Abstract
Purpose.
L-type voltage gated calcium channels in retina localize primarily at the presynaptic active zones of photoreceptors and bipolar cells where they modulate glutamate release. However, the pore forming subunit Cacna1s of certain L-type channels is also expressed postsynaptically at the tips of ON bipolar cell dendrites where it colocalizes with mGluR6, but has an unknown function. At these dendritic tips, the components of the mGluR6 signaling cascade cluster together in a macromolecular complex, and each one's localization often depends on that of the others. Thus, we explored if Cacna1s is part of the mGluR6 complex.
Methods.
We determined Cacna1s expression by PCR using an ON bipolar library, by Western blotting, and by standard immunohistochemistry.
Results.
The PCR amplification confirmed expression of the transcript in ON bipolar cells, and Western blotting showed the expected bands. Immunostaining for Cacna1s was stronger in the dendritic tips of rod bipolar cells than in those of ON cone bipolar cells. This staining severely decreased in mice missing various mGluR6 cascade elements (Grm6−/−, Gnao1−/−, Gnb3−/−, Gng13−/−, and Trpm1−/−). During development, the ratio of the number of Cacna1s puncta to the number of presynaptic ribbons followed a sigmoidal pattern, rising rapidly from P13 to P17. The mGluR6 expression preceded that of Cacna1s and RGS11.
Conclusions.
Our results show that the localization and stability of Cacna1s depend on the expression of mGluR6 and its cascade components, and they suggest that Cacna1s is part of the mGluR6 complex. We hypothesize that Cacna1s contributes to light adaptation by permeating calcium.
Keywords: rod bipolar cell, retina, adaptation, mGluR6, signaling cascade
The pore forming subunit of a L-type voltage gated calcium channel colocalizes with the components of the ON bipolar cell signaling cascade and its localization is dependent on the normal expression of these components.
Introduction
L-type voltage gated calcium channels are characterized by high voltage thresholds of activation, large single channel conductances, slow voltage-dependent inactivation, and sensitivity to dihydropyridines, phenylalkylamines, and benzothiazepines.1 These channels are expressed in skeletal muscle cells, where they mediate excitation-contraction coupling through direct interaction with the ryanodine receptor; in endocrine cells, where they regulate hormone release; and in neurons, where they have diverse roles in neural processing. In the retina, L-type channels localize at the presynaptic active zones of photoreceptor and bipolar cell ribbon synapses.2,3 There, they are necessary for glutamate release and, consequently, for synaptic transmission from these first and second order retinal neurons. More recently, Cacna1s, the pore-forming α subunit (also called Cav1.1) of some L-type voltage-gated calcium channels, was discovered at the tips of ON bipolar cell dendrites,4,5 where it colocalizes with mGluR6, the metabotropic glutamate receptor that generates the light response of these cells.6–10
The ON bipolar cells are a class of second order neurons in the retina that signal increments in light intensity through two primary pathways: the rod bipolar and ON cone bipolar pathways. Rod bipolar cells receive input exclusively from rod photoreceptors and constitute the visual pathway primarily responsible for night vision. The ON cone bipolar cells receive input from cone photoreceptors and constitute multiple (4–5) parallel pathways that transmit image characteristics in daylight.11 All types of ON bipolar cells use mGluR6 to detect changes in glutamate concentration.6,9,12 In the dark, glutamate released continuously by the photoreceptors binds mGluR6 and activates the heterotrimeric G-protein Go. This closes the nonselective melastatin-type transient receptor potential cation channel, TRPM1, thus hyperpolarizing the cell.13–16 The G-protein is inactivated by a GTPase activating protein (GAP) complex that is formed by the regulators of G-protein signaling RGS7 and RGS11, their membrane anchor R9AP (or GPR179), and a unique Gβ subunit Gβ5. This complex colocalizes with mGluR6 and catalyzes the hydrolysis of GTP.17–22 Light reduces the rate of glutamate release, inactivates Go, opens TRPM1 channels, and depolarizes the cell.
Recent studies indicate that the components of this mGluR6 cascade that are essential for the light response of ON bipolar cells cluster in a macromolecular complex at the dendritic tip, such that the proper localization of any component depends on the presence and proper localization of the others in the complex.10,20,23–27 We, therefore, hypothesized that if Cacna1s contributes to the light response of ON bipolar cells, it may be a part of the mGluR6 cascade complex. Here, we tested this idea by examining Cacna1s expression in knockout (KO) mice lacking a gene that encodes a key cascade element and in normal mice during development. Our results showed that Cacna1s localization dramatically changes in several KO mice, and that it is expressed concurrently with the key players in the mGluR6 cascade during development. These findings support the hypothesis that Cacna1s is part of the mGluR6 complex.
Methods
Animals
Wild-type mice (WT, C57BL/6J) or rats (Sprague Dawley) of both sexes and pregnant female mice were purchased from Charles River Laboratories (Wilmington, MA). Gnao1−/− (gene encoding Gαo1), Grm6-GFP (GFP driven under mGluR6 promoter), and Gnb3−/− (gene encoding Gβ3) mice were generated and maintained as described previously.13,24,28 The Gng13−/− (gene encoding Gγ13) mice were generated by the University of Pennsylvania Transgenic and Chimeric Mouse Facility, and will be described elsewhere (Ramakrishnan H, Dhingra A, Fina M, Lyubarsky A, Vardi N, manuscript in preparation, 2014). The Grm6−/− (gene encoding mGluR6) mouse9,10 was a gift from Shigetada Nakanishi (Kyoto University, Kyoto, Japan) and David Copenhagen (University of California, San Francisco, CA). Trpm1−/− (gene encoding TRPM1) retinas were obtained from Neal Peachey (Cleveland Clinic, Cleveland, OH). For developmental studies, WT retinas were harvested from litters born to six different mothers at the following postnatal (P) ages: litter 1: P0, P1, P2, P4, P17, P19, P21, P25, and P28; litter 2: P0, P3, P5, P6, P7, P8, and P9; litter 3: P11 and P14; litters 4 and 5 each: P9, P11, P13, P15, P17, and P28; and litter 6: P13, P14, P15, and P19. Two additional mice were euthanized at postnatal day P21 to increase the sample number at this age. Animal care and use was in compliance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) and Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. The protocol was approved by the Committee for Ethics of Animal Experiments of the University of Pennsylvania (Protocol Number 803174). Mice older than P5 were anesthetized with a mixture of ketamine (100 μg/g) and xylazine (10 μg/g), and pups younger than P5 were decapitated. Eyes were enucleated, and the cornea and lens were removed. Animals older than P5 were euthanized with an overdose (3-fold higher concentration) of the anesthetic drugs.
Amplification of Cacna1s Transcript
To determine if ON bipolar cells transcribe Cacna1s, we performed PCR on an ON bipolar cDNA library generated previously from a Grm6-GFP transgenic mouse (containing greater than 106 independent clones with no contamination from other cell types).28 Library DNA was isolated (QIAprep Spin Miniprep Kit; Qiagen, Inc., Valencia, CA) and PCR was performed at two dilutions for 35 cycles (denaturation at 95°C for 15 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 30 seconds). Molecular biology-grade water was used as a negative control, and no other tissue was processed at the same time to avoid false-positives. The following custom synthesized primer sequences were used for Cacna1s (NM_014193.2): Upper1 (U5264), 5′-TTC CCA GGA ATT CGG CTC ACA GGT -3′; Upper2 (U5070), 5′-GGC CTC AGG ACC TCA CAG CAG AT -3′; and Lower (L5546), 5′- GTT TGG GAG CCC CAA CGC AGA TT -3.′ These primer pairs were designed to amplify a sequence present in both splice variants of Cacna1s. The expected band sizes were 283 and 477 base pairs (bp). The PCR products were sequenced to confirm the correct message. For quantitative RT-PCR, after enucleation, retinas were removed and immediately frozen for RNA extraction. Retinal mRNA was reverse transcribed, and Cacna1s transcript was amplified with a Power SYBR Green kit (Applied Biosystems, Life Technologies, Carlsbad, CA) using an Applied Biosystems 7500 Fast Real-Time PCR System. GAPDH was used as a reference gene for normalization, and relative gene expression was analyzed using the relative standard curve method described in the manufacturer's protocol. The primers used for qRT-PCR for Cacna1s were: Upper1 (U5264) and Lower (L5546), and GAPDH (NM_008084.2): forward, (5) ACG GCC GCA TCT TCT TGT GCA; reverse, (85) ATA CGG CCA AAT CCG TTC ACA CCG.
Western Blotting
After enucleation, retinas of WT mice were isolated quickly and frozen in liquid nitrogen. For membrane preparation, tissue was homogenized in PBS buffer containing 150 mM NaCl and protease inhibitor (P 8340; Sigma-Aldrich, St. Louis, MO). The homogenate was centrifuged at 100,000g for 30 minutes at 4°C. The pellet was resuspended in PBS-based detergent buffer containing150 mM NaCl, 1% Triton X-100, and protease inhibitor, and was incubated on a rocker at 4°C for 1 hour. The sample was centrifuged again at 100,000g at 4°C for 30 minutes and the supernatant was collected. The supernatant was run on 7.5% SDS-PAGE gel and transferred to a nitrocellulose membrane using a wet transfer apparatus (Bio-Rad Laboratories, Inc., Hercules, CA). After a brief rinse in PBS, the blots were incubated sequentially in the following: blocking buffer containing 5% milk in PBST (PBS plus 0.1% Tween 20; 1 hour), primary antibody diluted in blocking buffer at 4°C overnight, PBST (3 × 10 minute washes), anti-mouse or goat-HRP secondary antibody diluted in blocking buffer for 3 hours, and PBST (3 × 10-minute washes). The blot was developed for visualization using SuperSignal West Pico Chemiluminescent Substrate (Pierce Protein Biology Products, Thermo Fisher Scientific, Inc., Rockford, IL). Cacna1s antibodies (diluted 1:100) used were mouse mAB 1a (MAB427; Chemicon, Millipore, Billerica, MA) and goat N-19 (sc-8160; Santa Cruz Biotechnology, Inc., Dallas, TX).
Immunohistochemistry
After enucleation, eyecups were fixed in 4% paraformaldehyde for 10 minutes, rinsed in phosphate buffer, soaked overnight in 30% sucrose phosphate buffer, and embedded in a 2:1 mixture of 20% sucrose and tissue freezing medium. The embedded eyecups were cryosectioned into 18-μm radial sections. To compare Cacna1s expression in KO and WT retinas, we performed immunohistochemistry simultaneously on retinas from a KO mouse and from its littermate or an age-matched WT animal (henceforth referred to as a set; three sets, therefore, imply retinas from three WT and three KO animals). To determine Cacna1s expression during development, we performed immunohistochemistry simultaneously on retinas of mice of multiple ages (henceforth referred to as a series). Sections were immersed in a blocking solution consisting of 10% normal goat or donkey serum, 5% sucrose, and 0.5% Triton X-100 in phosphate buffer for 1 hour at room temperature. They then were incubated with primary antibodies overnight at 4°C, rinsed with 5% sucrose in phosphate buffer, and incubated with fluorescently tagged secondary antibodies for 3 hours at room temperature. Sections were rinsed again and mounted in Vectashield (Vector Laboratories, Burlingame, CA). The antibodies used were: mouse anti-Cacna1s (mAB 1a; 1:500, Millipore), rabbit polyclonal anti-Ribeye (1:500; gift from Thomas Sudhof, Stanford University, CA), sheep polyclonal anti-mGluR6 (1:400; gift from Kirill Martemyanov, The Scripps Research Institute, FL), and rabbit polyclonal antibody against RGS11 (1:1000; gift from Theodore Wensel, Baylor College of Medicine, Houston, TX).
Imaging and Quantification
Stained sections were imaged using an Olympus FV1000 confocal microscope with a ×60 oil-immersion objective zoom 2 (resolving 0.6 μm in the z-axis). Representative images shown here were incorporated into figures using Adobe Photoshop CS5 and Adobe Illustrator CS5 (Adobe Systems, San Jose, CA). To compare Cacna1s expression in WT and KO retinas, we imaged sections from each set using the same settings. To study expression during development, we acquired images at the different postnatal ages in a series at the acquisition parameters used for the oldest retina in the series (P28).
We used several metrics to quantify staining. To compare intensities of mGluR6 and Cacna1s staining in rod bipolar cells relative to ON cone bipolar cells, we specified small regions of interest (ROIs) around the stains in rod and ON cone bipolar dendrites using Volocity (Perkin Elmer, Waltham, MA) software. The background subtracted average intensities for each ROI were determined, and the values for each cell type were averaged and statistically compared (Fig. 1A). To obtain the average intensity in the outer plexiform layer (OPL), we specified a ROI encompassing the whole OPL using Volocity software (Fig. 1B), and subtracted the average background intensity per pixel (taken from the outer nuclear layer [ONL]) from the average intensity per pixel in the ROI. The number, size, and intensity of Cacna1s-stained puncta were measured with a user-developed MATLAB (Mathworks, Natick, MA) program. The program isolates puncta in a single focal plane using a user-specified intensity threshold (thresholds were selected to include all visible puncta). For each isolated punctum, we fit a 2-D Gaussian and determined its size as the number of pixels with intensities above the half maximum intensity of the punctum (FWHM; full width at half maximum), and its intensity as the average intensity of these pixels (Fig. 1C). We then averaged these metrics for all the puncta in multiple images of the retina. Statistical comparison was done using 2-tailed, equal variances Student's t-test (n, the number of sets, ranged from 3–4). A P value of less than 0.05 was considered significant. The MATLAB program was also used to quantify the ratio of the number of puncta to the number of ribbons.
Figure 1.
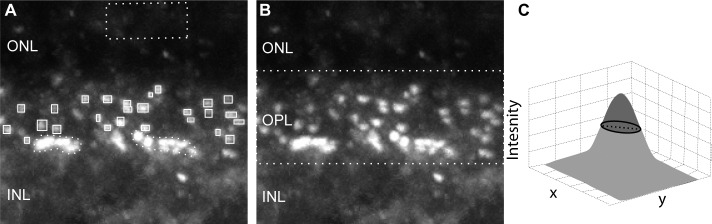
Methods used for quantification. (A) Image showing mGluR6 staining. To compare the average staining intensity in rod bipolar dendritic tips versus that in ON cone bipolar dendritic tips, we drew ROIs around these structures. Puncta in the upper part of the OPL represent the dendritic tips of rod bipolar cells (small rectangles) and characteristic row of puncta lower in the OPL represent the dendritic tips of ON cone bipolar cells (dotted irregular regions). The mean intensity was taken as the average per pixel in the ROIs minus the average background (taken from the ONL, dotted square). These averages were computed from single confocal images. (B) Same image as in (A). Average staining intensity per pixel in the OPL was calculated by encircling the whole OPL (dotted rectangle) and subtracting the average background taken from the ONL (as in [A]). This operation was computed from the captured volume (approximately 4-μm depth). (C) A hypothetical 3D surface showing the intensity (as height) of each pixel in the stained puncta in the x-y dimension (horizontal plane). The dashed black line shows the level of half maximum intensity, and the dark circle indicates the size of the puncta (FWHM). The punctum intensity was calculated as the average of the intensities within the circle above the half maximum width (darker shade).
Results
All ON Bipolar Cells Express Cacna1s, but Expression in ON Cone Bipolar Cells Is Weaker
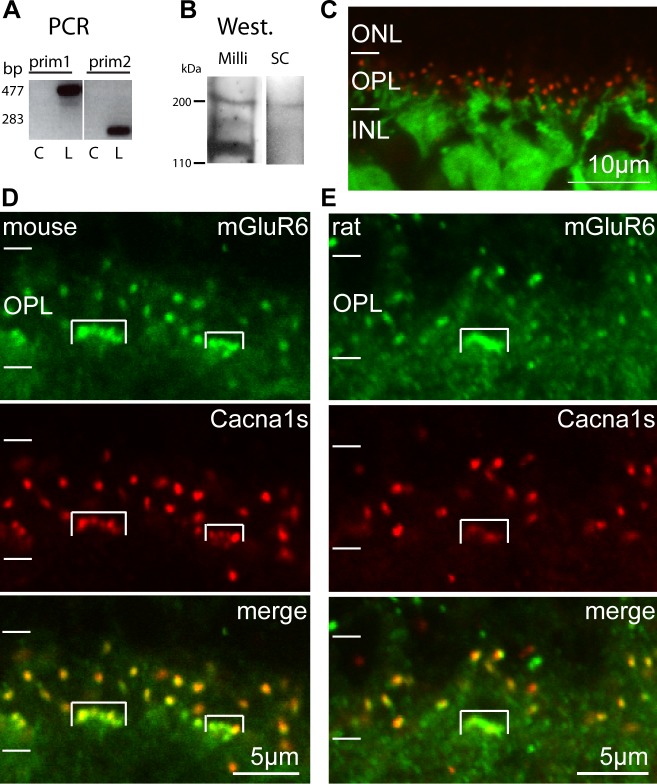
In a previous study, immunostaining showed that Cacna1s colocalizes with mGluR6 at the tips of ON bipolar cell dendrites, but the protein was barely detected by Western blotting of retinal sample.4 To confirm that Cacna1s is expressed by ON bipolar cells, we first tested if Cacna1s is transcribed by these cells. Using our ON bipolar–specific cDNA library28 and two sets of Cacna1s-specific primers, we amplified the message and obtained bands at the expected sizes (Fig. 2A). Furthermore, sequencing the PCR products showed the correct transcript (data not shown). No bands were observed in the water sample used as a negative control. To determine if the protein is expressed in retina, we performed Western blots on membrane fractions using two different antibodies, mouse mAB 1a (MAB427; Chemicon, Millipore) and goat N-19 (sc-8160; Santa-Cruz Biotechnology, Inc.). Both antibodies gave a band at the expected size of approximately 200 kDa; the Millipore antibody gave an additional band at approximately 120 kDa (Fig. 2B). As a comparison, we performed Western blots in muscle tissue that displays high expression of this protein, and found two strong bands that extended from approximately 200 to 110 kDa (Supplementary Fig. S1A). To verify that the Millipore antibody is suitable for immunohistochemical localization of the protein under our experimental conditions, we stained skeletal muscles and, indeed, observed the expected localization in the transverse tubules (Supplementary Figs. S1B, S1C). We then confirmed localization in retina by staining Grm6-GFP retina, where GFP labels all ON bipolar cells. We found that Cacna1s staining was restricted to the outer plexiform layer (OPL) and was present in discrete puncta located at the tips of the GFP-expressing dendrites (Fig. 2C). This set of experiments shows that ON bipolar cells express Cacna1s in their dendritic tips.
Figure 2.
Cacna1s is expressed by ON bipolar cells and colocalizes with mGluR6 at their dendritic tips. (A) The PCR products amplified from ON bipolar cDNA library using two sets of primers for Cacna1s. The lanes shown were cropped from the same gel. L, library sample; C, a control water sample. (B) Western blots of retinal membrane fractions probed with Millipore monoclonal anti-Cacna1s (Mill; also used for the immunocytochemical experiments) and with N-19 from Santa Cruz Biotechnology, Inc. (SC). (C) Cross-section of a Grm6-GFP mouse retina stained for Cacna1s. Cacna1s staining (red) is restricted to the OPL in discrete puncta located at the tips of the GFP-expressing ON bipolar cells dendrites (green). (D) Cross-section of a mouse retina double stained for mGluR6 and Cacna1s. The stained puncta colocalize well in all ON bipolar cell dendrites. However, mGluR6 staining is more intense and Cacna1s staining is less intense in ON cone bipolar dendritic tips (enclosed by brackets) than in rod bipolar dendritic tips. (E) Cross-section of a rat retina double stained for mGluR6 and Cacna1s. Rat retina displays patterns similar to mouse retina for both stains.
We also used immunohistochemistry to examine the colocalization of Cacna1s with mGluR6. We found that the Cacna1s puncta were well correlated with mGluR6 puncta in rod and ON cone bipolar cells (Fig. 2D). However, the puncta of ON cone bipolar cells appeared greener than those of rod bipolar cells, suggesting that either mGluR6 staining is stronger and/or Cacna1s staining is weaker in ON cone bipolar cells than in rod bipolar cells. To determine which was the case, we quantified the intensities of mGluR6 and Cacna1s staining in rod and ON cone bipolar dendrites. We found that for mGluR6 staining, the average intensity per pixel in ON cone bipolar cells (CB) was significantly higher than that in rod bipolar cells (RB, CB/RB = 1.65 ± 0.31, P = 0.03), while for Cacna1s staining, the reverse was true (CB/RB = 0.54 ± 0.13, P = 0.03). Similar staining patterns were seen in rat retina (Fig. 2E). Thus, ON cone bipolar cells showed stronger mGluR6 staining and weaker Cacna1s staining than rod bipolar cells.
Cacna1s Expression Depends on Several mGluR6-Cascade Elements
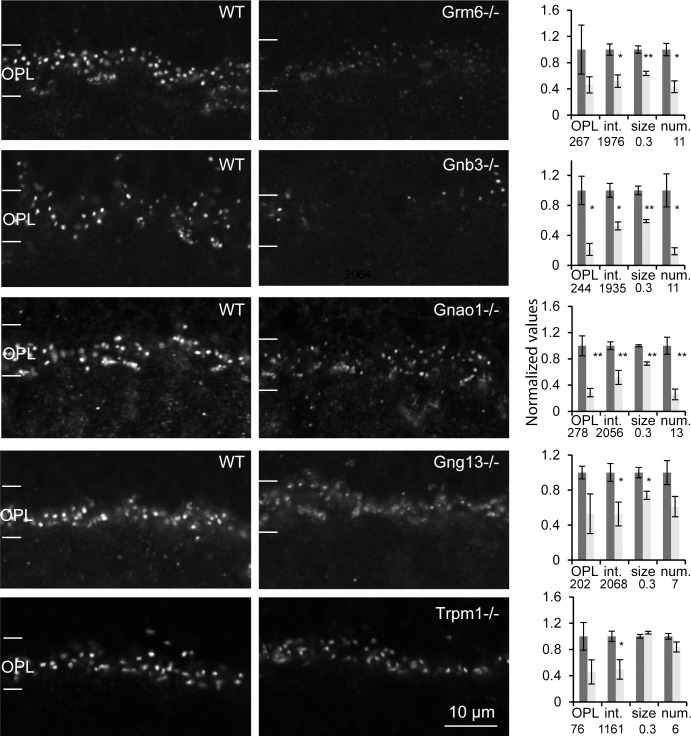
The components of the mGluR6 cascade cluster in a macromolecular complex at the ON bipolar cell's dendritic tip. As more of these components are identified, it becomes increasingly clear that each one's localization often depends on that of the others. To determine if Cacna1s is a component of the mGluR6 macromolecular complex, we tested its expression in mice lacking the gene encoding mGluR6 (Grm6−/−), Gαo1 (Gnao1−/−, the predominant splice variant of the α subunit of Go), Gβ3 (Gnb3−/−, the β subunit of Go), Gγ13 (Gng13−/−), the best candidate for the γ subunit), or TRPM1 (Trpm1−/−). We performed immunohistochemistry on retinas taken from sets of littermates or from age-matched WT and KO animals; staining was performed at the same time, and images were obtained under identical acquisition settings (Fig. 3). Staining for Cacna1s in all five genotypes was reduced greatly, albeit to a different degree and depending on the used metric (Fig. 3, right graphs). The staining intensity in the whole OPL reached significance only in Gnb3−/− and Gnao1−/− (staining in the other genotypes was reduced, but failed to reach significance). The intensity per pixel of puncta was significantly lower for all genotypes. The number of visible puncta (given same threshold for WT and KO) was lower for Grm6−/−, Gnao1−/−, and Gnb3−/−. The size of the puncta (an indication of intensity strength rather than actual size) was smaller in all genotypes except for Trpm1−/−. To test if transcription of Cacna1s was affected, we performed qPCR on cDNA isolated from Gnao1−/− and Gnb3−/− retinas. For the WT and Gnb3−/− sets, the relative levels of Cacna1s transcripts were 1.15 ± 0.73 and 0.95 ± 0.25, respectively, and for the WT and Gnao1−/− sets, the levels were 0.9 ± 0.12 and 1.17 ± 0.11 (3 sets for each genotype). Thus, the transcript of Cacna1s was not significantly different between KO and WT for both KOs. These results showed that localization of Cacna1s at the dendritic tips greatly depends on normal expression of several of the mGluR6 cascade proteins, suggesting that it is a part of the mGluR6 complex at the dendritic tips.
Figure 3.
Cacna1s expression decreases dramatically in retinas lacking any of several cascade elements (Grm6−/−, Gnao1−/,− Gnb3−/−, Gng13−/−, and Trpm1−/− retinas). Images show single confocal optical planes cropped at the level of the OPL. For each pair, WT and KO retinas were processed at the same time, and images were acquired with the same settings. All images were selected from the top of the stack. Bar graph on right of each pair shows normalized data for four metrics that were averaged from three (Grm6−/−, Gnb3−/, Gng13−/−, and Trpm1−/−) or four (Gnao1−/−) sets. The numbers below the bars indicate the average values for WT that were used to normalize the data for each parameter. The size is given in μm2, and the number of puncta is per 10 μm length of OPL. Error bars represent SEM. OPL, average pixel intensity across the whole OPL; int., average pixel intensity in the stained puncta; size, area of a punctum determined from the number of pixels with intensities greater than the half maximum staining intensity; num., number of puncta. *P < 0.05, **P < 0.01.
Expression of Cacna1s Follows the Same Timeline as That of mGluR6 and RGS11
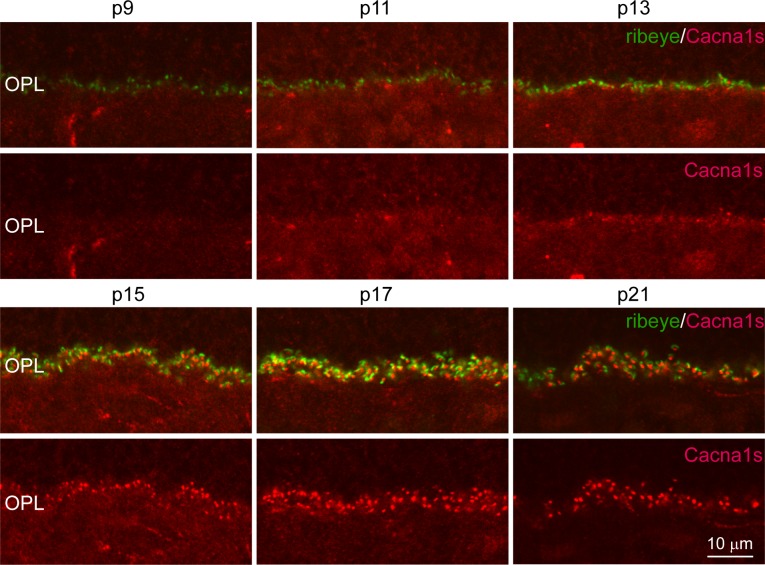
During development, horizontal cell processes and ON bipolar cell dendrites invaginate the presynaptic photoreceptor terminal and terminate postsynaptic to the photoreceptor's ribbon. To determine when and where Cacna1s first appears in the developing retina, we double-stained mouse retinas at ages ranging from postnatal day 6 to 28 (P6-P28) for Cacna1s and ribeye, a component of the presynaptic ribbon that marks the location of developing photoreceptor synaptic complexes (Fig. 4). We first quantified the total number of ribbons at each developmental stage per 10 μm length of the OPL. At P9, the age at which three postsynaptic elements of the rod synapse are first observed in electron micrographs of mouse retina (although four processes terminate at the rod synapse, typically only three are seen in an EM section, we will, therefore, refer to it as triad), there were approximately five ribbons in 10 μm length of OPL. This number increased gradually between P9 and P15 to nine ribbons, after which it settled (Fig. 5A). We then examined Cacna1s staining across the same ages. At P13, Cacna1s staining was apparent at the tips of a few dendrites. At all stages of development, staining appeared to be restricted to the dendritic tips and was not discernible in either the somas or primary dendrites. The percentage of Cacna1s puncta that were associated with ribbons increased rapidly from 17% at P13 to 60% at P17, and hit a plateau of approximately 80% at approximately P21 (Fig. 5B). The intensity of Cacna1s staining in the OPL increased gradually between P11 and P19 from 25% to 70% of the adult staining intensity, peaked at approximately P21 at 150% and decreased by P28 to the adult intensity level (that was normalized to 100%, Fig. 5C). Thus, Cacna1s is recruited to the dendritic tip after ribbon formation.
Figure 4.
Expression of Cacna1s appears after that of presynaptic ribeye and increases rapidly following eye opening. Representative images of single confocal planes of retinas at P9-21 stained for Cacna1s and ribeye. In this series, although Cacna1s intensity in the OPL is getting brighter at P11, clear puncta are discernible only at P13.
Figure 5.
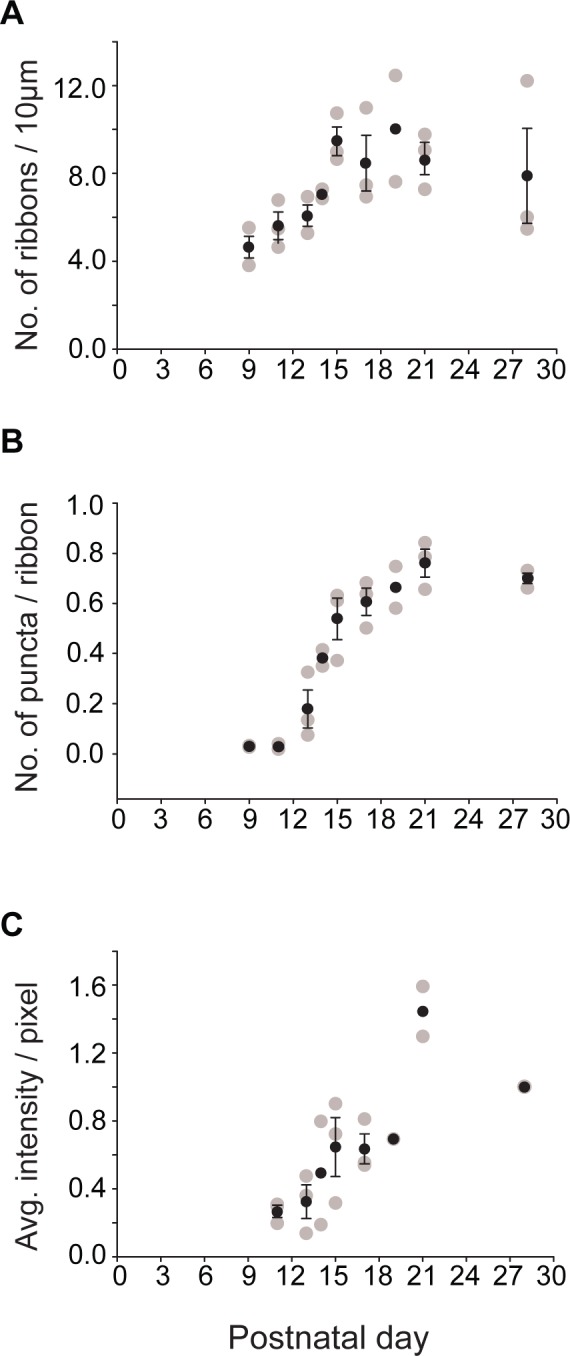
Cacna1s expression increases rapidly between postnatal day 13 and 17. (A) Number of ribbons in a 10-μm length of OPL during development. This number increases gradually and then plateaus. (B) Ratio of Cacna1s puncta to number of ribbons (labeled for ribeye) during development. This value increases as a sigmoid. Note, the ratio of puncta apposed to ribbons in the adult is less than 1, because this number was estimated from single confocal planes, and the larger sized ribbons were visible in more planes than the smaller bipolar dendrites. (C) Average Cacna1s intensity per pixel in the OPL during development normalized to the adult (P28) intensity. Staining intensity increases gradually. Gray circles represent data from individual animals. Black circles represent the average ± SEM. Number of retinas (n) = 3 for all time points except P14 and P19 (n = 2). Note that at P28 all three data points lie at 1 because they are normalized.
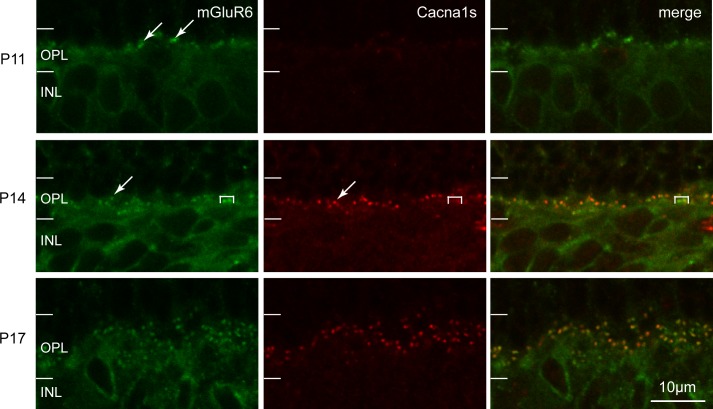
To determine the pattern of Cacna1s expression relative to that of mGluR6 and RGS11, we performed double labeling. Double labeling for Cacna1s and mGluR6 revealed a very small number of mGluR6 puncta at the tips of dendrites at P11 that increased rapidly between P14 and P17 (Fig. 6). The punctate staining of Cacna1s after its expression at P13 colocalized well with the mGluR6 puncta. Furthermore, as with adult, at P14, mGluR6 staining in the dendrites of ON cone bipolar cells appeared more intense than that in the dendrites of rod bipolar cells, while Cacna1s staining in cone bipolar cells was less intense. Double labeling for Cacna1s and RGS11 showed that RGS11 staining in the early stages was diffuse throughout the inner nuclear layer (INL) and OPL with no discernible puncta at P11. Distinct puncta for both proteins first appeared at approximately P13, and they were colocalized (Fig. 7). These puncta displayed low intensity and were detectable only at greatly increased contrast. Close examination of the two stains showed that the RGS puncta were more diffuse than the Cacna1s puncta, suggesting that Cacna1s clustered at the tip slightly earlier than RGS11 did (Fig. 7, bottom row). Similarly at P15, RGS11 puncta, which were now easily detectable, were large and diffuse, while Cacna1s puncta were more compact. At P17 and more so at P21, the characteristic punctate staining for RGS11 at the dendritic tips became more pronounced, and all puncta were labeled for RGS11 and Cacna1s. However, the Cacna1s staining in ON cone bipolar dendrites was fainter than in rod bipolar dendritic tips, again suggesting differential expression of mGluR6 cascade proteins and Cacna1s in the ON bipolar cell types. Together, these results indicate that recruitment of Cacna1s to the dendritic tip may follow that of mGluR6 and may coincide or precede recruitment of the GAP complex (of which RGS11 is a component).
Figure 6.
Expression pattern of Cacna1s during development closely follows that of mGluR6. Double labeling of mGluR6 and Cacna1s in mouse retina. As in adult, during development mGluR6 staining is more intense and Cacna1s staining is less intense in the ON cone bipolar dendrites than in the rod bipolar dendrites. Brackets enclose ON cone bipolar dendritic tips, arrows point to rod bipolar dendritic tips.
Figure 7.
The RGS11 and Cacna1s localize to dendritic tips at approximately the same time. Representative images of retinas stained for RGS11 (green) and Cacna1s (red) during development. At P13, staining for RGS11 and Cacna1s is hardly visible. Staining intensity and number of observed puncta rise with age. Bottom row is a magnified and contrast-enhanced region from P13 (denoted by dotted square in top row) showing that well-defined Cacna1s puncta (indicated by arrows) display diffuse staining for RGS11. Brackets in P21 indicate dendrites of ON cone bipolar cells illustrating that Cacna1s staining is less intense in ON cone bipolar dendrites than in rod bipolar dendrites.
Discussion
This study adds two critical pieces of evidence to support the idea that Cacna1s is expressed by ON bipolar cells: that the transcript for this protein is present in ON bipolar cells, and that the protein is expressed by retinal membranes, as would be expected from a channel protein. This study further shows that Cacna1s staining in rod bipolar dendritic tips is stronger than in ON cone bipolar dendritic tips. Significantly, the staining intensity of Cacna1s in the dendritic tips dramatically decreases in the absence of components of the mGluR6 cascade – mGluR6, Gαo1, Gβ3, Gγ13, or TRPM1. Moreover, the expression of Cacna1s puncta closely follows the time courses displayed by components of the mGluR6 cascade—mGluR6 and RGS11. Together, our findings strongly suggest that Cacna1s is part of the mGluR6 macromolecular complex.
Cacna1s Localization at Rod Bipolar Dendritic Tips Requires mGluR6-Cascade Components
Deletion of important molecules in signaling cascades often affects expression or localization of an array of other molecules. These effects often indicate that the affected molecules are either trafficked together, belong to the same macromolecule, or that they depend on the deleted ones for their function. However, the affected components and the degree to which they are affected vary depending on the deleted molecule. For example, when Gβ3 is deleted, the G-protein subunits Gαo and Gγ13, as well as mGluR6, TRPM1, and the GAP complex are greatly reduced.24 When mGluR6 is deleted or mutated, the effect is less severe. The TRPM1 and the GAP complex are greatly reduced, but the G-protein subunits are unaffected.10,27 Interestingly, among the known mGluR6 component KO mice tested here, we noticed that the most susceptible molecules (i.e., those that were the most reduced) are R9AP, RGS11, and Gβ5, all elements of the GAP complex. The current study showed that Cacna1s is also highly susceptible. Its staining is reduced in all 5 tested KO lines, albeit to a different degree. Notably, staining intensity is reduced more greatly in KO lines that display greater effects on the RGS molecules (such as Grm6−/− and Gnb3−/−). It is unclear why some molecules are more susceptible than others, but their labile localization may represent mechanisms that adapt the cell to new cellular environments. In a related study, Cacna1s was shown to be reduced dramatically in a Cacna1f mutant mouse. Since Cacna1f normally is localized to the active zone in the rod terminal, the effect on Cacna1s is transynaptic.4 In this mouse model, mGluR6 also was reduced, raising the possibility that Cacna1s was affected subsequent to mGluR6. Double staining of these mice mutants would be required to see if all the postsynaptic elements are affected at the same time or progress like in a domino effect.
Possible Function of Cacna1s in ON Bipolar Cells
Clustering the components of a signaling cascade into a macromolecular complex can improve the efficacy of the signaling system as was shown for the β2-adrenergic receptor system in hippocampal neurons.29 Assuming that the components of the mGluR6 cascade are clustered at the dendritic tip for the same reason, the inclusion of Cacna1s in this macromolecular complex suggests that it contributes to the light response of ON bipolar cells. Cacna1s is the pore-forming subunit of certain L-type voltage-gated calcium channels and, thus, could have two possible functions. The first and most likely function, as suggested also by Specht et al.,4 involves permeating calcium into the dendrites in a voltage-dependent manner; that is, in response to a strong light stimulus. Calcium influx evoked by a step increment in light intensity has been inferred in rod bipolar cells of various species, because adding BAPTA to the pipette changes the light response.30–33 The resulting increase in internal calcium concentration appears to shape the step and flash responses of rod bipolar cells by desensitizing or inactivating the inward current after the initial transient. Thus, calcium mediates adaptation by reducing the synaptic gain, increasing the cell's dynamic range and, thus, preserving the fidelity of the single photon response.33,34 In all species where this effect is observed, calcium is thought to enter through TRPM1, the obligatory nonspecific cation channel that is coupled to mGluR6. However, it is possible that Cacna1s contributes to the inactivation by also allowing calcium into the cell. Support for the role of Cacna1s in adaptation arises from our finding that Cacna1s expression in ON cone bipolar cells is weaker than in rod bipolar cells. This is consistent with the previously reported observation in mouse that ON cone bipolar cells exhibit different calcium-dependent transient responses than rod bipolar cells.33 The second and less likely function of Cacna1s is that current through this channel amplifies the signal and contributes to the observed depolarization. Probing the possible contribution of Cacna1s to adaptation will require electrophysiological experiments with pharmacologic substances that can block Cacna1s from the intracellular side or with Cacna1s conditional KOs.
Development of the Postsynaptic Complex in ON Bipolar Cells
Figure 8 summarizes the sequence of events in the developing outer plexiform layer. The ON bipolar cells begin to be born at P2, a process that mostly is complete by P7,35 the age at which the OPL is distinguishable under light microscopy. These cells first appear as neuroepithelial-like cells at P3 with a single apical and basal process extending to the outer and inner limiting membranes (OLM and ILM), respectively.36 Dendrites develop from lateral processes that emerge from the shaft of the apical process in the region of the OPL between P5 and P9, the period in which horizontal cell dendrites invaginate the spherules and form dyads with the rod terminals.36,37 The ON bipolar cell dendrites are dynamically remodeled between P9 and P19. Triad synapses first appear in electron microscopy at P10, increase rapidly in number between P10 and P14 (eye opening), and then stabilize.37–39 The earliest age at which we observed mGluR6 puncta was P11; before this time mGluR6 staining was diffusely localized to the somas and immature dendrites. This timeline is consistent with an earlier study examining the expression of mGluR6 through development.8 Thus, the assembly of the mGluR6 complex at the tip of the ON bipolar dendrite appears to occur subsequent to the dendrite's invagination. Cacna1s puncta appeared slightly after mGluR6. They were observed first at approximately P13 (close to eye opening), approximately the same time that RGS11 puncta were first observed. Interestingly, preliminary results from our lab show that expression of the α (Gαo1) and β (Gβ3) subunits of the heterotrimer Go precedes mGluR6 expression, occurring sometime between P6 and P9, and localizing to somas, immature dendrites, and axons. The lag in Cacna1s expression behind that of mGluR6 and Go suggests that it probably does not have a developmental function. Moreover, restriction of Cacna1s to the dendritic tips, unlike the diffuse distribution of mGluR6 and RGS11 at early stages of ON bipolar dendrite development, suggests that this protein is stable only at the tip and only in the presence of mGluR6.
Figure 8.
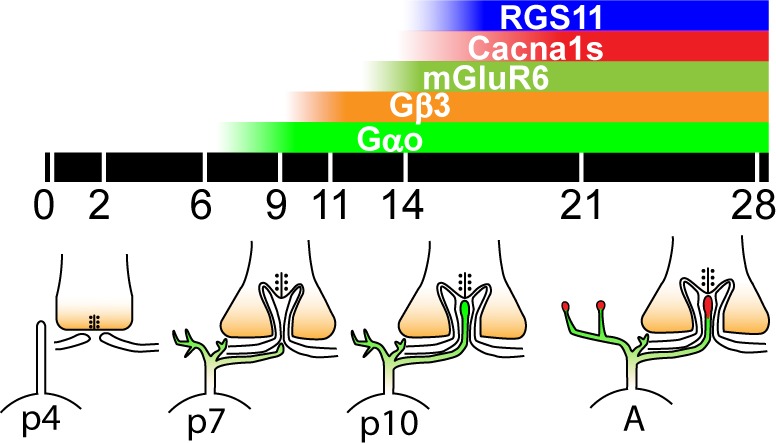
Developmental timeline of the mGluR6 cascade components at the rod bipolar dendritic tip. Postnatal days are indicated below the black bar. Color bars at top represent time course of expression for the various components of the mGluR6 complex. The schematics below the black bar show representative developmental stages of the rod synapse. P0 to P4: Rod bipolar cells have differentiated, and have an apical process and a basal process (only the apical is shown). Horizontal cell lateral processes appose the rod terminal. P5 to P9: Rod bipolar dendrites emerge from the apical process. Both subunits of Go are present throughout the cell (green). Horizontal cells invaginate the terminal and form dyad synapses. P10 to P14: Rod bipolar dendrites invaginate the terminal and form triad synapses. All triad synapses are formed by P14, coinciding with eye opening and the appearance of the electroretinogram (ERG) b-wave. mGluR6 puncta are seen at P11 first, and other punctate components of the complex (Cacna1s and RGS11, red) are recruited rapidly thereafter. Staining intensity of puncta continues to increase, as does the ERG b-wave amplitude, and it matures to adult (A) between P21 and P28.
Acknowledgments
The authors thank Kirill Martemyanov, Thomas Sudhof, and Theodore Wensel for antibodies against mGluR6, ribeye, and RGS11, respectively; Shigetada Nakanishi and David Copenhagen for the Grm6−/− mice; and Lutz Birnbaumer for the Gnao1−/− mice. The manuscript was edited by Mirotznik Editing Services.
Supported by EY11105 (NV), R21 EY021308 (NV), and NEI P30 EY01583 (Vision Research Core of the University of Pennsylvania).
Disclosure: S.R. Tummala, None; A. Neinstein, None; M.E. Fina, None; A. Dhingra, None; N. Vardi, None
References
- 1. Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011; 3: a003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 2001; 42: 2414–2418 [PubMed] [Google Scholar]
- 3. Berntson A, Taylor WR, Morgans CW. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J Neurosci Res. 2003; 71: 146–151 [DOI] [PubMed] [Google Scholar]
- 4. Specht D, Wu SB, Turner P, et al. Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Invest Ophthalmol Vis Sci. 2009; 50: 505–515 [DOI] [PubMed] [Google Scholar]
- 5. Soto F, Ma X, Cecil JL, Vo BQ, Culican SM, Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci. 2012; 32: 5426–5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000; 423: 402–412 [DOI] [PubMed] [Google Scholar]
- 7. Vardi T, Fina M, Zhang L, Dhingra A. Vardi N. mGluR6 transcripts in non-neuronal tissues. J Histochem Cytochem. 2011; 59: 1076–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally-regulated postsynaptic localization of a metabotropic glutamate-receptor in rat rod bipolar cells. Cell. 1994; 77: 361–369 [DOI] [PubMed] [Google Scholar]
- 9. Masu M, Iwakabe H, Tagawa Y, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995; 80: 757–765 [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T. Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012; 107: 948–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004; 5: 747–757 [DOI] [PubMed] [Google Scholar]
- 12. Vardi N, Morigiwa K. ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Vis Neurosci. 1997; 14: 789–794 [DOI] [PubMed] [Google Scholar]
- 13. Dhingra A, Jiang M, Wang TL, et al. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o). J Neurosci. 2002; 22: 4878–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhingra A, Lyubarsky A, Jiang M, et al. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000; 20: 9053–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009; 106: 19174–19178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koike C, Obara T, Uriu Y, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010; 107: 332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shim H, Wang CT, Chen YL, et al. Defective retinal depolarizing bipolar cells in regulators of G protein signaling (RGS) 7 and 11 double null mice. J Biol Chem. 2012; 287: 14873–14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgans CW, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, Duvoisin RM. Gbeta5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. Eur J Neurosci. 2007; 26: 2899–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao Y, Pahlberg J, Sarria I, Kamasawa N, Sampath AP, Martemyanov KA. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc Natl Acad Sci U S A. 2012; 109: 7905–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007; 27: 14199–14204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeffrey BG, Morgans CW, Puthussery T, et al. R9AP stabilizes RGS11-G beta5 and accelerates the early light response of ON-bipolar cells. Vis Neurosci. 2010; 27: 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masuho I, Celver J, Kovoor A, Martemyanov KA. Membrane anchor R9AP potentiates GTPase-accelerating protein activity of RGS11 x Gbeta5 complex and accelerates inactivation of the mGluR6-G(o) signaling. J Biol Chem. 2010; 285: 4781–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pearring JN, Bojang P Jr, Shen Y, et al. A role for nyctalopin, a small leucine-rich repeat protein, in localizing the TRP melastatin 1 channel to retinal depolarizing bipolar cell dendrites. J Neurosci. 2011; 31: 10060–10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhingra A, Ramakrishnan H, Neinstein A, et al. Gbeta3 is required for normal light on responses and synaptic maintenance. J Neurosci. 2012; 32: 11343–11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orlandi C, Posokhova E, Masuho I, et al. GPR158/179 regulate G protein signaling by controlling localization and activity of the RGS7 complexes. J Cell Biol. 2012; 197: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao Y, Song H, Okawa H, Sampath AP, Sokolov M, Martemyanov KA. Targeting of RGS7/Gbeta5 to the dendritic tips of ON-bipolar cells is independent of its association with membrane anchor R7BP. J Neurosci. 2008; 28: 10443–10449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao Y, Masuho I, Okawa H, et al. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009; 29: 9301–9313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhingra A, Sulaiman P, Xu Y, Fina ME, Veh RW, Vardi N. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008; 510: 484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davare MA, Avdonin V, Hall DD, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001; 293: 98–101 [DOI] [PubMed] [Google Scholar]
- 30. Snellman J, Nawy S. Regulation of the retinal bipolar cell mGluR6 pathway by calcineurin. J Neurophysiol. 2002; 88: 1088–1096 [DOI] [PubMed] [Google Scholar]
- 31. Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999; 19: 2938–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiells RA, Falk G. A rise in intracellular Ca2+ underlies light adaptation in dogfish retinal ‘on' bipolar cells. J Physiol. 1999; 514: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004; 21: 913–924 [DOI] [PubMed] [Google Scholar]
- 34. Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008; 27: 450–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996; 93: 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006; 9: 85–92 [DOI] [PubMed] [Google Scholar]
- 37. Blanks JC, Adinolfi AM, Lolley RN. Synaptogenesis in the photoreceptor terminal of the mouse retina. J Comp Neurol. 1974; 156: 81–93 [DOI] [PubMed] [Google Scholar]
- 38. Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968; 7: 250–268 [PubMed] [Google Scholar]
- 39. Olney JW. Centripetal sequence of appearance of receptor-bipolar synaptic structures in developing mouse retina. Nature. 1968; 218: 281–282 [DOI] [PubMed] [Google Scholar]