Abstract
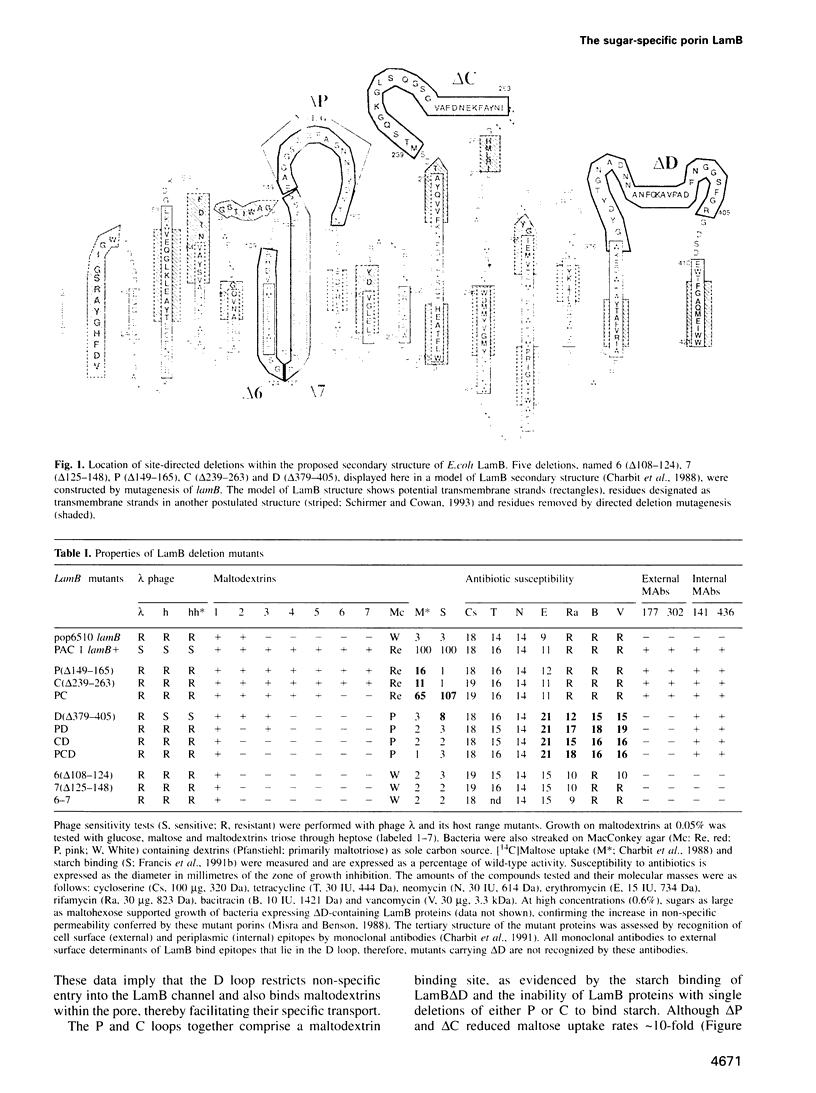
LamB facilitates the uptake of maltose and maltodextrins across the bacterial outer membrane and acts as a general porin for small molecules. Using directed deletion mutagenesis we removed several regions of the LamB polypeptide and identified a polypeptide loop that both constricts the maltoporin channel and binds maltodextrins. In conjunction with a second sugar binding site that we identified at the rim of the channel, these data clarify, for the first time, the mechanism of transport through a substrate-specific porin. Furthermore, unlike the transverse loops of general porins, which originate from a central location in their primary structure, the loop that regulates LamB permeability originates from a C-terminal site. Thus LamB represents a second distinct class of porins in the bacterial outer membrane that is differently organized and separately evolved from OmpF-type, general porins.
Full text
PDF
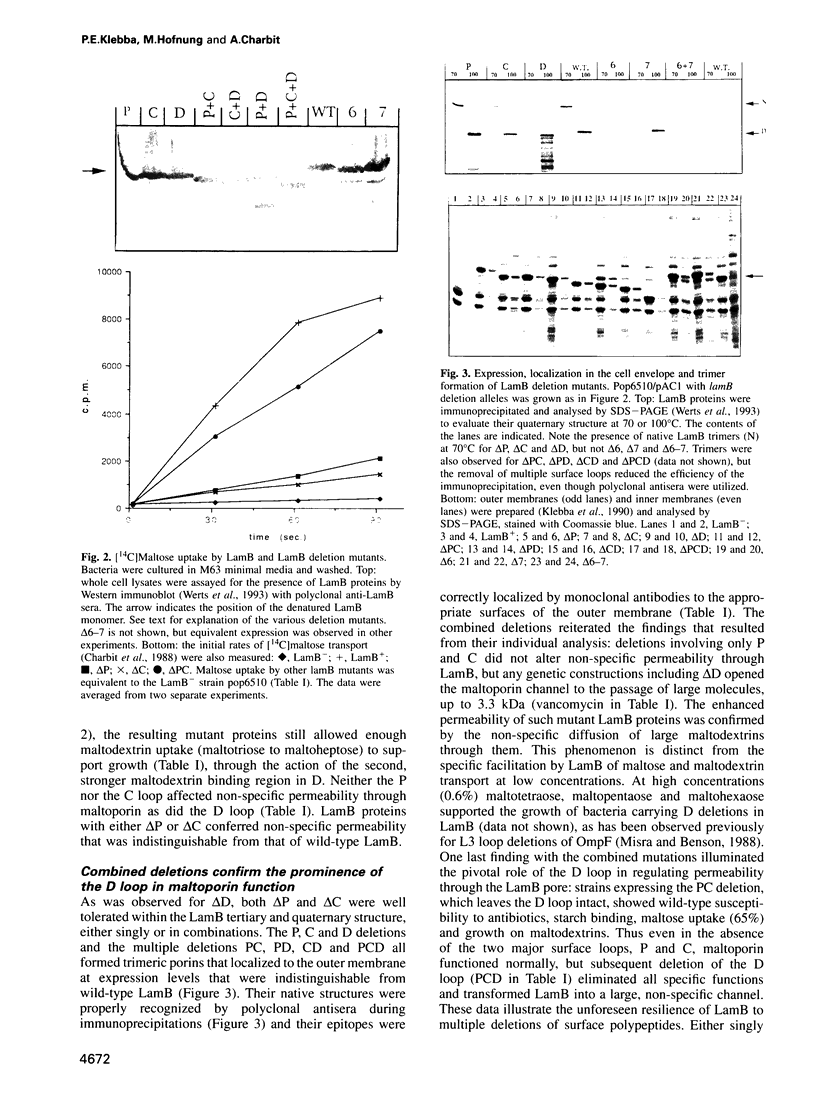



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K., Struyvé M., Bosch D., Benz R., Tommassen J. One single lysine residue is responsible for the special interaction between polyphosphate and the outer membrane porin PhoE of Escherichia coli. J Biol Chem. 1989 Oct 5;264(28):16393–16398. [PubMed] [Google Scholar]
- Benz R., Francis G., Nakae T., Ferenci T. Investigation of the selectivity of maltoporin channels using mutant LamB proteins: mutations changing the maltodextrin binding site. Biochim Biophys Acta. 1992 Mar 2;1104(2):299–307. doi: 10.1016/0005-2736(92)90044-m. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Nakae T., Vos-Scheperkeuter G. H. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986 Mar;165(3):978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Gehring K., Nikaido H., Ferenci T., Hofnung M. Maltose transport and starch binding in phage-resistant point mutants of maltoporin. Functional and topological implications. J Mol Biol. 1988 Jun 5;201(3):487–496. doi: 10.1016/0022-2836(88)90630-4. [DOI] [PubMed] [Google Scholar]
- Charbit A., Ronco J., Michel V., Werts C., Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991 Jan;173(1):262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Dargent B., Charbit A., Hofnung M., Pattus F. Effect of point mutations on the in-vitro pore properties of maltoporin, a protein of Escherichia coli outer membrane. J Mol Biol. 1988 Jun 5;201(3):497–506. doi: 10.1016/0022-2836(88)90632-8. [DOI] [PubMed] [Google Scholar]
- Francis G., Brennan L., Ferenci T. Affinity-chromatographic purification of sixteen cysteine-substituted maltoporin variants: thiol reactivity and cross-linking in an outer membrane protein of Escherichia coli. Biochim Biophys Acta. 1991 Aug 5;1067(1):89–96. doi: 10.1016/0005-2736(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Francis G., Brennan L., Stretton S., Ferenci T. Genetic mapping of starch- and lambda-receptor sites in maltoporin: identification of substitutions causing direct and indirect effects on binding sites by cysteine mutagenesis. Mol Microbiol. 1991 Sep;5(9):2293–2301. doi: 10.1111/j.1365-2958.1991.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Hofnung M., Jezierska A., Braun-Breton C. lamB mutations in E. coli K12: growth of lambda host range mutants and effect of nonsense suppressors. Mol Gen Genet. 1976 May 7;145(2):207–213. doi: 10.1007/BF00269595. [DOI] [PubMed] [Google Scholar]
- Killmann H., Benz R., Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993 Aug;12(8):3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebba P. E., Benson S. A., Bala S., Abdullah T., Reid J., Singh S. P., Nikaido H. Determinants of OmpF porin antigenicity and structure. J Biol Chem. 1990 Apr 25;265(12):6800–6810. [PubMed] [Google Scholar]
- Kreusch A., Neubüser A., Schiltz E., Weckesser J., Schulz G. E. Structure of the membrane channel porin from Rhodopseudomonas blastica at 2.0 A resolution. Protein Sci. 1994 Jan;3(1):58–63. doi: 10.1002/pro.5560030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rutz J. M., Feix J. B., Klebba P. E. Permeability properties of a large gated channel within the ferric enterobactin receptor, FepA. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10653–10657. doi: 10.1073/pnas.90.22.10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Benson S. A. Genetic identification of the pore domain of the OmpC porin of Escherichia coli K-12. J Bacteriol. 1988 Aug;170(8):3611–3617. doi: 10.1128/jb.170.8.3611-3617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol Chem. 1994 Feb 11;269(6):3905–3908. [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Wu H. C. Amino acid sequence homology among the major outer membrane proteins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1048–1052. doi: 10.1073/pnas.81.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauptit R. A., Schirmer T., Jansonius J. N., Rosenbusch J. P., Parker M. W., Tucker A. D., Tsernoglou D., Weiss M. S., Schultz G. E. A common channel-forming motif in evolutionarily distant porins. J Struct Biol. 1991 Oct;107(2):136–145. doi: 10.1016/1047-8477(91)90017-q. [DOI] [PubMed] [Google Scholar]
- Rutz J. M., Liu J., Lyons J. A., Goranson J., Armstrong S. K., McIntosh M. A., Feix J. B., Klebba P. E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992 Oct 16;258(5081):471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Cowan S. W. Prediction of membrane-spanning beta-strands and its application to maltoporin. Protein Sci. 1993 Aug;2(8):1361–1363. doi: 10.1002/pro.5560020820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Thirion J. P., Hofnung M. On some genetic aspects of phage lambda resistance in E. coli K12. Genetics. 1972 Jun;71(2):207–216. doi: 10.1093/genetics/71.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Werts C., Michel V., Hofnung M., Charbit A. Adsorption of bacteriophage lambda on the LamB protein of Escherichia coli K-12: point mutations in gene J of lambda responsible for extended host range. J Bacteriol. 1994 Feb;176(4):941–947. doi: 10.1128/jb.176.4.941-947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts C., O'Callaghan D., Hofnung M., Charbit A. Immunological relatedness of the LamB proteins among members of Enterobacteriaceae. J Gen Microbiol. 1993 Apr;139(4):881–887. doi: 10.1099/00221287-139-4-881. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Analysis and prediction of the different types of beta-turn in proteins. J Mol Biol. 1988 Sep 5;203(1):221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]