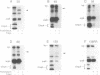Abstract
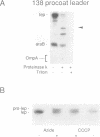
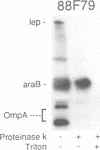
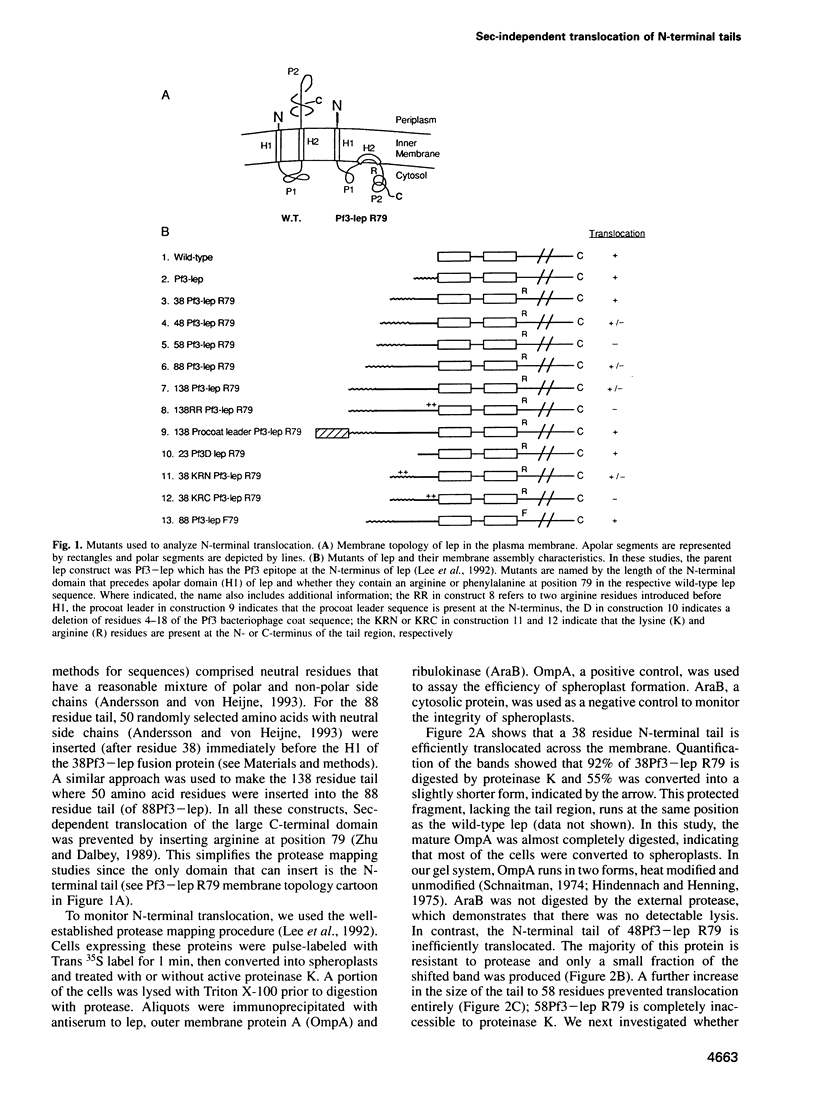
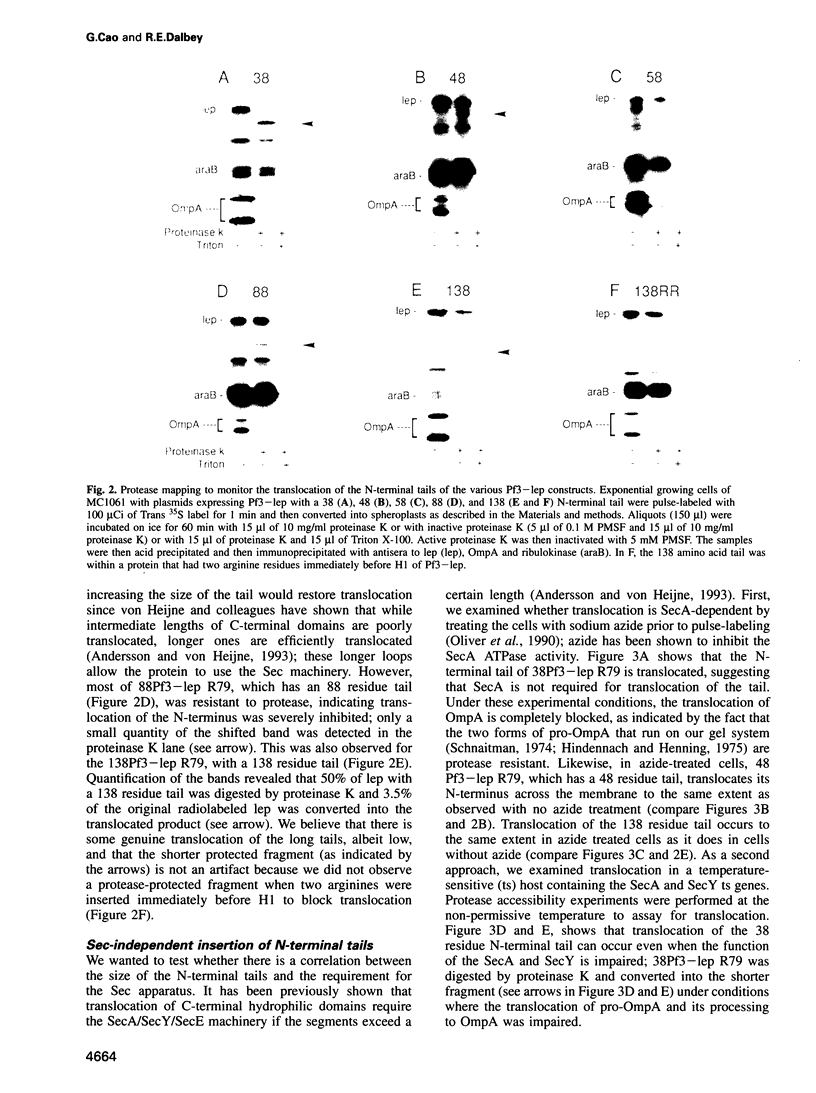
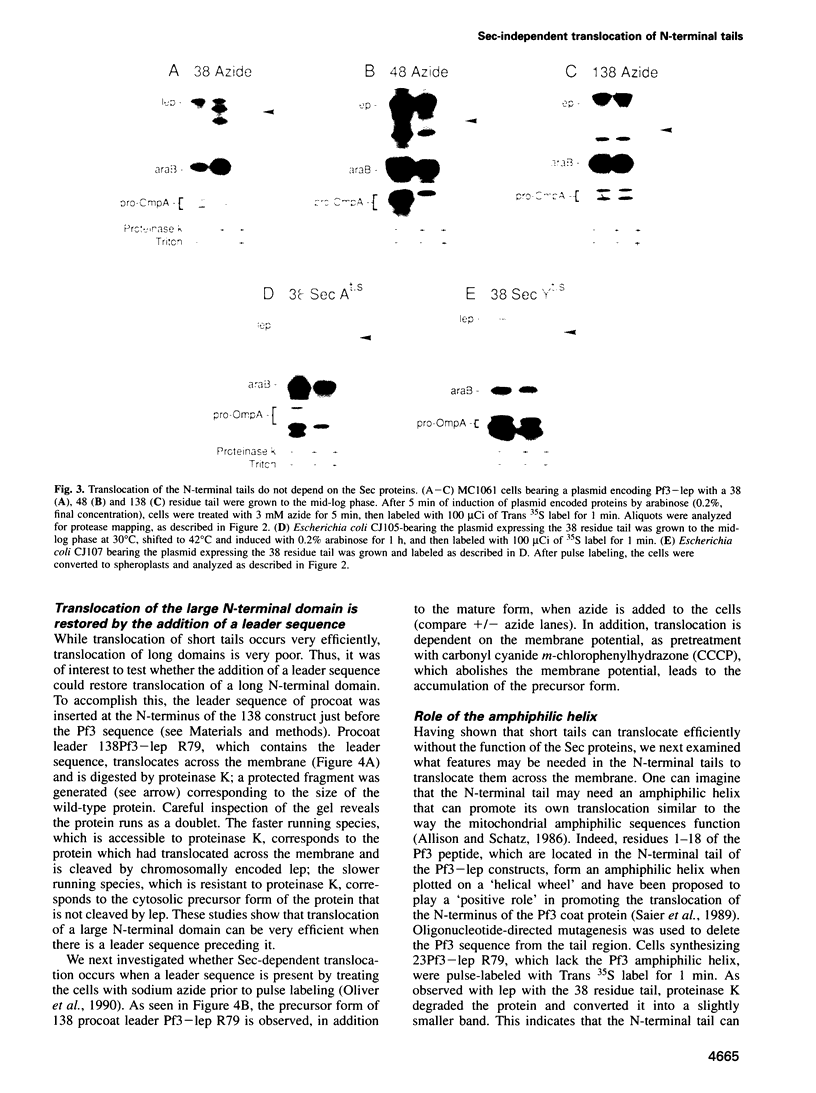
Previously we have shown that the first hydrophobic domain of leader peptidase (lep) can function to translocate a short N-terminal 18 residue antigenic peptide from the phage Pf3 coat protein across the plasma membrane of Escherichia coli. We have now examined the mechanism of insertion of N-terminal periplasmic tails and have defined the features needed to translocate these regions. We find that short tails of up to 38 residues are efficiently translocated in a SecA- and SecY-independent manner while longer tails are very poorly inserted. Efficient translocation of a 138 residue tail is restored and is Sec-dependent by the addition of a leader sequence to the N-terminus of the protein. We also find that while there is no amphiphilic helix requirement for N-terminal translocation, there is a charge requirement that is needed within the tail; an arginine and lysine residue can inhibit or completely block translocation when introduced into the tail region. Intriguingly, the membrane potential is required for insertion of a 38 residue tail but not for a 23 residue tail.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. S., Schatz G. Artificial mitochondrial presequences. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9011–9015. doi: 10.1073/pnas.83.23.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson H., von Heijne G. Sec dependent and sec independent assembly of E. coli inner membrane proteins: the topological rules depend on chain length. EMBO J. 1993 Feb;12(2):683–691. doi: 10.1002/j.1460-2075.1993.tb05702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Beckwith J. Positively charged amino acid residues can act as topogenic determinants in membrane proteins. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9446–9450. doi: 10.1073/pnas.86.23.9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Beckwith J. The role of charged amino acids in the localization of secreted and membrane proteins. Cell. 1990 Sep 21;62(6):1031–1033. doi: 10.1016/0092-8674(90)90378-r. [DOI] [PubMed] [Google Scholar]
- Boyd D., Manoil C., Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Kuhn A., Wickner W. The internal signal sequence of Escherichia coli leader peptidase is necessary, but not sufficient, for its rapid membrane assembly. J Biol Chem. 1987 Sep 25;262(27):13241–13245. [PubMed] [Google Scholar]
- Dalbey R. E. Positively charged residues are important determinants of membrane protein topology. Trends Biochem Sci. 1990 Jul;15(7):253–257. doi: 10.1016/0968-0004(90)90047-f. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase of Escherichia coli: critical role of a small domain in membrane assembly. Science. 1987 Feb 13;235(4790):783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- Ehrmann M., Beckwith J. Proper insertion of a complex membrane protein in the absence of its amino-terminal export signal. J Biol Chem. 1991 Sep 5;266(25):16530–16533. [PubMed] [Google Scholar]
- Emr S. D., Schwartz M., Silhavy T. J. Mutations altering the cellular localization of the phage lambda receptor, an Escherichia coli outer membrane protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5802–5806. doi: 10.1073/pnas.75.12.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey E. S., Hashemzadeh-Bonehi L., Hunt L. A., Broome-Smith J. K. Assembly of eukaryotic class III (N-out, C-in) membrane proteins into the Escherichia coli cytoplasmic membrane. FEBS Lett. 1993 Sep 27;331(1-2):159–161. doi: 10.1016/0014-5793(93)80317-n. [DOI] [PubMed] [Google Scholar]
- Hindennach I., Henning U. The major proteins of the Excherichia coli outer cell envelope membrane. Preparative isolation of all major membrane proteins. Eur J Biochem. 1975 Nov 1;59(1):207–213. doi: 10.1111/j.1432-1033.1975.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Ito K., Date T., Wickner W. Synthesis, assembly into the cytoplasmic membrane, and proteolytic processing of the precursor of coliphage M13 coat protein. J Biol Chem. 1980 Mar 10;255(5):2123–2130. [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kuhn A. Alterations in the extracellular domain of M13 procoat protein make its membrane insertion dependent on secA and secY. Eur J Biochem. 1988 Nov 1;177(2):267–271. doi: 10.1111/j.1432-1033.1988.tb14372.x. [DOI] [PubMed] [Google Scholar]
- Kuhn A., Zhu H. Y., Dalbey R. E. Efficient translocation of positively charged residues of M13 procoat protein across the membrane excludes electrophoresis as the primary force for membrane insertion. EMBO J. 1990 Aug;9(8):2385–2389. doi: 10.1002/j.1460-2075.1990.tb07413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws J. K., Dalbey R. E. Positive charges in the cytoplasmic domain of Escherichia coli leader peptidase prevent an apolar domain from functioning as a signal. EMBO J. 1989 Jul;8(7):2095–2099. doi: 10.1002/j.1460-2075.1989.tb03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. I., Kuhn A., Dalbey R. E. Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J Biol Chem. 1992 Jan 15;267(2):938–943. [PubMed] [Google Scholar]
- Lewis M. J., Chang J. A., Simoni R. D. A topological analysis of subunit alpha from Escherichia coli F1F0-ATP synthase predicts eight transmembrane segments. J Biol Chem. 1990 Jun 25;265(18):10541–10550. [PubMed] [Google Scholar]
- McGovern K., Ehrmann M., Beckwith J. Decoding signals for membrane protein assembly using alkaline phosphatase fusions. EMBO J. 1991 Oct;10(10):2773–2782. doi: 10.1002/j.1460-2075.1991.tb07826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Moore K. E., Miura S. A small hydrophobic domain anchors leader peptidase to the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1987 Jun 25;262(18):8806–8813. [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990 Sep 21;62(6):1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler B., Boyd D., Beckwith J. The topological analysis of integral cytoplasmic membrane proteins. J Membr Biol. 1993 Feb;132(1):1–11. doi: 10.1007/BF00233047. [DOI] [PubMed] [Google Scholar]
- Whitley P., Nilsson L., von Heijne G. Three-dimensional model for the membrane domain of Escherichia coli leader peptidase based on disulfide mapping. Biochemistry. 1993 Aug 24;32(33):8534–8539. doi: 10.1021/bi00084a020. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Rice M., Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985 Feb 10;260(3):1836–1841. [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W. Bacterial leader peptidase, a membrane protein without a leader peptide, uses the same export pathway as pre-secretory proteins. Cell. 1984 Apr;36(4):1067–1072. doi: 10.1016/0092-8674(84)90056-4. [DOI] [PubMed] [Google Scholar]
- Zhu H. Y., Dalbey R. E. Both a short hydrophobic domain and a carboxyl-terminal hydrophilic region are important for signal function in the Escherichia coli leader peptidase. J Biol Chem. 1989 Jul 15;264(20):11833–11838. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]