinically relevant additional benefit
A key pre-requisite for anticoagulation management is to ensure above all that patients learn how to adjust the dosage in structured, validated educational programs.
Anticoagulation management is an option for all patients with the necessary mental abilities and fine motor skills as well as adequate vision.
Studies directly comparing coumarin-based anticoagulation plus patient self-management with the recently approved new thrombin inhibitors are currently lacking.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
We would like to thank Ms. Antonia Zengerer for her support with collecting and checking the data.
Footnotes
Conflict of interest statement
At the Institute of General Practice, Goethe University Frankfurt, the BMBF-sponsored study (FKZ: 01GY1145) on “Primary Care Management for Optimized Antithrombotic Treatment (PICANT)” has been conducted since March 2012. The HEALTH Institute of JOANNEUM RESEARCH Forschungs-GmbH prepared a health economic analysis regarding the self-management of oral anticoagulation for ROCHE Diagnostics in 2011.
The full report of this manuscript was prepared by the authors for the Federation of Austrian Social Insurance Institutions (HVB) and is available on its homepage under www.hauptverband.at/mediaDB/953046_Bericht_Selbstmanagement_orale_Antikoagulation.pdf
References
- 1.Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324:1865–1875. doi: 10.1056/NEJM199106273242606. [DOI] [PubMed] [Google Scholar]
- 2.Hein L. Antithrombotika und Antihämorrhagika. In: Schwabe U, Paffrath D, editors. Arzneiverordnungs-Report 2011. Berlin, Heidelberg: Springer; 2011. pp. 421–439. [Google Scholar]
- 3.Sawicki PT. A structured teaching and self-management program for patients receiving oral anticoagulation: a randomized controlled trial. Working Group for the Study of Patient Self-Management of Oral Anticoagulation. JAMA. 1999;281:145–150. doi: 10.1001/jama.281.2.145. [DOI] [PubMed] [Google Scholar]
- 4.Voller H, Dovifat C, Glatz J, Kortke H, Taborski U, Wegscheider K. Self management of oral anticoagulation with the IN Ratio system: impact of a structured teaching program on patient’s knowledge of medical background and procedures. Eur J Cardiovasc Prev Rehabil. 2004;11:442–447. doi: 10.1097/00149831-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Watzke H. Patienten-Compliance beim Gerinnungs-Selbstmanagement. Die Punkte - DFP Literatur. 2011 Angiologie 2/2011:8. [Google Scholar]
- 6.Siebenhofer A, Hemkens LG, Rakovac I, Spat S, Didjurgeit U. Self-management of oral anticoagulation in elderly patients - effects on treatment-related quality of life. Thromb Res. 2012;130:e60–e66. doi: 10.1016/j.thromres.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Heneghan C, Ward A, Perera R, et al. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. [Review] [Erratum appears in Lancet 2012 379 1102] Lancet. 2012;379:322–334. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
- 8.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) Allgemeine Methoden: Version 4.0. www.iqwig.de/download/IQWiG_Methoden_Version_4_0.pdf. 2011. Last accessed on 13 March 2013.
- 9.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 51.0. 2011. The Cochrane Collaboration. www.cochrane-handbook.org. Last accessed on 13. March 2013. [Google Scholar]
- 10.Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44:1271–1278. doi: 10.1016/0895-4356(91)90160-b. [DOI] [PubMed] [Google Scholar]
- 11.Oxman AD, Guyatt GH, Singer J, et al. Agreement among reviewers of review articles. J Clin Epidemiol. 1991;44:91–98. doi: 10.1016/0895-4356(91)90205-n. [DOI] [PubMed] [Google Scholar]
- 12.Siebenhofer-Kroitzsch A, Semlitsch T. Selbstmanagement der oralen Antikoagulation - Evidenz und Ist-Analyse in Österreich 2013. Hauptverband der österreichischen Sozialversicherungsträger. www.hauptverband.at/mediaDB/953046_Bericht_Selbstmanagement_orale_Antikoagulation.pdf. Last accessed on 13. March 2013. [Google Scholar]
- 13.Bloomfield HE, Krause A, Greer N, et al. Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Annals of Internal Medicine. 2011;154:472–482. doi: 10.7326/0003-4819-154-7-201104050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Alamino JM, Ward AM, Alonso-Coello P, et al. Self-monitoring and self-management of oral anticoagulation. Review] [83 refs] [Cochrane Database of Systematic Reviews. 2010;(4) doi: 10.1002/14651858.CD003839.pub2. CD003839. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Wang Z, Ou J, Xu Y, Yang S, Zhang X. Two monitoring methods of oral anticoagulant therapy in patients with mechanical heart valve prothesis: a meta-analysis. Journal of Thrombosis & Thrombolysis. 2012;33:38–47. doi: 10.1007/s11239-011-0626-1. [DOI] [PubMed] [Google Scholar]
- 16.Christensen TD, Johnsen SP, Hjortdal VE, Hasenkam JM. Self-management of oral anticoagulant therapy: a systematic review and meta-analysis. International Journal of Cardiology. 2007;118:54–61. doi: 10.1016/j.ijcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Connock M, Stevens C, Fry-Smith A, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. 2007;11 doi: 10.3310/hta11380. iii-iv, ix-66. [DOI] [PubMed] [Google Scholar]
- 18.Health Quality O. An evidence-based analysis. Vol. 9. Ontario: Health Technology Assessment Series; 2009. Point-of-care international normalized ratio (INR) monitoring devices for patients onlong-term oral anticoagulation therapy; pp. 1–114. [PMC free article] [PubMed] [Google Scholar]
- 19.Wells PS, Brown A, Jaffey J, McGahan L, Poon MC, Cimon K. Safety and effectiveness of point-of-care monitoring devices in patients on oral anticoagulant therapy: a meta-analysis. Open Med. 2007;1:e131–e146. Epub 2007 Oct 16. [PMC free article] [PubMed] [Google Scholar]
- 20.Siebenhofer A, Rakovac I, Kleespies C, Piso B, Didjurgeit U. Self-management of oral anticoagulation reduces major outcomes in the elderly. A randomized controlled trial. Thromb Haemost. 2008;100:1089–1098. [PubMed] [Google Scholar]
- 21.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 23.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 24.Saal K, Hofmann B, Blauth E, et al. Identifying safety problems in the General Practice - analysis of the treatment process of oral anticoagulation therapy. Zeitschrift für Allgemeinmedizin. 2009;85:148–155. [Google Scholar]
- 25.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus Warfarin in patients with mechanical heart valves. N Engl J Med. 2013 doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 26.Neue Indikationen für Faktor Xa-Hemmstoff Rivaroxaban (Xarelto) Arznei-Telegramm. 2012;43:2–12. [Google Scholar]
- 27.Schwabe U, Paffrath D, editors. Berlin: Springer-Verlag GmbH; 2013. Arzneiverordnungs-Report 2013 - Aktuelle Daten, Kosten, Trends und Kommentare. [Google Scholar]
- 28.Arzneimittelkommission der deutschen Ärztschaft (AkdÄ) Orale Antikoagulation bei nicht valvulären Vorhofflimmern - Empfehlungen zum Einsatz der neuen Antikoagulantien Dabigatran (Pradaxa) und Rivaroxaban (Xarelto) www.akdae.de/Arzneimitteltherapie/TE/LF/PDF/OAKVHF.pdf. 2012. Last accessed on 8. November 2012.
- 29.Siebenhofer A, Ulrich LR, Mergenthal K, et al. Primary care management for optimized antithrombotic treatment [PICANT]: study protocol for a cluster-randomized controlled trial. Implement Sci. 2012;7 doi: 10.1186/1748-5908-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.White RH, McCurdy SA, von Marensdorff H, et al. Home prothrombin time monitoring after the initiation of warfarin therapy. A randomized, prospective study. Annals of Internal Medicine. 1989;111:730–737. doi: 10.7326/0003-4819-111-9-730. [DOI] [PubMed] [Google Scholar]
- e2.Horstkotte D, Piper C, Wiemer M. Optimal Frequency of Patient Monitoring and Intensity of Oral Anticoagulation Therapy in Valvular Heart Disease. Journal of Thrombosis and Thrombolysis. 1998 5;(Suppl 1):19–24. doi: 10.1023/a:1013228718768. [DOI] [PubMed] [Google Scholar]
- e3.Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Annals of Internal Medicine. 2000;133:687–695. doi: 10.7326/0003-4819-133-9-200011070-00010. [DOI] [PubMed] [Google Scholar]
- e4.Cromheecke ME, Levi M, Colly LP, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet. 2000;356:97–102. doi: 10.1016/S0140-6736(00)02470-3. [DOI] [PubMed] [Google Scholar]
- e5.Kaatz S, Elston-Lafata J, Gooldy S. Anticoagulation therapy home and office monitoring evaluation study. J Thromb Thrombolysis. 2001;12 [Google Scholar]
- e6.Kortke H, Minami K, Breymann T, et al. [INR self-management after mechanical heart valve replacement: ESCAT (Early Self-Controlled Anticoagulation Trial)] Zeitschrift fur Kardiologie. 2001 90;(Suppl 6):118–124. doi: 10.1007/s003920170019. [DOI] [PubMed] [Google Scholar]
- e7.Sidhu P, O’Kane HO. Self-managed anticoagulation: results from a two-year prospective randomized trial with heart valve patients. The Annals of Thoracic Surgery. 2001;72:1523–1527. doi: 10.1016/s0003-4975(01)03049-1. [DOI] [PubMed] [Google Scholar]
- e8.Fitzmaurice DA, Murray ET, Gee KM, et al. A randomised controlled trial of patient self management of oral anticoagulation treatment compared with primary care management. Journal of Clinical Pathology. 2002;55:845–849. doi: 10.1136/jcp.55.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Gadisseur AP, Breukink-Engbers WG, van der Meer FJ, et al. Comparison of the quality of oral anticoagulant therapy through patient self-management and management by specialized anticoagulation clinics in the Netherlands: a randomized clinical trial. Archives of Internal Medicine. 2003;163:2639–2646. doi: 10.1001/archinte.163.21.2639. [DOI] [PubMed] [Google Scholar]
- e10.Khan TI, Kamali F, Kesteven P, et al. The value of education and self-monitoring in the management of warfarin therapy in older patients with unstable control of anticoagulation. British Journal of Haematology. 2004;126(4):557–564. doi: 10.1111/j.1365-2141.2004.05074.x. [DOI] [PubMed] [Google Scholar]
- e11.Sunderji R, Gin K, Shalansky K, et al. A randomized trial of patient self-managed versus physician-managed oral anticoagulation. The Canadian Journal of Cardiology. 2004;20:1117–1123. [PubMed] [Google Scholar]
- e12.Claes N, Buntinx F, Vijgen J, et al. The Belgian Improvement Study on Oral Anticoagulation Therapy: a randomized clinical trial. European Heart Journal. 2005;26:2159–2165. doi: 10.1093/eurheartj/ehi327. [DOI] [PubMed] [Google Scholar]
- e13.Fitzmaurice DA, Murray ET, McCahon D, et al. Self management of oral anticoagulation: randomised trial. BMJ (Clinical researched) 2005;331 doi: 10.1136/bmj.38618.580903.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Gardiner C, Williams K, Mackie IJ, et al. Patient self-testing is a reliable and acceptable alternative to laboratory INR monitoring. British Journal of Haematology. 2005;128:242–247. doi: 10.1111/j.1365-2141.2004.05300.x. [DOI] [PubMed] [Google Scholar]
- e15.Menendez-Jandula B, Souto JC, Oliver A, et al. Comparing self-management of oral anticoagulant therapy with clinic management: a randomized trial. Annals of Internal Medicine. 2005;142:1–10. doi: 10.7326/0003-4819-142-1-200501040-00006. [DOI] [PubMed] [Google Scholar]
- e16.Voller H, Glatz J, Taborski U, et al. Self-management of oral anticoagulation in nonvalvular atrial fibrillation (SMAAF study) Zeitschrift fur Kardiologie. 2005;94:182–186. doi: 10.1007/s00392-005-0199-0. [DOI] [PubMed] [Google Scholar]
- e17.Christensen TD, Maegaard M, Sorensen HT, et al. Self-management versus conventional management of oral anticoagulant therapy: A randomized, controlled trial. European Journal of Internal Medicine. 2006;17:260–266. doi: 10.1016/j.ejim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- e18.Gardiner C, Williams K, Longair I, et al. A randomised control trial of patient self-management of oral anticoagulation compared with patient self-testing. British Journal of Haematology. 2006;132:598–603. doi: 10.1111/j.1365-2141.2005.05899.x. [DOI] [PubMed] [Google Scholar]
- e19.Dauphin C, Legault B, Jaffeux P, et al. Comparison of INR stability between self-monitoring and standard laboratory method: preliminary results of a prospective study in 67 mechanical heart valve patients. Archives of Cardiovascular Diseases. 2008;101:753–761. doi: 10.1016/j.acvd.2008.10.007. [DOI] [PubMed] [Google Scholar]
- e20.Eitz T, Schenk S, Fritzsche D, et al. International normalized ratio self-management lowers the risk of thromboembolic events after prosthetic heart valve replacement. The Annals of Thoracic Surgery. 2008;85:949–54. doi: 10.1016/j.athoracsur.2007.08.071. discussion 55. [DOI] [PubMed] [Google Scholar]
- e21.Ryan F, Byrne S, O’Shea S. Randomized controlled trial of supervised patient self-testing of warfarin therapy using an internet-based expert system. Journal of Thrombosis and Haemostasis. 2009;7:1284–1290. doi: 10.1111/j.1538-7836.2009.03497.x. [DOI] [PubMed] [Google Scholar]
- e22.Soliman Hamad MA, van Eekelen E, van Agt T, et al. Self-management program improves anticoagulation control and quality of life: a prospective randomized study. European Journal of Cardio-Thoracic Surgery. 2009;35:265–269. doi: 10.1016/j.ejcts.2008.10.020. [DOI] [PubMed] [Google Scholar]
- e23.Matchar DB, Jacobson A, Dolor R, et al. Effect of home testing of international normalized ratio on clinical events. NEJM. 2010;363:1608–1620. doi: 10.1056/NEJMoa1002617. [DOI] [PubMed] [Google Scholar]