Abstract
Background
Recurrent urinary tract infections are important in children and adults with diabetes mellitus and/or incontinence due to risk of pyelonephritis (PYN) and renal damage. There is a positive correlation released free radicals during PYN and renal damage. Experimental studies showed that antioxidant agents improve renal damage when used immediately after bacterial inoculation.
Objective
The aim of the present study was to evaluate whether treatment by thymoquinone (TQ) before or during Escherichia coli inoculation prevents oxidative damage in acute pyelonephritis (PYN) in an ascending obstructive rat model.
Methods
In this study, 42 Wistar rats were grouped as follows: control, PYN (24, 48, and 72 hours), and TQ-PYN (24, 48, and 72 hours). E. coli (1 ×109 colony forming units) was inoculated into the bladder via urethral catheterization in both the PYN and TQ groups. TQ injections were performed 24 hours before bacteria inoculation and repeated at 24-hour intervals during the indicated time at a dose of 10 mg/kg body weight intraperitoneally in TQ groups.
Results
Superoxide dismutase activity was statistically lower in the TQ-PYN-48 and -72 groups than the PYN-48 and -72 groups (P < 0.001, P = 0.004, respectively). Catalase activity was significantly higher in PYN-24, -48, and -72 groups than the control group (P < 0.001). In addition, there was a significant difference between the TQ-PYN-24, -48, and -72 groups and PYN groups in terms of glutathione peroxidase activity (P < 0.001, P = 0.026, P = 0.046, respectively). When the TQ-PYN-72 group was compared with the PYN-72 group, malondialdehyde levels were significantly lower in the TQ-PYN-72 group than in the PYN-72 group (P = 0.033). A histologic examination also confirmed the protective effect of TQ. In statistical analysis of histopathologic findings, there were significant differences between the PYN-24 and TQ-PYN-24, PYN-48 and TQ-PYN-48, and PYN-72 and TQ-PYN-72 groups (P = 0.008, P < 0.001, P < 0.001, respectively).
Conclusions
The results indicate that TQ administration attenuated the oxidative damage that occurred in PYN and, therefore, could be used as a supportive agent to protect the kidneys from oxidative damage caused by PYN.
Key words: kidney, oxidative stress, pyelonephritis, thymoquinone, Wistar rats
Introduction
Urinary tract infection is one of the most common diseases encountered in medical practice. Escherichia coli is the most frequent cause of urinary tract infection. Clinical manifestations of these infections range in severity from cystitis to acute or chronic pyelonephritis (PYN). If not treated properly, permanent renal damage is possible.1
Renal scarring is the worst result of PYN. This event depends on the inflammatory damage followed by a bacterial infection in the kidneys. In experimental and clinical studies, it has been reported that PYN should be treated urgently with antibiotics, such as aminoglycosides or β-lactam antibiotics, to prevent renal scar formation. A late start of antibiotic treatment can lead to renal scarring.2–5
Recurrent urinary tract infections are important in children and adults with diabetes mellitus and/or incontinence due to the risk of PYN and renal damage.6,7 There is a positive correlation between acute PYN and renal failure secondary to chronic scarring due to the released free radicals during PYN.8 In experimental studies regarding rat models of PYN, antioxidant agents such as vitamins A, E, and pentoxifylline improved E. coli–induced renal scar formation when used immediately after bacterial inoculation.9,10
Nigella sativa was shown to have many therapeutic effects, including immunomodulatory,11 antibacterial,12 antidiabetic,13,14 antihistaminic, and antioxidant.15 Thymoquinone (TQ; 2-isopropyl-5-methyl-1,4-ben-zoquinone) is the main active component of the volatile oil of the black seed (Nigella sativa L.) and may have a protective effect against leukocytes, membrane lipid peroxidation, and cisplatin-induced nephrotoxicity.16,17 However, the therapeutic or preventive effect of TQ on E. coli–induced acute PYN remains unknown.
The aim of the present study was to investigate whether treatment by TQ before or during E. coli inoculation prevents oxidative damage in acute PYN in an ascending obstructive rat model.
Materials and Methods
Animals and Experimental Infection
A total of 42 male Wistar albino rats weighing 200 to 250 g (average body weight of groups, 236 [19] g) and averaging 12 weeks of age were obtained from the Laboratory Animal Production Unit of Gaziantep University (Gaziantep, Turkey). They were kept in an environment with controlled temperature (21 [2]°C), humidity (55%–60%), and photoperiod (12:12-hour light–dark cycle) for 1 week before the start of the experiment. A commercial balanced diet (Gaziantep Yem Sanayi, Gaziantep, Turkey) and tap water were provided ad libitum. Experimental procedures were approved by Mustafa Kemal University's Local Ethics Committee for the use and care of laboratory animals.
The rats were divided into 7 groups to be equal to the average body weights (6 animals in each group). In the control group (n = 6), saline was given to the rats intraperitoneally and into the urinary bladder via urethral catheterization at once; they were euthanized 24 hours later. In the PYN groups (n = 18), bacterial inoculation was introduced into the bladder of the rats at 24 hours after intraperitoneal sterile saline injection. The rats were followed at 24 (PYN-24, n = 6), 48 (PYN-48, n = 6), and 72 (PYN-72, n = 6) hours in terms of PYN development. Sterile saline injection was repeated each day. The rats were euthanized at the end of follow-up. In the TQ-PYN groups (n = 18), bacterial inoculation was introduced into the bladder of the rats 24 hours after the intraperitoneal TQ injection. The rats were followed at 24 (TQ-PYN-24, n = 6), 48 (TQ-PYN-48, n = 6), and 72 (TQ-PYN-72, n = 6) hours in terms of PYN development. TQ injection was repeated each day. The rats were euthanized at the end of follow-up.
The rats were anesthetized with ether. The urethra was catheterized with a sterile 22-gauge angiocatheter sheath and the bladder was emptied, and 0.4 mL of bacterial solution (1 × 109 colony forming units, all of the groups except the control group) or sterile saline solution (control group) was instilled into the bladder via urethra. The angiocatheter was removed and the urethra was occluded with collodium. The collodium was removed by acetone 4 hours after the bacterial inoculation. TQ injections were performed at a dose of 10 mg/kg/d body weight intraperitoneally in the TQ-PYN groups.18 Under ether anesthesia, animals were euthanized by intracardiac puncture for blood sampling at the indicated times (24, 48, and 72 hours) after bacterial inoculation. Both kidneys were removed aseptically, and one of them was stored in a 10% formalin solution for blind histopathologic evaluation, whereas the other was stored (–30°C) for blind biochemical analysis.
Bacterial Strain
E. coli strain ATCC 25922 was used to induce PYN. This E. coli strain was grown in trypticase soy broth at 37°C overnight. Organisms were harvested and diluted to a final concentration of approximately 1010 organisms per milliliter in 1 × phosphate buffered saline as determined by optical density at 600 nm in accordance with the method of Bennett et al.10 The solution containing 1010 bacteria/mL was diluted 4 times in sterile saline solution. Bacterial suspensions were administered to the rats on the day they were prepared. The rats were anesthetized with ether. The urethra was catheterized with a sterile 22-gauge angiocatheter sheath and the bladder was emptied, and 0.4 mL bacterial solution (1 × 109 colony forming units) or sterile saline solution was instilled. The angiocatheter was removed and the urethra was occluded with collodium. The collodium was removed by acetone 4 hours after bacterial inoculation.
Tissue Homogenization and Protein Determination
The kidney tissue samples were stored at –30°C until assayed for kidney tissue malondialdehyde (MDA) levels, and catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities. The kidney tissues were homogenized (for 2 minutes at 5000 rpm) in 4 volumes of ice-cold Tris-hydrogen chloride buffer (50 mmol, pH 7.4) using a glass Teflon homogenizer (Ultra Turrax IKA T10 Basic, Staufen, Germany). MDA and protein levels were measured at homogenate. The homogenate was then centrifuged at 5000g for 60 minutes to remove debris. The supernatant fluid was collected, analyses of CAT and GSH-Px activities were conducted, and protein concentration was measured. The supernatant solutions were used for the assay, mixed with an equal volume of an ethanol/chloroform (5/3, volume per volume [v/v]). After centrifugation at 5000g for 30 minutes, the clear upper layer (the ethanol phase) was collected and used in the analysis of SOD activity and protein assays. All preparation procedures were carried out at +4°C.
Determination of Malondialdehyde Levels
The thiobarbituric acid reactive (TBA) substances level was determined by a method based on the reaction with TBA at 90°C to 100°C.19 In the TBA test reaction, MDA or MDA-like substances (ie, the byproduct of the lipid peroxidation process of the polyunsaturated fatty acid) and TBA reacted together, producing a pink pigment with an absorption maximum of 532 nm. The reaction was performed at pH 2 to 3 at 90°C for 15 minutes. The sample was mixed with 2 volumes of cold 10% (w/v) trichloroacetic acid to precipitate protein. The precipitate was pelleted by centrifugation, and an aliquot of the supernatant was reacted with an equal volume of 0.67% (w/v) TBA in a boiling water bath for 10 minutes. After cooling, the absorbance was read at 532 nm (Ultra Spec Plus, Pharmacia LKB Biochrom Ltd, Cambridge, United Kingdom). The results were expressed as nanomoles per gram wet tissue by reference to a standard curve prepared from measurements made with a standard solution (1,1,3,3-tetramethoxypropane).
Determination of Catalase Activity
CAT (Enzyme Commission Number [EC] 1.11.1.6) activity was measured according to the method of Aebi.20 The principle of the assay is based on the determination of the rate constant k (dimension: s–1, k) of hydrogen peroxide decomposition. By measuring the absorbance changes per minute, the rate constant of the enzyme was determined. Activities were expressed as k (rate constant) per gram of protein.
Determination of Glutathione Peroxidase Activity
GSH-Px (EC 1.6.4.2) activity was measured using the method of Paglia and Valentine.21 The enzymatic reaction was initiated by the addition of hydrogen peroxide to the reaction mixture containing GSH, nicotinamide adenosine dinucleotide phosphate, and glutathione reductase. The change in the absorbance at 340 nm was monitored by a spectrophotometer. The activity was expressed as units per gram protein.
Determination of Protein Level
Protein measurements were performed in the tissue homogenate in accordance with the method of Lowry et al.22
Determination of Superoxide Dismutase Activity
The principle of the total SOD (EC 1.15.1.1) activity method is based, briefly, on the inhibition of nitroblue tetrazolium reduction by O2− generated by a xanthine/xanthine oxidase system.23 Activity was assessed in the ethanol phase of the serum after 1.0 mL of an ethanol/chloroform mixture (5/3, v/v) was added to the same volume of the serum and centrifuged. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the nitroblue tetrazolium reduction rate. The SOD activity was expressed as unit per gram protein.
Histologic Examination
For light microscopic evaluation, renal samples were fixed in 10% neutral buffered formalin and dehydrated in a graded alcohol series from 70% to 100%. The tissue samples were then embedded in paraffin. Sections of 4 to 6 μm were prepared from paraffin blocks using a rotary microtome. These sections were then stained with hematoxylin and eosin and photographed using an Olympus CX-41 photomicroscope (Olympus Corporation, Tokyo, Japan) powered with an Olympus DP20 digital camera (Olympus Corporation, Tokyo, Japan). All sections of the kidney samples were examined by a histologist blinded to the group in which the rats were randomized for characteristic histologic changes, including tubular epithelial alterations (vacuolization, atrophy, and cellular desquamation) and glomerular pathology (collapse, edema). To obtain a histologic score, histopathologic changes were graded according to cortical involvement and the severity of the lesions, modified from Karahan et al24 and Celik et al.25
Statistical Analysis
Biochemical data were analyzed using a commercially available statistics software package (SPSS for Windows 15.0; SPSS Inc, Chicago, Illinois). Distribution of the groups was analyzed using 1-sample Kolmogorov-Smirnov test. The groups showed normal distributions for the level of MDA and the activities of CAT, SOD, and GSH-Px, so parametric statistical methods were used to analyze the data. A 1-way ANOVA test was performed, and post hoc multiple comparisons were made using least-squares differences. The results are presented as mean (SEM); P < 0.05 was considered statistically significant. One-way ANOVA and post hoc multiple comparisons by least-square difference test were used to analyze the histopathologic changes.
Results
When the total PYN group (24 + 48 + 72 hours) was compared with the total TQ-PYN group (24 + 48 + 72 hours), serum SOD, CAT, and GSH-Px activities were statistically lower in the TQ-PYN group than in the total PYN group (P < 0.001, P = 0.002, and P < 0.001, respectively). However, serum MDA levels were not significant (P > 0.05) (Figure 1).
Figure 1.
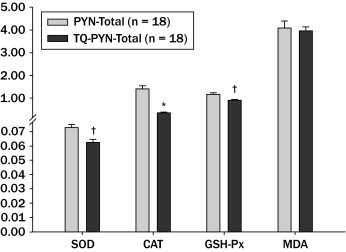
Malondialdehyde (MDA) levels and superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activity in kidney homogenates. Results are presented as mean (SEM). Student t test was used to test for differences between groups. *P < 0.01; †P < 0.001 compared with pyelonephritis (PYN) total group. TQ = thymoquinone.
In addition, control, PYN, and TQ-PYN groups were compared for aspects of serum SOD, CAT, GSH-Px activity, and MDA levels. There was no statistical difference between control and PYN groups in terms of SOD activity (P > 0.05). However, SOD activity was statistically lower in the TQ-PYN-48 and -72 groups than the PYN-48 and -72 groups (P < 0.001, P = 0.004, respectively). CAT activity was significantly higher in the PYN-24, -48, and -72 groups than in the control group (P < 0.001). However, there was no significant increase in the TQ groups (P > 0.05). GSH-Px activity was not statistically different between the control group and PYN groups. However, there was a significant difference between TQ-PYN-24, -48, and -72 groups and PYN groups in terms of GSH-Px activity (P < 0.001, P = 0.026, and P = 0.046, respectively). MDA levels were statistically higher in the PYN-72 group than in the control group (P = 0.002). When the TQ-PYN-72 group was compared with the PYN-72 group, MDA levels were significantly lower in the TQ-PYN-72 group than the PYN-72 group (P = 0.033) (Figure 2).
Figure 2.
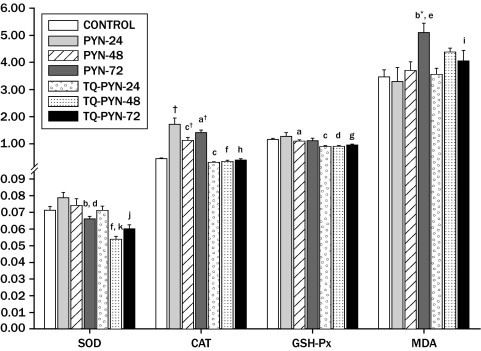
Malondialdehyde (MDA) levels and superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activity in kidney homogenates. Results are presented as means (SEM). One-way ANOVA and post hoc by least-square difference was used to test for differences between groups. *P < 0.01; †P < 0.001 compared with control group. aP < 0.05, bP < 0.01, cP < 0.001 compared with pyelonephritis (PYN)-24 group. dP < 0.05, eP < 0.01, fP < 0.001 compared with PYN-48 group. gP < 0.05, hP < 0.001 compared with PYN-72 group. iP < 0.05, jP < 0.01, kP < 0.001 compared with thymoquinone (TQ)-PYN-24 group.
The control group showed normal renal architecture in the histologic examination (Figure 3A), but there were neutrophil casts in the tubuli and signs of tubular degeneration such as vacuolization in the tubular epithelium in group 2 (Figure 3B). PYN caused diffuse damage in the glomeruli (Figure 3C) and the medullar region in 48 hours. Extensive vascular congestion, tubular degeneration, and dilatation were noticed in the medulla (Figure 3D). The most severe findings were noted in group 4. Hyalinization with fibrosis occurred in the cortical area (Figure 3E). TQ application reversed the damage in renal tissues, to some extent, in the TQ groups. Cortical lesions, such as glomerular shrinkage, cellular casts, or vacuolization in the tubular epithelium, were fewer in these groups (data not shown). Microscopic examination also showed mild tubular degeneration, dilatation, and hemorrhage in the medullar region (Figure 3F). In statistical analysis of histopathologic findings, there were significant differences between PYN-24 and TQ-24, PYN-48 and TQ-48, and PYN-72 and TQ-72 groups (P = 0.008, P < 0.001, and P < 0.001, respectively). In addition, when we combined all PYN subgroups into one PYN group and all TQ subgroups into one TQ group and compared the two, we obtained a significant difference between the two groups, as shown in Table (P < 0.001).
Figure 3.
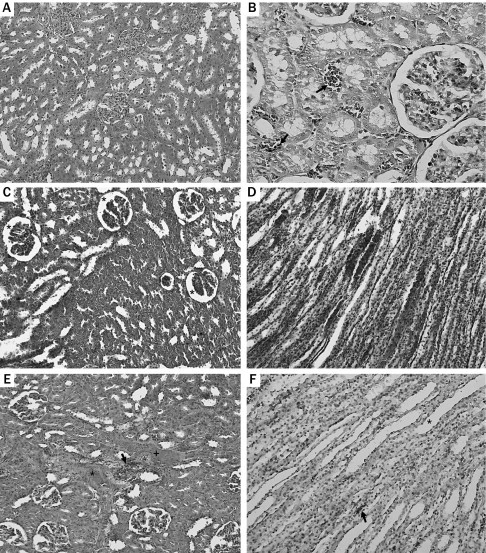
(A) Normal glomerular and tubular structure (hematoxylin and eosin [HE] ×100). (B) Neutrophil casts (arrow) (HE ×400). (C) Diffuse glomerular shrinkage (asterisk) (HE ×200). (D) Extensive vascular congestion, tubular degeneration, and dilatation (asterisk) (HE ×200). (E) Interstitial fibrosis (asterisk), cellular casts (arrow), hyalinization (plus sign), and degenerated glomeruli (HE ×200). (F) Mild tubular degeneration, dilatation (asterisk), and hemorrhage (arrow) (HE ×200).
Table.
Grading levels of inflammation in pyelonephritis (PYN)– and thymoquinone (TQ)–treated groups as determined by histopathologic examination.
| Grade | Histopathologic Changes |
|---|---|
| Grade 0 | No pathologic changes |
| Grade I | Slight degenerative changes in tubuli and glomeruli with cortical involvement <25% |
| Grade II | Mild degenerative changes with cortical involvement of 25%–50% |
| Grade III | Tubular and glomerular necrosis at different foci throughout the cortex with cortical involvement of 50%–75% |
| Grade IV | Extensive and marked necrosis throughout the cortex with cortical involvement >75% |
| Between Group Comparisons | P |
|---|---|
| Control vs PYN-24 | <0.001 |
| Control vs PYN-48 | <0.001 |
| Control vs PYN-72 | <0.001 |
| PYN-24 vs TQ-PYN-24 | 0.008 |
| PYN-48 vs TQ-PYN-48 | <0.001 |
| PYN-72 vs TQ-PYN-72 | <0.001 |
| PYN total vs TQ-PYN total) | <0.001 |
One-way ANOVA and post hoc test by least-square difference test was used to analyze the histopathologic changes.
Discussion
Infection of the kidney is one of the most common serious conditions in humans, particularly in childhood. E. coli may often cause cystitis or PYN. The activities of CAT, SOD, and GSH-Px, and lipid peroxidation in E. coli-induced PYN were evaluated in an experimental rat model.26
Some studies demonstrated that TQ shows a potent superoxide radical scavenger effect, with scavenging power being as effective as SOD against superoxide. Badary et al27 evaluated the antioxidant and pro-oxidant effects of TQ in vitro. Their results showed that TQ is a very active superoxide anion scavenging agent. Nagi et al28 reported a protective effect of TQ with respect to doxorubicin-induced cardiotoxicity in rats. Their results suggested that the peroral administration of TQ provided potent superoxide radical scavenger activity. In this study, there were no increases in the SOD and GSH-Px enzyme activity when the control group was compared with the PYN groups, whereas CAT activity for the PYN groups was significantly higher than that for the control group. Significant attenuates in antioxidant enzyme activities were observed in the TQ groups versus the control and PYN groups. It was thought that these attenuated antioxidant enzyme activities might be the consequence of the direct scavenger activity of TQ in kidney tissue with E. coli–induced PYN.
Another parameter that has been used widely in demonstrating oxidative injury is MDA,29 a product of lipid peroxidation. In a study that carried out isolated rat kidney homogenates, Celik et al25 reported that E. coli–induced PYN caused oxidative injury. In the present study, there was a statistically significant rise in MDA activity in rat renal tissue with PYN. This increase in MDA levels demonstrated that E. coli–induced PYN led to lipid peroxidation followed naturally by oxidative injury in the renal tissues. According to early studies, TQ decreased the level of lipid peroxidation products such as MDA. Abdelmeguid et al30 observed a significant decrease in the levels of MDA in the TQ groups in rats made diabetic with streptozotocin.
Treatment with TQ showed a therapeutic and protective effect by attenuating oxidative stress. Fouda et al31 reported that the maximal protection offered by TQ treatment was particularly noticeable 48 and 72 hours after administration of the toxic agent at the time when histologic damage, apoptotic events, and proliferative reactions reached their maximum. There was a similar result in our study. MDA levels significantly attenuated in TQ-72 hour treatment versus the PYN-72 hour group. This result suggests that TQ might be used in E. coli–induced PYN. In addition, the significant attenuate in the MDA level in the TQ-72 group showed that TQ might affect the MDA levels over a longer period, such as 48 to 72 hours.
In the present study, a histologic examination also confirmed the protective effect of TQ in kidney tissue with E. coli–induced PYN. Microscopic examination revealed a better renal histology in the TQ-administered group. Complete improvement was not seen, but both cortical and medullar lesions were reversed with TQ application. Fewer hemorrhagic areas and inflammatory cells and better glomerular and tubular architecture were also observed. The protective effect of TQ has been shown in various pathologic conditions. For example, Fouda et al31 demonstrated that treatment of rats with TQ reduced the number of superoxide-positive cells by 70% and offered imperative protection from mercury chloride–induced renal damage. Kanter et al32 showed that TQ therapy caused renal morphologic and functional improvement after streptozotocin-induced diabetes in rats. Also, Sayed-Ahmet et al33 reported that peroral TQ supplementation completely prevented an increase in nephrotoxicity indexes and histopathologic lesions induced by gentamicin. Although various toxic or injuring agents were used in those studies, TQ provided a significant improvement in renal lesions.
Study Limitations
There are some limitations to the present study. First, we did not measure plasma levels of lipid peroxidation. In the present study, our aim was to investigate oxidative injury in tissue and histopatologic evaluation. However, we did not use different TQ doses and longer duration of the drug. Also, we did not study other parameters, such as the nitrogen oxide, carbonyl, and sulfidryl group, and 8-hydroxy guanosine in terms of oxidative injury. These parameters were important for understanding the efficacy of the drugs on oxidative injury.
Conclusion
To our knowledge, this is the first report demonstrating a protective effect of TQ against E. coli–induced renal oxidative damage in a rat model of lower tract obstruction based on histopathologic and biochemical findings. This protective effect could be crucial in adult patients with diabetes and urinary incontinence and pediatric patients with recurrent urinary tract infections, because PYN may cause permanent renal damage in these patients.
Acknowledgments
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Dr. Evirgen was responsible for the general organization and writing. Dr. Gökçe was responsible for the study design and writing. Dr. Hasan Ozturk was responsible for the Biochemical analysis. Mr. Nacar was responsible for the histological study, Dr. Onlen was responsible for the literature search and hypothesis formation. Drs. Ozer and Koksaldi Motoer were responsible for the data collection and analysis.
References
- 1.Sobel J.D., Kaye D. Urinary tract infections. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and Practice of Infectious Diseases. 7th ed. Churchill Livingstone; Philadelphia: 2010. pp. 957–985. [Google Scholar]
- 2.Leroy S., Chalumeau M., Ulinski T. Impressive renal damage after acute pyelonephritis in a child. Pediatr Nephrol. 2010;25:1365–1368. doi: 10.1007/s00467-010-1461-x. [DOI] [PubMed] [Google Scholar]
- 3.Haraoka M., Matsumoto T., Takahashi K. Suppression of renal scarring by prednisolone combined with ciprofloxacin in ascending pyelonephritis in rats. J Urol. 1994;151:1078–1080. doi: 10.1016/s0022-5347(17)35187-x. [DOI] [PubMed] [Google Scholar]
- 4.Garin E.H., Olavarria F., Garcia Nieto V. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117:626–632. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 5.Gordon I., Barkovics M., Pindoria S. Primary vesicoureteric reflux as a predictor of renal damage in children hospitalized with urinary tract infection: a systematic review and meta-analysis. J Am Soc Nephrol. 2003;14:739–744. doi: 10.1097/01.asn.0000053416.93518.63. [DOI] [PubMed] [Google Scholar]
- 6.Glauser M.P., Meylan P., Bille J. The inflammatory response and tissue damage: The example of renal scars following acute renal infection. Pediatr Nephrol. 1987;1:615–622. doi: 10.1007/BF00853599. [DOI] [PubMed] [Google Scholar]
- 7.Scholes D., Hooton T.M., Roberts P.L. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005;142:20–27. doi: 10.7326/0003-4819-142-1-200501040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mundi H., Bjorksten B., Svanborg C. Extracellular release of reactive oxygen species from human neutrophils upon interaction with Escherichia coli strains causing renal scarring. Infect Immun. 1991;59:4168–4172. doi: 10.1128/iai.59.11.4168-4172.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagmurlu A., Boleken M.E., Ertoy D. Preventive effect of pentoxifylline on renal scarring in rat model of pyelonephritis. Urology. 2003;61:1037–1041. doi: 10.1016/s0090-4295(02)02428-7. [DOI] [PubMed] [Google Scholar]
- 10.Bennett R.T., Mazzaccaro R.J., Chopra N. Suppression of renal inflammation with vitamins A and E in ascending pyelonephritis in rats. J Urol. 1999;161:1681–1684. [PubMed] [Google Scholar]
- 11.El-Kadi A., Kandil O. The black seed (Nigella sativa) and immunity: its effect on human T cell subset. Fed Proc. 1987;46:1222. [Google Scholar]
- 12.Hanafy M.S., Hatem M.E. Studies on the antimicrobial activity of Nigella sativa seed (black cumin) J Ethnopharmacol. 1991;34:275–278. doi: 10.1016/0378-8741(91)90047-h. [DOI] [PubMed] [Google Scholar]
- 13.Kanter M., Coskun O., Korkmaz A., Oter S. Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:685–691. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- 14.Kanter M., Meral I., Yener Z. Partial regeneration/proliferation of the beta-cells in the islets of Langerhans by Nigella sativa L. in streptozotocin-induced diabetic rats. Tohoku J Exp Med. 2003;201:213–219. doi: 10.1620/tjem.201.213. [DOI] [PubMed] [Google Scholar]
- 15.Kanter M., Coskun O., Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80:217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 16.Badary O.A., Nagi M.N., al-Shabanah O.A. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can J Physiol Pharmacol. 1997;75:1356–1361. [PubMed] [Google Scholar]
- 17.Houghton P.J., Zarka R., de las Heras B., Hoult J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 18.Al-Ali A., Alkhawajah A.A., Randhawa M.A., Shaikh N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad. 2008;20:25–27. [PubMed] [Google Scholar]
- 19.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase. In: Bergmeyer H.U., editor. Methods of Enzymatic Analysis. Academic Press; New York: 1974. pp. 673–677. [Google Scholar]
- 21.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 22.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Sun Y., Oberley L.W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 24.Karahan I., Atessahin A., Yilmaz S. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215:198–204. doi: 10.1016/j.tox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Celik S., Gorur S., Aslantas O. Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli-induced pyelonephritis in rats. Mol Cell Biochem. 2007;297:131–138. doi: 10.1007/s11010-006-9337-x. [DOI] [PubMed] [Google Scholar]
- 26.Kucheria R., Dasgupta P., Sacks S.H. Urinary tract infections: new insights into a common problem. Postgrad Med J. 2005;81:83–86. doi: 10.1136/pgmj.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badary O.A., Taha R.A., Gamal el-Din A.M., Abdel-Wahab M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 28.Nagi M.N., Mansour M.A. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41:283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 29.Pompella A. Biochemistry and histochemistry of oxidant stress and lipid peroxidation. Int J Vitam Nutr Res. 1997;67:289–297. [PubMed] [Google Scholar]
- 30.Abdelmeguid N.E., Fakhoury R., Kamal S.M., Al Wafai R.J. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic beta-cells of streptozotocin-induced diabetic rats. J Diabetes. 2010;2:256–266. doi: 10.1111/j.1753-0407.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 31.Fouda A.M., Daba M.H., Dahab G.M., Sharaf El-Din O.A. Thymoquinone ameliorates renal oxidative damage and proliferative response induced by mercuric chloride in rats. Basic Clin Pharmacol Toxicol. 2008;103:109–118. doi: 10.1111/j.1742-7843.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanter M. Protective effects of thymoquinone on streptozotocin-induced diabetic nephropathy. J Mol Histol. 2009;40:107–115. doi: 10.1007/s10735-009-9220-7. [DOI] [PubMed] [Google Scholar]
- 33.Sayed-Ahmed M.M., Nagi M.N. Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol. 2007;34:399–405. doi: 10.1111/j.1440-1681.2007.04560.x. [DOI] [PubMed] [Google Scholar]


