Abstract
Background
Pancuronium, vecuronium, rocuronium, and mivacurium are nondepolarizing neuromuscular blocking agents that affect the cardiovascular system with different potencies. Their cardiovascular effects are clinically significant in the anesthetic management of patients, particularly those undergoing cardiac surgery.
Objective
We aimed to compare the cardiac effects of these compounds, such as heart rate and developed force, in one species under identical experimental conditions in isolated rat atria.
Methods
The left or right atria of rats were removed and suspended in organ baths. Pancuronium, vecuronium, rocuronium, or mivacurium were added cumulatively (10–9–10–5 M) in the presence and absence of the nonselective β-blocker propranolol (10–8 M) and the noradrenaline reuptake inhibitor desipramine (10–7 M), and heart rate changes were recorded in spontaneously beating right atria. Left atrial preparations were stimulated by electrical field stimulation using a bipolar platinum electrode, and the effects of cumulative concentrations of these nondepolarizing neuromuscular blocking agents on the developed force in the presence and absence of propranolol (10–8 M) and desipramine (10–7 M) were recorded.
Results
Pancuronium increased heart rate in a dose-dependent manner compared with the control group (P < 0.027). Vecuronium, rocuronium, and mivacurium also increased heart rate in a dose-dependent manner, but the changes were not statistically significant. Although propranolol decreased the pancuronium heart rate effect (P < 0.05), it did not change the heart rate effects with vecuronium, rocuronium, or mivacurium. Desipramine did not change the heart rate effects of vecuronium, rocuronium, mivacurium, or pancuronium. All 4 drugs increased developed force in a dose-dependent manner; the increases were significant at 10–5 M concentration for pancuronium and at 10–6 and 10–5 M concentrations for vecuronium, rocuronium, and mivacurium (P < 0.038). These increases in developed force were abolished with the addition of propranolol. Desipramine did not change the developed force effects of any of the 4 drugs.
Conclusions
The heart rate effect of pancuronium and developed force effects of pancuronium, vecuronium, rocuronium, and mivacurium may occur via direct stimulation of β receptors. Although our investigation was an in vitro study, the effects found may be important especially under pathologic conditions, such as hypertension, in which patients usually use β-blocking agents, which cause β receptor upregulation.
Key words: atrium, mivacurium, neuromuscular blockers, pancuronium, rat, rocuronium, vecuronium
Introduction
Pancuronium, vecuronium, rocuronium, and mivacurium are nondepolarizing neuromuscular blocking agents that affect the cardiovascular system with different potencies.1 Their cardiovascular effects are clinically significant in the anesthetic management of patients, particularly those undergoing cardiac surgery. Although neuromuscular blocking drugs are designed to specifically block nicotinic cholinergic receptors at the neuromuscular junction, many bind to muscarinic cholinergic receptors on ganglia, nerve endings, and smooth muscle and alter parasympathetically mediated heart rate.2
The cardiovascular effects of these neuromuscular blocking agents have been investigated in different studies. Both positive inotropic and chronotropic effects have been reported after pancuronium administration3,4 by increased heart rate and arterial blood pressure.5,6 In patients with coronary artery disease, pancuronium caused tachycardia, which may result in myocardial ischemia.7 These effects of pancuronium on heart rate were reported to be due to the increased release and decreased reuptake of catecholamines at the adrenergic nerve terminal.8 Vecuronium has been reported to possess sympathomimetic properties that are less pronounced than those produced by pancuronium,9 and, in contrast to pancuronium, it has been reported to induce bradycardia.10–12 Vecuronium has been used more often than pancuronium because it has a relatively short duration of action, minimal cardiovascular effects, and an apparent lack of histamine-releasing properties.13–14
The absence of cardiovascular effects has led investigators to propose using a large dose of vecuronium to achieve a shorter onset of action,15–17 and it has been offered as a better alternative to pancuronium for long-lasting neuromuscular blockade in patients with coronary artery disease during opioid anesthesia.18
Clinical use of rocuronium has shown that it does not produce significant cardiac effects,6 except minor hemodynamic changes.19 Mivacurium has been reported to cause transient tachycardia and hypotension,20,21 and has been offered in patients who require hemodynamic stability throughout the surgical procedure.22
In the present study we aimed to compare the cardiac effects of neuromuscular blockers pancuronium, vecuronium, rocuronium, and mivacurium under identical experimental conditions. We evaluated the cardiac effects of heart rate and developed force and the possible underlying mechanisms at rat atrial tissue after exposure to cumulative doses of these drugs.
Methods
Animals
All experimental procedures were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and approved by the Animal Care Committee of Cumhuriyet University Medical Faculty. Twenty male albino Wistar rats (weighing 180–220 g) were allocated into 3 groups: control (n = 6), right atrium for heart rate measurement (n = 7), and left atrium for developed force measurement (n = 7). The rats were injected with pentobarbital sodium (100 mg/kg) and sodium heparin (5000 IU/kg) intraperitoneally, and each heart was rapidly and carefully excised and placed into Ringer solution at room temperature. The left or right atrium was then removed and suspended in a water-jacketed organ bath containing 10 mL of Ringer solution at 37°C with an applied preload of 600 mg. The Ringer solution contained (mmol/L): 144 Na+ (sodium ion), 5.3 K+ (potassium ion), 1.98 Ca2+ (calcium ion), 1.18 Mg2+ (magnesium ion), 123.93 Cl– (chloride ion), 2.35 H2PO4– (dihydrogen phosphate ion), 25 HCO3– (bicarbonate ion), 1.18 SO42– (sulfate ion), and 10 glucose. The pH of the solution was 7.4, and the solution was gassed with a mixture of 95% oxygen and 5% carbon dioxide.
Isometric Measurements
The investigators measuring the effects of these agents were blind to the treatment groups. After mounting in the organ bath, each preparation was allowed to stabilize for 60 minutes. During this period, the solution in the bath was changed every 15 minutes. The control group received no treatment. The spontaneously beating right atria received cumulative concentrations (10–9–10–5 mol/L) of pancuronium, vecuronium, rocuronium, or mivacurium in the presence and absence of nonselective β-blocker propranolol (10–8 M) and noradrenaline reuptake inhibitor desipramine (10–7 M). These antagonists had no effect on heart rate or developed force in these concentrations during preliminary experiments (data not shown). The right atrium preparations were exposed to each concentration for 20 minutes, and the heart rate changes were recorded. After the exposure of the highest concentration, the preparation was washed by Ringer solution and rested for 20 minutes before the next drug exposure. Left atrial preparations were stimulated by electrical field stimulation using a bipolar platinum electrode with 2-millisecond constant current square pulses. The intensity of these stimuli was set at 75 mA, and the applied frequency was 1 Hz (60 beats per minute).1 Every preparation was exposed to electrical impulses for 30 minutes, 5 minutes of exposure for each concentration of drugs. Biological response of heart tissue (heart rate and developed force) was converted to electrical stimulus and recorded on a Grass model 79E polygraph (Grass Technologies, Astro-Med, Inc., West Warwick, Rhode Island).
Drugs
Drugs used were propranolol and desipramine (Sigma Chemical, St. Louis, Missouri); vecuronium and rocuronium (NV Organon, Oss, Netherlands); mivacurium (GlaxoSmithKline, Parma, Italy); and pancuronium (Organon Teknika, Istanbul, Turkey). All drugs were dissolved in deionized water freshly on the day of the experiment. Stock solutions were stored at −20°C.
Statistical Analysis
All data are expressed as mean (SEM). Groups were compared statistically using general linear models of ANOVA followed by Newman-Keuls test and t test, when appropriate. Differences were considered to be significant at P < 0.05. All analyses were performed with Statistica for Windows 6.0 (Statsoft, Inc, Tulsa, Oklahoma).
Results
Pancuronium increased heart rate in a dose-dependent manner compared with the control group, especially at 10–7, 10–6, and 10–5 M concentrations (P < 0.05). This increase was even more obvious at 10–6 and 10–5 M concentrations. Vecuronium, rocuronium, and mivacurium also increased heart rate in a dose-dependent manner, but the changes were not statistically significant. Although propranolol (10–8 M), a nonselective β-blocker, decreased the pancuronium heart rate effect (P < 0.05), it did not change that of vecuronium, rocuronium, or mivacurium. Desipramine (10–7 M), a noradrenaline reuptake inhibitor, did not change the heart rate effect of vecuronium, rocuronium, mivacurium, or pancuronium (Figure 1).
Figure 1.
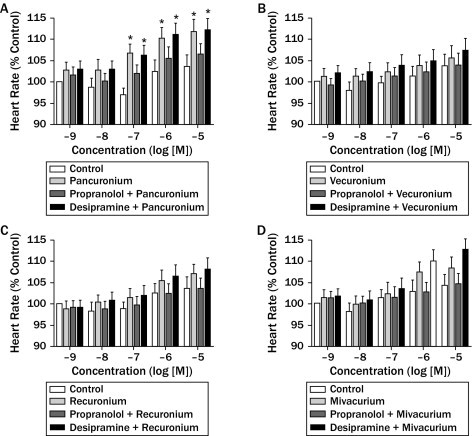
The effects of (A) pancuronium (10–9–10–5 M), (B) vecuronium (10–9–10–5 M), (C) rocuronium (10–9–10–5 M), and (D) mivacurium (10–9–10–5 M) on heart rate at rat atrial tissue. Data are expressed as mean (SEM) (n = 5 for each drug). *P < 0.05 denotes significant difference from the pancuronium control group.
Separately, all 4 drugs increased developed force in a dose-dependent manner; the increases were significant at 10–5 M concentration for pancuronium and 10–6 and 10–5 M concentrations for vecuronium, rocuronium, and mivacurium (P < 0.05). The increases in developed force were abolished by the addition of propranolol. Desipramine (10–7 M) did not change the developed force effects of pancuronium, vecuronium, rocuronium, or mivacurium (Figure 2).
Figure 2.
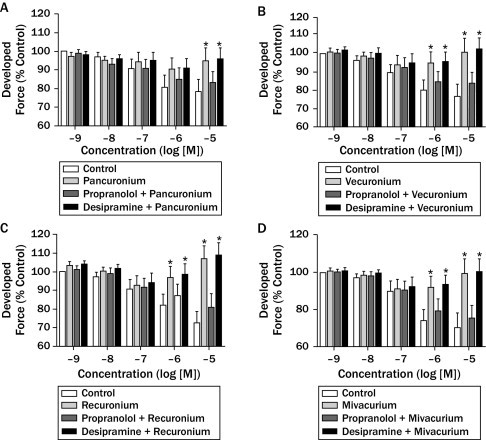
The effects of (A) pancuronium (10–9–10–5 M), (B) vecuronium (10–9–10–5 M), (C) rocuronium (10–9–10–5 M), and (D) mivacurium (10–9–10–5 M) on developed force at rat atrial tissue. Data are expressed as mean (SEM) (n = 5 for each drug). *P < 0.05 denotes significant difference from each control group.
The maximum effects of these drugs on heart rate and developed force are summarized in the Table.
Table.
Maximum effects of all 4 drugs on heart rate and developed force. Data are given as mean (SEM).
| Group | Heart Rate | Developed Force |
|---|---|---|
| Control | 103.6 (2.9) | 78.2 (7.2) |
| Pancuronium | 111.7 (3.0)⁎ | 90.4 (6.0)⁎ |
| Pancuronium + Propranolol | 106.4 (2.5) | 83.2 (6.4) |
| Pancuronium + Desipramine | 112.2 (2.6)⁎ | 91.0 (51)⁎ |
| Control | 103.6 (2.7) | 76.6 (6.8) |
| Vecuronium | 105.6 (2.7) | 93.7 (5.3)⁎ |
| Vecuronium + Propranolol | 103.8 (2.8) | 84.0 (6.2) |
| Vecuronium + Desipramine | 107.2 (2.8) | 95.0 (5.0)⁎ |
| Control | 103.7 (2.3) | 72.4 (6.1) |
| Rocuronium | 106.9 (2.4) | 92.5 (5.1)⁎ |
| Rocuronium + Propranolol | 103.7 (2.3) | 80.8 (7.4) |
| Rocuronium + Desipramine | 108.2 (2.4) | 94.0 (5.0)⁎ |
| Control | 104.1 (2.6) | 70.2 (8.1)⁎ |
| Mivacurium | 108.2 (2.6) | 91.2 (5.4) |
| Mivacurium + Propranolol | 104.4 (2.5) | 75.5 (7.2) |
| Mivacurium + Desipramine | 112.6 (2.4) | 92.6 (4.9)⁎ |
P < 0.05 denotes significant difference from control of each group.
Discussion
The cardiovascular effects of nondepolarizing muscle relaxants have been studied in different animals and also in humans. The isolated heart muscle preparation is useful in evaluation of the effects of drugs upon myocardial contractility, because such a preparation allows preload and afterload to be controlled and because it is free of the influence of neurohumoral responses.3 Pancuronium produced tachycardia and increased arterial pressure; this cardiac stimulation has been attributed to vagolytic action, a release of norepinephrine from the sympathetic nerve terminals, or inhibition of neuronal uptake of norepinephrine.9 In another study, the effect of pancuronium on heart rate was connected to, in part, increased release and decreased reuptake of catecholamines at the adrenergic nerve terminal.8 Plasma adrenaline and noradrenaline concentrations increased when pancuronium was administered to facilitate tracheal intubation23 or after administration of pancuronium to ill neonates.24
In contrast, vecuronium has been reported to have minimal effects on the cardiovascular system in experimental animals and in clinical practice.4,25 However, vecuronium has been suggested to have positive chronotropic and inotropic effects, but it is much less potent than pancuronium in some experimental animals.9 It has been suggested that the bradycardia effect of vecuronium was due to its direct action or the increase in vagal tone induced by either surgical procedures or other drugs.9 In patients undergoing coronary artery bypass grafting, rocuronium administration was associated with significant increases in cardiac index and stroke volume index, but not with a change in heart rate.26 In a study performed in elderly patients, the authors reported that rocuronium did not cause tachycardia or hypertension.27 However, Booth et al28 demonstrated a marked tachycardia after administration of rocuronium. Appadu and Lambert29 showed an interaction with cardiac muscarinic receptors with a potency rank order of pancuronium, vecuronium, and rocuronium, respectively. Savarese et al20 showed a transient decrease in blood pressure after mivacurium administration, but Okanlami et al30 reported that mivacurium inhibited bradycardia and bronchoconstriction induced by either intravenous acetylcholine (Ach) or vagal nerve stimulation. They suggested that mivacurium blocks both M2 and M3 receptors and that it is a muscarinic antagonist with similar potencies for both M2 and M3 receptors.
The plasma concentration of pancuronium administered to humans during the first 3 hours is reportedly 500 to 100 ng/mL (∼90% free form), which is equivalent to 7 × 10–7 and 1.4 × 10–7 M, respectively.31,32 In the present study we used all neuromuscular blocking drugs at concentrations harmonious with the study of Cannon et al.17
In the present study, pancuronium increased heart rate in a dose-dependent manner compared with the control group, especially at high concentrations, but vecuronium, rocuronium, and mivacurium did not cause a significant increase. Although propranolol decreased the heart rate effect of pancuronium, desipramine did not. All 4 drugs increased developed force in a dose-dependent manner. The increases in developed force were abolished by the addition of propranolol, but desipramine did not change the developed force effect of any of the 4 drugs.
The decreased heart rate effect of pancuronium and developed force effects of all 4 agents in the presence of propranolol points to the effects occurring via β receptor stimulation. If we had not used desipramine, we might hypothesize that the effects may occur by direct stimulation of β receptors, increased release of endogen noradrenaline from sympathetic nerve terminals, and/or decreased reuptake of noradrenaline at nerve terminals. Desipramine did not change their effects, so the effects may be due to direct stimulation of β receptors.
Conclusions
Cardiovascular effects of nondepolarizing muscle relaxants are clinically significant in the anesthetic management of patients in whom long-duration neuromuscular blockade is usually required. Pancuronium increased heart rate, and pancuronium, vecuronium, rocuronium, and mivacurium had positive inotropic effects. A positive inotropic effect of pancuronium was seen at a higher concentration. These effects may be due to direct stimulation of β receptors. The effects may be important, especially under pathologic conditions such as hypertension, in which patients usually use β-blocking agents causing β receptor upregulation. A limitation of the present study is that it was an in vitro investigation. Further in vivo experimental studies and controlled clinical trials are needed to determine whether these findings are correct.
Acknowledgments
The authors have indicated that they have no conflicts of interest regarding the content of this article. All authors contributed equally to the conduct of the study and creation of the manuscript.
References
- 1.Meinikov A.L., Malakhov K.Y., Helgesen K.G., Lathrop D.A. Cardiac effects of non-depolarizing neuromuscular blocking agents pancuronium, vecuronium, and rocuronium in isolated rat atria. Gen Pharmacol. 1999;33:313–317. doi: 10.1016/s0306-3623(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 2.Bowman W.C. Non-relaxant properties of neuromuscular blocking drugs. Br J Anaesth. 1982;54:147–160. doi: 10.1093/bja/54.2.147. [DOI] [PubMed] [Google Scholar]
- 3.Iwatsuki N., Hashimoto Y., Amaha K. Inotropic effects of non-depolarizing muscle relaxants in isolated canine heart muscle. Anesth Analg. 1980;59:717–721. [PubMed] [Google Scholar]
- 4.Alvarez L., Escudero C., Silva L., Castillo-Olivares J.L. Electrophysiological effects of atracurium and vecuronium on normal and denervated hearts. J Cardiothorac Vasc Anesth. 1992;6:304–307. doi: 10.1016/1053-0770(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 5.Krol T., Siondalska-Kunicka E. Haemodynamic side-effects of pancuronium. Anaesth Resusc Intensive Ther. 1974;2:161–165. [PubMed] [Google Scholar]
- 6.Cornet J.P., Abiad M., Coriat P. Evaluation of the effects of rocuronium bromide on haemodynamics and left ventricular function in patients undergoing abdominal aortic surgery. Eur J Anesthesiol Suppl. 1994;9:78–81. [PubMed] [Google Scholar]
- 7.Thomson I.R., Putnins C.L. Adverse effects of pancuronium during high-dose fentanyl anesthesia for coronary artery bypass grafting. Anesthesiology. 1985;62:708–713. doi: 10.1097/00000542-198506000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Docherty J.R., McGrath J.C. Sympathomimetic effects of pancuronium bromide on the cardiovascular system of the pithed rat: a comparison with the effects of drugs blocking the neuronal uptake of noradrenaline. Br J Pharmacol. 1978;64:589–599. doi: 10.1111/j.1476-5381.1978.tb17321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narita M., Furukawa Y., Ren L.M. Cardiac effects of vecuronium and its interaction with autonomic nervous system in isolated perfused canine hearts. J Cardiovasc Pharmacol. 1992;19:1000–1008. doi: 10.1097/00005344-199206000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Milligan K.R., Beers H.T. Vecuronium-associated cardiac arrest. Anaesthesia. 1985;42:192–194. [Google Scholar]
- 11.Inoue K., el-Banayosy A., Stolarski L., Reichelt W. Vecuronium induced bradycardia following induction of anaesthesia with etomidate or thiopentone, with or without fentanyl. Br J Anaesth. 1988;60:10–17. doi: 10.1093/bja/60.1.10. [DOI] [PubMed] [Google Scholar]
- 12.Cozanitis D.A., Lindgren L., Rosenberg P.H. Bradycardia in patients receiving atracurium or vecuronium in conditions of low vagal stimulation. Anaesthesia. 1989;44:303–305. doi: 10.1111/j.1365-2044.1989.tb11282.x. [DOI] [PubMed] [Google Scholar]
- 13.Morris R.B., Cahalan M.K., Miller R.D. The cardiovascular effects of vecuronium (ORG NC45) and pancuronium in patients undergoing coronary artery bypass grafting. Anesthesiology. 1983;58 doi: 10.1097/00000542-198305000-00008. 43–40. [DOI] [PubMed] [Google Scholar]
- 14.Tullock W.C., Diana P., Cook D.R. Neuromuscular and cardiovascular effects of high-dose vecuronium. Anesth Analg. 1990;70:86–90. doi: 10.1213/00000539-199001000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Lennon R.L., Olson R.A., Gronert G.A. Atracurium or vecuronium for rapid sequence endotracheal intubation. Anesthesiology. 1986;64:510–513. doi: 10.1097/00000542-198604000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz S., Ilias W., Lackner F. Rapid tracheal intubation with vecuronium: the priming principle. Anesthesiology. 1985;62:388–391. doi: 10.1097/00000542-198504000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cannon J.E., Fahey M.R., Moss J., Miller R.D. Large doses of vecuronium and plasma histamine concentrations. Can J Anaesth. 1988;35:350–353. doi: 10.1007/BF03010854. [DOI] [PubMed] [Google Scholar]
- 18.Husby P., Gramstad L., Rosland J.H. Haemodynamic effects of high-dose vecuronium compared with pancuronium in beta-blocked patients with coronary artery disease during fentanyl-diazepam-nitrous oxide anaesthesia. Acta Anaesthesiol Scand. 1996;40:26–31. doi: 10.1111/j.1399-6576.1996.tb04384.x. [DOI] [PubMed] [Google Scholar]
- 19.Marshall R.J., Muir A.W., Sleigh T., Savage D.S. An overview of the pharmacology of rocuronium bromide in experimental animals. Eur J Anaesthesiol Suppl. 1994;9:9–15. [PubMed] [Google Scholar]
- 20.Savarese J.J., Ali H.H., Basta S.J. The clinical neuromuscular pharmacology of mivacurium chloride (BW B1090U): a short-acting nondepolarizing ester neuromuscular blocking drug. Anesthesiology. 1988;68:723–732. doi: 10.1097/00000542-198805000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Savarese J.J., Caldwell J.E., Lien C.A., Miller R.D. Pharmacology of muscle relaxants and their antagonists. In: Miller R.D., editor. Anesthesia. 5th ed. Churchill Livingstone; Philadelphia: 2000. pp. 412–490. [Google Scholar]
- 22.Plaud B., Marty J., Debaene B. The cardiovascular effects of mivacurium in hypertensive patients. Anesth Analg. 2002;95:379–384. doi: 10.1097/00000539-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Cummings M.F., Russell W.J., Frewin D.B. Effects of pancuronium and alcuronium on the changes in arterial pressure and plasma catecholamine concentrations during tracheal intubation. Br J Anaesth. 1983;55:619–623. doi: 10.1093/bja/55.7.619. [DOI] [PubMed] [Google Scholar]
- 24.Cabal L.A., Siassi B., Artal R. Cardiovascular and catecholamine changes after administration of pancuronium in distressed neonates. Pediatrics. 1985;75:284–287. [PubMed] [Google Scholar]
- 25.Lavery G.G., Mirakhur R.K., Clarke R.S., Gibson F.M. The effect of atracurium, vecuronium and pancuronium on heart rate and arterial pressure in normal individuals. Eur J Anaesthesiol. 1986;3:459–467. [PubMed] [Google Scholar]
- 26.McCoy E.P., Maddineni V.R., Elliott P. Haemodynamic effects of rocuronium during fentanyl anaesthesia: comparison with vecuronium. Can J Anaesth. 1993;40:703–708. doi: 10.1007/BF03009764. [DOI] [PubMed] [Google Scholar]
- 27.Shorten G.D., Uppington J., Comunale M.E. Changes in plasma catecholamine concentrations and haemodynamic effects of rocuronium and vecuronium in elderly patients. Eur J Anaesthesiol. 1998;15:335–341. doi: 10.1046/j.1365-2346.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 28.Booth M.G., Marsh B., Bryden F.M. A comparison of the pharmacodynamics of rocuronium and vecuronium during halothane anesthesia. Anaesthesia. 1992;47:832–834. doi: 10.1111/j.1365-2044.1992.tb03139.x. [DOI] [PubMed] [Google Scholar]
- 29.Appadu B.L., Lambert D.G. Studies on the interaction of steroidal neuromuscular blocking drugs with cardiac muscarinic receptors. Br J Anaesth. 1994;72:86–88. doi: 10.1093/bja/72.1.86. [DOI] [PubMed] [Google Scholar]
- 30.Okanlami O.A., Fryer A.D., Hirshman C. Interaction of nondepolarizing muscle relaxants with M2 and M3 muscarinic receptors in guinea pig lung and heart. Anesthesiology. 1996;84:155–161. doi: 10.1097/00000542-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 31.McLeod K., Watson M.J., Rawlins M.D. Pharmacokinetics of pancuronium in patients with normal and impaired renal function. Br J Anaesth. 1976;48:341–345. doi: 10.1093/bja/48.4.341. [DOI] [PubMed] [Google Scholar]
- 32.Wood M. Neuromuscular blocking agents. In: Wood M., Wood A.J., editors. Drugs and Anesthesia: Pharmacology for Anesthesiologists. Williams & Wilkins; Baltimore, Md: 1982. pp. 299–340. [Google Scholar]


